Electroconvulsive therapy (ECT) remains the most effective treatment for severe depression, although it was first introduced in 1938 as a treatment for schizophrenia. A recent meta-analysis reconfirmed the efficacy and safety of this therapy in depression (UK ECT Review Group, 2003). Despite its established utility, however, the precise mechanisms of action of ECT remain unknown (Reference SackeimSackeim, 1994; Reference AbramsAbrams, 2002). One useful approach to determine these mechanisms would be neuroimaging techniques that could elucidate the functional anatomy of ECT. Blumenfeld et al (Reference Blumenfeld, Westerveld and Ostroff2003) measured cerebral blood flow during ECT by using single photon emission computed tomography (SPECT). However, for each patient, ictal and interictal SPECT scans were performed on separate days of ECT. In this study, we examined the acute effects of ECT on the regional cebral blood flow (rCBF) in patients with major depression by performing serial high-resolution [15O]H2O positron emission tomography (PET) scanning during the same session of ECT.
METHOD
Participants
Six people undergoing in-patient treatment (4 men and 2 women; mean age 55.0 years, s.d.=16.1, range 35–70) who fulfilled DSM–IV criteria (American Psychiatric Association, 1994) for major depressive disorder (five recurrent episodes and one single episode) participated in this study. The mean of their total scores on the Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1960) was 27.3 (s.d.= 9.8, range 20–46). All patients were right-handed and had no history of major medical illness, psychotic disorder (other than as part of a mood disorder), cognitive disorder, psychoactive substance misuse or dependence, and had not received ECT up to 6 months prior to the study. Their medication for anxiety and sleep disturbances included lorazepam (1–3 mg per day), flunitrazepam (2–4 mg per day) and trazodone (50–150 mg per day). All patients provided written informed consent prior to their participation in the study. The study was approved by the Intramural Research Board of the National Centre of Neurology and Psychiatry.
All patients experienced clinical improvement after the ECT course (mean 8.5 sessions per patient, range 5–12); this was demonstrated by a reduced HRSD score (before ECT, mean=27.3, s.d.=9.8; after ECT, mean=14.5, s.d.=9.0; paired t-test, P=0.02).
Experimental procedure
A PET scan was performed on each patient during the first session of the ECT course. On the day of the experiment the patients fasted and did not take any medication after breakfast. The experiment began at 14.00 h, which was more than 16 h after the last intake of medication. Electroencephalograms (EEGs) were recorded from the disc electrodes placed at F3, F4, P3, P4, Fz, Cz and Pz; the A1 and A2 electrodes were used as references. All inter-electrode impedances were maintained below 10 kΩ. The EEG was amplified by a multichannel EEG amplifier (Neurotop, Nihon Kohden, Tokyo, Japan) and filtered using high-cut (low-pass) and low-cut (high-pass) filters with frequencies of 60 Hz and 0.53 Hz respectively. A venous line was inserted into the right median antebrachial vein for transfusion and injecting a tracer, and an arterial line was inserted into the left radial artery to measure the radioactivity in the blood sample throughout the scanning period. The blood pressure, pulse rate and arterial blood gas were monitored throughout the experiment. Propofol (5 mg/kg per h) and vecuronium bromide (0.15 mg/kg initially and 0.04 mg/kg later) were used for anaesthesia. A laryngeal mask was inserted and the patient was kept under controlled ventilation with a respirator (tidal volume 10 mL/kg; 8 times per min). The current was administered through the bilateral temporal regions by means of a Thymatron DGx with disposable electrodes (Somatics Inc., Lake Bluff, Illinois, USA). The initial stimulus dose was 101.2 mC, and this was later increased in cases of aborted seizures (no spike and wave complex observed on the EEG, i.e. there was no seizure generalisation). In the case of complete seizures, the mean duration of seizure activity on the EEG was 67.5 s (s.d.=26.1, range 50–120). A maximum of 12 intravenous injections of the radioisotope were administered during relaxed wakefulness (3 injections), prior to ECT (3 injections), during ECT (1–3 injections) and after ECT (3 injections) under anaesthesia. In order to perform a scan during ECT, the radioisotope was injected just prior to the electrical stimulation. The interval between each scan was approximately 10 min.
Scanning procedure
The PET images were acquired on a Siemens ECAT EXACT HR 961 scanner (http://www.medical.siemens.com) in the three-dimensional mode, as described in a previous report (Reference Kajimura, Uchiyama and TakayamaKajimura et al, 1999). In brief, a camera with a 150 mm axial field of view was used to acquire data simultaneously from 47 consecutive axial planes. An image resolution of 3.8×3.8×4.7 mm was obtained after back projection and filtering (Hanning filter; cut-off frequency 0.5 cycles per pixel). The reconstructed image was displayed in a matrix of 128×128×47 voxel format (voxel size 1.7×1.7×3.1 mm). Prior to the acquisition of emission data, a 10 min transmission scan was carried out using a retractable rotating 68Ga/68Ge source with three rods to correct for tissue attenuation and background activity. For each scan, 259 MBq of [15O]H2O was automatically flushed intravenously in a bolus manner over a period of 15 s. The total radioactive dose per patient was less than 1 mSv. The scanning was started manually 1 s after the initial increase in head counts and was continued for 90 s. The arterial blood was sampled automatically throughout the scanning period using a Pico-Count flow-through radioactivity monitor (Bioscan Inc., Washington, DC, USA). Absolute rCBF images were produced based on the arterial time activity data obtained using an autoradiographic method (Reference Herscovitch, Markham and RaichleHerscovitch et al, 1983; Reference Raichle, Martin and HerscovitchRaichle et al, 1983).
Data analysis
The PET images were analysed using the Statistical Parametric Mapping 2 (SPM2) software (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB version 6.5 (MathWorks, Inc., Sherborn, Massachusetts, USA) for Windows XP on a personal computer. Spatial normalisation was employed to fit each individual brain to a standard template brain in a three-dimensional space to correct for differences in the brain size and shape and to facilitate inter-individual averaging. The stereotaxically normalised scans contained 68 planes (voxel size 2×2×2 mm) and a final image with a resolution of 17×17×20 mm was produced by smoothing with a 10 mm Gaussian kernel. Since SPM2 uses a standard brain from the Montreal Neurological Institute, Canada, the precise anatomical localisations of significant changes were indicated in accordance with the atlas of Talairach & Tournoux (Reference Talairach and Tournoux1988) using a numerical transformation formula. Global cerebral blood flow (gCBF) was calculated as the sum of the grey-matter blood flow, including that of the region of interest after spatial normalisation. First, the absolute gCBF rates during, before and after ECT as well as during wakefulness were analysed and compared in this study; global normalisation with proportional scaling was then used to compare the relative changes in the rCBF rate.
After specifying the appropriate design matrix, the condition of each voxel in each patient was assessed in accordance with the theory of Gaussian fields. The exact significance level of the difference between the conditions was characterised by the peak amplitude. In this study we focused on the cluster level to detect significantly different regions because our sample size was too small to be analysed by the random field theory, and such an analysis would lead to type II errors (false negative). Since we had some data on the neural mechanism of action of ECT (Reference Bajc, Medved and BasicBajc et al, 1989; Reference Blumenfeld, Westerveld and OstroffBlumenfeld et al, 2003), we performed a priori studies. In general, the significance level was thresholded at P<0.05 with a false discovery rate correction (Reference Genovese, Lazar and NicholsGenovese et al, 2002), and the minimum cluster size (k) was set at 100 voxels. Finally, the resulting T-values were converted to Z-scores for interpretation. In order to compare the physiological variables and the gCBF, one-way analysis of variance was performed followed by Bonferroni's multiple comparison test.
RESULTS
We successfully performed a total of 16 scans at rest, 18 scans pre-ECT under anaesthesia, 6 scans during ECT (generalised seizures) and 15 scans post-ECT.
The physiological variables are listed in Table 1. The systolic blood pressure values increased during ECT compared with pre-ECT values (P=0.042). Other variables showed no significant change across the four different states (at rest, pre-ECT, during ECT and post-ECT).
Table 1 Physiological variables

| Awake | Pre-ECT | During ECT | Post-ECT | |
|---|---|---|---|---|
| Heart rate, beats/min: mean (s.d.) | 64 (5) | 63 (7) | 67 (8) | 61 (13) |
| Systolic blood pressure, mmHg: mean (s.d.) | 128 (14) | 115 (7) | 136 (10)* | 123 (14) |
| Diastolic blood pressure, mmHg: mean (s.d.) | 77 (6) | 73 (9) | 85 (6) | 78 (11) |
| Paco2, mmHg: mean (s.d.) | 44 (3) | 39 (5) | 41 (6) | 37 (4) |
The gCBF decreased significantly when the patients were under propofol anaesthesia compared with when they were awake: mean 45.1 ml/100 g per min (s.d.=5.5) v. mean 20.5 ml/100 g per min (s.d.=4.8); P=0.0001. During ECT of generalised seizures, the gCBF value increased significantly compared with the baseline pre-ECT values: mean 37.5 ml/100 g per min (s.d.=8.9) during ECT; P=0.0001. Approximately 10–30 min post-ECT the gCBF value returned to the baseline pre-ECT values: mean 21.2 ml/100 g per min (s.d.=4.7); P=1.0. The averaged images obtained at each stage are shown in Fig. 1.

Fig. 1 The averaged positron emission tomography images of the regional cerebral blood flow (rCBF) include those of (a) 16 scans at rest, (b) 18 scans before electroconvulsive therapy (ECT), (c) 6 scans during ECT and (d) 15 scans post-ECT. The brain slices are oriented in the plane of the Talairach atlas; the distances (mm) above or below the anterior–posterior commissure line are –34, –18, –2, 14 and 38, from left to right. The right side of the brain is depicted by the right side of each slice. The colour scale on the right side of the figure indicates the degree of the rCBF (ml/100 g per min).
In order to clarify the neural systems related to the acute effects of ECT, we examined the distribution patterns of the rCBF in patients at rest, pre-ECT, during ECT and post-ECT. Compared with the baseline pre-ECT values under anaesthesia, during ECT the relative rCBF significantly increased in the basal ganglia, midbrain, pontine tegmentum, thalamus, amygdala, hypothalamus and vermis as well as in the inferior frontal, parietal and temporal cortices (Fig. 2, Table 2). Identical trends were observed when the values were compared with the relative rCBF at rest (data not shown).

Fig. 2 Upper row: transverse sections of the brain areas with significantly higher relative regional cerebral blood flow values during electroconvulsive therapy (ECT) than the pre-ECT value. The brain slices are oriented in the plane of the Talairach atlas; the distances (mm) above or below the anterior–posterior commissure line are –24, –6, 6 and 24, from left to right. The right side of the brain is depicted by the right side of each slice. Lower row: left side, sagittal section; right side, coronal section. The colour scale on the right side of the figure indicates the T-value (Ant., anterior).
Table 2 Local statistical maxima in the pattern of increased cerebral blood flow before v. during electroconvulsive therapy
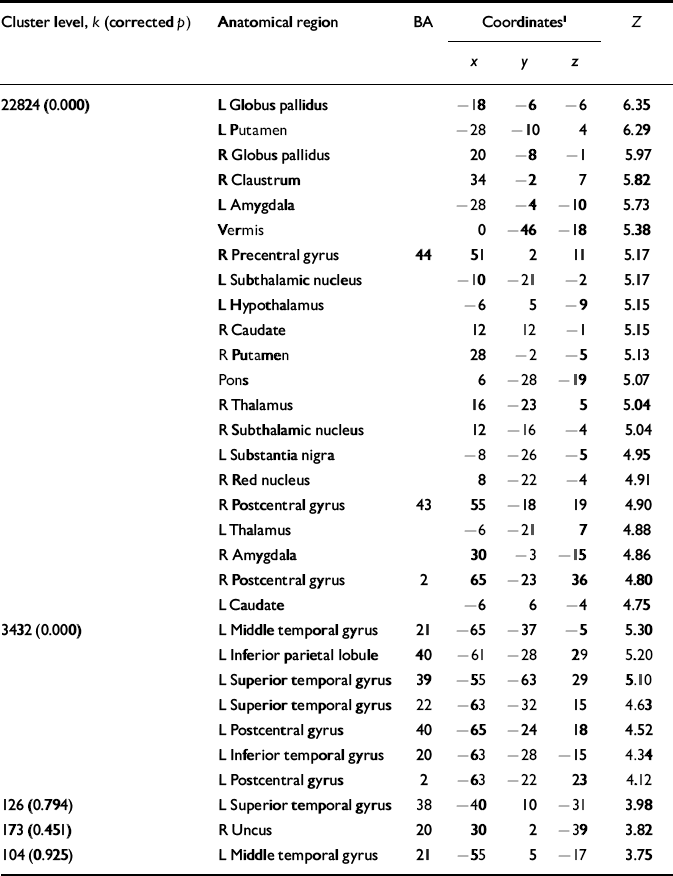
| Cluster level, k (corrected p) | Anatomical region | BA | Coordinates1 | Z | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 22824 (0.000) | L Globus pallidus | -18 | -6 | -6 | 6.35 | |
| L Putamen | -28 | -10 | 4 | 6.29 | ||
| R Globus pallidus | 20 | -8 | -1 | 5.97 | ||
| R Claustrum | 34 | -2 | 7 | 5.82 | ||
| L Amygdala | -28 | -4 | -10 | 5.73 | ||
| Vermis | 0 | -46 | -18 | 5.38 | ||
| R Precentral gyrus | 44 | 51 | 2 | 11 | 5.17 | |
| L Subthalamic nucleus | -10 | -21 | -2 | 5.17 | ||
| L Hypothalamus | -6 | 5 | -9 | 5.15 | ||
| R Caudate | 12 | 12 | -1 | 5.15 | ||
| R Putamen | 28 | -2 | -5 | 5.13 | ||
| Pons | 6 | -28 | -19 | 5.07 | ||
| R Thalamus | 16 | -23 | 5 | 5.04 | ||
| R Subthalamic nucleus | 12 | -16 | -4 | 5.04 | ||
| L Substantia nigra | -8 | -26 | -5 | 4.95 | ||
| R Red nucleus | 8 | -22 | -4 | 4.91 | ||
| R Postcentral gyrus | 43 | 55 | -18 | 19 | 4.90 | |
| L Thalamus | -6 | -21 | 7 | 4.88 | ||
| R Amygdala | 30 | -3 | -15 | 4.86 | ||
| R Postcentral gyrus | 2 | 65 | -23 | 36 | 4.80 | |
| L Caudate | -6 | 6 | -4 | 4.75 | ||
| 3432 (0.000) | L Middle temporal gyrus | 21 | -65 | -37 | -5 | 5.30 |
| L Inferior parietal lobule | 40 | -61 | -28 | 29 | 5.20 | |
| L Superior temporal gyrus | 39 | -55 | -63 | 29 | 5.10 | |
| L Superior temporal gyrus | 22 | -63 | -32 | 15 | 4.63 | |
| L Postcentral gyrus | 40 | -65 | -24 | 18 | 4.52 | |
| L Inferior temporal gyrus | 20 | -63 | -28 | -15 | 4.34 | |
| L Postcentral gyrus | 2 | -63 | -22 | 23 | 4.12 | |
| 126 (0.794) | L Superior temporal gyrus | 38 | -40 | 10 | -31 | 3.98 |
| 173 (0.451) | R Uncus | 20 | 30 | 2 | -39 | 3.82 |
| 104 (0.925) | L Middle temporal gyrus | 21 | -55 | 5 | -17 | 3.75 |
Post-ECT, the rCBF increased in the thalamus and decreased in the anterior cingulate (Brodmann's areas 24 and 32) and dorsolateral and medial frontal cortices (Brodmann's areas 6, 8, 9, 10 and 11) compared with the pre-ECT rCBF (Fig. 3).

Fig. 3 Transverse sections of the brain areas with significantly higher (upper row) and lower regional cerebral blood flow (lower row) values following electroconvulsive therapy (ECT) compared with the pre-ECT value. The brain slices are oriented in the plane of the Talairach atlas; the distances (mm) above or below the anterior–posterior commissure line are 10 (upper row), 24 and 38 (lower row, from left to right). The right side of the brain is depicted by the right side of each slice. The colour scale on the right side of the figure indicates the T-value.
DISCUSSION
In this study, we were able to completely induce generalised seizures in all six patients in a safe and reliable manner by using ECT; we also examined the temporal changes in the cerebral blood flow using [15O]H2O PET scanning. We observed changes in the absolute values of the gCBF and the relative values of the rCBF during the same session of ECT.
Previous research
Thus far, the acute effects of ECT or generalised seizures on cerebral blood flow have been examined in a limited number of human studies by using the xenon-133 inhalation method, PET and SPECT (Reference Bajc, Medved and BasicBajc et al, 1989; Reference Nobler, Sackeim and ProhovnikmNobler et al, 1994; Reference Scott, Dougall and RossScott et al, 1994). Previous reports have revealed that the cerebral blood flow or cerebral glucose uptake increases during ECT or generalised seizures (Reference Engel, Kuhl and PhelpsEngel et al, 1982; Reference Bajc, Medved and BasicBajc et al, 1989; Reference Theodore, Balish and LeidermanTheodore et al, 1996) and decreases after ECT (Reference Nobler, Sackeim and ProhovnikmNobler et al, 1994; Reference Scott, Dougall and RossScott et al, 1994).
With regard to the regional distribution, blood flow increased in the frontal cortex, temporal cortex and basal ganglia and decreased in the parietal or occipital cortex during ECT (Reference Bajc, Medved and BasicBajc et al, 1989). Moreover, the rCBF decreased in the inferior anterior cingulate cortex 45 min after ECT (Reference Scott, Dougall and RossScott et al, 1994). One PET study revealed that the global cerebral metabolic rate increased during an ECT-induced seizure and reduced post-ictally. The pattern of post-ictal hypometabolism was more prominent in the cortical structures than in the grey-matter structures (Reference Engel, Kuhl and PhelpsEngel et al, 1982). Further, only one group of researchers serially measured cerebral blood flow by using [15O]H2O PET in chemically induced seizures in people with epilepsy. They demonstrated that in two patients with generalised tonic–clonic seizures, the CBF increased, particularly in the thalamus (Reference Theodore, Balish and LeidermanTheodore et al, 1996). More recently, Blumenfeld et al (Reference Blumenfeld, Westerveld and Ostroff2003) reported that the focal regions of the frontal and parietal association cortices show the greatest relative signal increase in SPECT during ECT.
Our finding of a significant increase in the gCBF during ECT and a decrease in the gCBF following ECT are in agreement with results reported previously (Reference Engel, Kuhl and PhelpsEngel et al, 1982; Reference Bajc, Medved and BasicBajc et al, 1989; Reference Nobler, Sackeim and ProhovnikmNobler et al, 1994; Reference Blumenfeld, Westerveld and OstroffBlumenfeld et al, 2003). With regard to the regional distribution patterns, Bajc et al (Reference Bajc, Medved and Basic1989) demonstrated relative increases in blood flow in the frontal and frontotemporal regions as well as in the basal ganglia in SPECT during ECT under anaesthesia. Our findings obtained during generalised ECT support these results. Further, the high-resolution PET used in our study facilitated the observation of the blood flow changes during ECT in the subcortical structures. In our study the generalised seizures increased the cerebral blood flow, particularly in the basal ganglia and reticular formation. These results are similar to those obtained in a recent SPECT study (Reference Blumenfeld, Westerveld and OstroffBlumenfeld et al, 2003) and are in agreement with the viewpoint that the reticular formation is involved in the generalisation of seizure activity (Reference FrommFromm, 1991).
Importance of the centrencephalic system during generalised ECT
Approximately 50 years ago, Penfield & Jasper (Reference Penfield and Jasper1954) proposed that the centrencephalic system is involved in seizure generalisation. In contrast to the slow progression observed in the Jacksonian motor seizure, the sudden rapid generalisation observed in grand mal seizures does not appear to occur by the spread of excitation via the cortical circuits. These authors defined the centrencephalic system as ‘the neuronal system in the higher brain-stem that demonstrated a functional relationship with the two hemispheres’ (Reference Penfield and JasperPenfield & Jasper, 1954). Although this system has been theoretically defined, the precise neural circuits of the centrencephalic system have not been clarified. Thus, our study in humans further supports this centrencephalic theory of seizure generalisation.
Since a chemically induced convulsion was also effective, generalised seizures of adequate duration appear to be closely related to the efficacy of ECT (Reference SackeimSackeim, 1994). Recent studies on deep brain stimulation and vagus nerve stimulation have demonstrated that the brain-stem structures are closely related to the pathophysiology of depression (Reference Bejjani, Damier and ArnulfBejjani et al, 1999; Reference Rush, George and SackeimRush et al, 2000). Thus, further studies on the centrencephalic system, including the brain-stem, are required to elucidate the mechanisms of action of ECT.
Cerebral blood flow in the post-ECT state
In our study, the rCBF level remained elevated in the thalamus and decreased in the anterior cingulate and medial frontal cortex following ECT. These results are in agreement with those reported previously with regard to the degree of rCBF after ECT or generalised tonic–clonic seizures in humans (Reference Nobler, Sackeim and ProhovnikmNobler et al, 1994; Reference Scott, Dougall and RossScott et al, 1994; Reference Theodore, Balish and LeidermanTheodore et al, 1996). Both the anterior cingulate and medial frontal cortex have long been thought to be involved in the pathophysiology of depression (see Reference DrevetsDrevets, 2000, for review). In particular, Nobler et al (Reference Nobler, Sackeim and Prohovnikm1994) demonstrated that post-ictal blood flow reductions in anterior cortical regions were associated with a positive clinical response. This finding was in line with the hypothesis proposed by Sackeim (Reference Sackeim1999) that diminished activity in these regions might be a reflection of the anticonvulsant effect of ECT – that is, triggering endogenous brain processes to terminate generalised seizures. Furthermore, it is interesting to note that metabolic activity decreased in the anterior cingulate cortex after treatment with different classes of antidepressants (e.g. selective serotonin uptake inhibitors) and interpersonal therapy (Reference Buchsbaum, Wu and SiegelBuchsbaum et al, 1997; Reference Mayberg, Brannan and MahurinMayberg et al, 2000; Reference Brody, Saxena and StoesselBrody et al, 2001). In addition, we are inclined to speculate that the increased blood flow in the thalamus following ECT may also be related to the therapeutic effects of ECT because depression is characterised by symptoms of diencephalic disturbances (Reference Carney and SheffieldCarney & Sheffield, 1973; Reference Abrams and TaylorAbrams & Taylor, 1976). Although repeated ECT is generally necessary for ameliorating depressive symptoms, the antidepressant effects of ECT are probably associated with changes in blood flow in the anterior cingulate and medial frontal cortex and thalamus.
Study limitations
This study has several limitations, including the relatively small sample size. In addition, the age range of our patients was wide, and they were not all of the same gender. However, PET scans were obtained from each patient prior to, during and following ECT, and we carefully noted the time course of the changes in cerebral blood flow during ECT. Another limitation arises from the fact that the patients were receiving some medication. The effects of the medication on cerebral blood flow cannot be ruled out, although the patients had fasted for more than 16 h between the last intake of medication and the time of the experiment. Moreover, we think that there is a relationship between muscle tone and blood pressure, although vecuronium (a muscle relaxant) was used to minimise changes in muscle tone, and the blood pressure would not be directly influenced owing to the mechanism of autoregulation in the brain.
In conclusion, to our knowledge this is the first PET study that has serially measured cerebral blood flow during acute ECT. Our results suggest that acute ECT increases cerebral blood flow, particularly in the centrencephalic system, and one of the mechanisms of action of ECT may be related to the brain regions that include the anterior cingulate and medial frontal cortex and the thalamus.
Acknowledgements
We thank Dr Richard Abrams for reviewing the manuscript and providing his comments. A part of this work was supported by research grants from the Ministry of Health, Labour and Welfare of Japan; the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Welfide Medical Research Foundation, Japan.








eLetters
No eLetters have been published for this article.