Fuel cells are electrochemical devices that convert chemical energy stored in fuels (e.g., hydrogen gas) into electricity. Their functionality relies on a rapid oxygen reduction reaction (ORR) for energy generation. Since ORR is a non-spontaneous process with a high reaction potential barrier, electrocatalysts containing noble metals (e.g., Pt) are typically used to reduce this barrier and improve the fuel cell energy efficiency. The scarcity and high cost of Pt is one of the reasons hindering broad adoption of fuel cells. A research team led by Di-Jia Liu, a senior chemist at Argonne National Laboratory, recently developed a novel ORR catalyst with record low Pt mass (0.035 mgPt/cm2) that outperformed its commercial counterparts. This breakthrough was reported in a recent issue of Science (doi:10.1126/science.aau0630).
“This discovery happened to us serendipitously,” Liu says. The researchers initially focused on the development of a heterogeneous catalyst for gas-phase biofuel production. They synthesized several materials consisting of Pt-Co core–shell nanoparticles sprinkled on N-doped porous carbon substrates by thermally annealing Co/Zn zeolitic imidazolate frameworks and Pt precursors. Afterward, “we could not help but to test their ORR catalytic activities in fuel cells since we are an electrocatalysis-fuel cell group, and the experimental setup was already there,” Liu says; “we were glad we did it.”
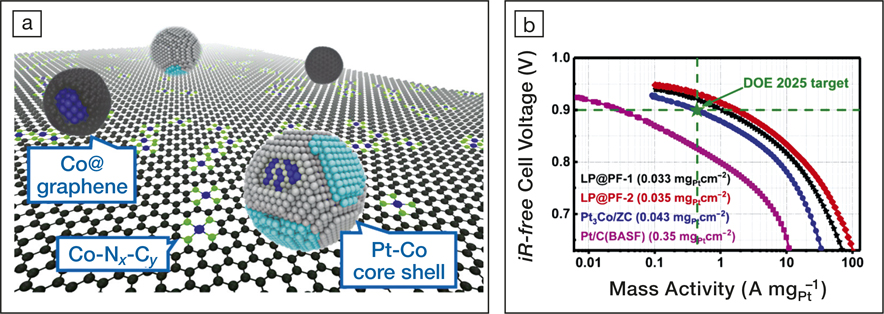
(a) An illustration of the microstructure of the developed catalyst: Pt-Co nanoparticles and graphene-wrapped Co nanoparticles anchored on a Co, N-containing carbon surface. (b) Tafel plots of output voltage versus mass activities of four oxygen reduction reaction catalysts. LP@PF-2 is the best catalyst the researchers synthesized. The green star marks the performance set by the 2025 target of the US Department of Energy (DOE), Fuel Cell Technologies Office. Credit: Di-Jia Liu.
The developed ORR catalysts were fabricated into fuel cell membrane electrodes for performance evaluation. The electrodes contained ultralow Pt loadings, approximately one tenth of those used in commercial electrodes, while still exhibiting excellent ORR catalytic activity. At an output voltage of 0.9 V, the highest mass activity (current generated per milligram of Pt) was 1.77 A/mgPt, which exceeds a 2025 target (0.44 A/mgPt) set by the US Department of Energy. The improved ORR catalytic activity was attributed to the synergistic catalysis between the Pt-Co nanoparticles and the Co, N-containing carbon support. Specifically, in addition to directly reducing O2 to water over the Pt-Co nanoparticles, the N-coordinated cobalt (Co-Nx-Cy) sites on the substrate can also reduce O2 to water and H2O2. The generated H2O2 then diffuses to the surface of nearby Pt-Co nanoparticles where it is eventually reduced to water.
Bao Yu Xia of Huazhong University of Science & Technology, China, says that the key deliverables of this work, “developing cost-effective and scalable approaches for some of the most promising ORR catalysts with ultralow Pt contents,” as well as understanding their catalytic activities in fuel cells are vital to large-scale implementation of fuel cells. Xia was not involved in this study.
“This work brings out a new research direction and is far from complete,” Liu says. The research group is investigating various issues to further enhance the performance of their catalysts, including the optimal distance between the Pt-Co nanoparticles and the Co-Nx-Cy coordination sites, the influence of humidity on the synergistic catalysis, and the minimal Pt loading possible without sacrificing catalytic activity.


