1 Introduction
Several macroevolutionary hypotheses, including Reference Van Valenvan Valen’s (1973) Red Queen and Reference VermeijVermeij’s (1977, Reference Vermeij1987) Escalation, posit that ecological interactions are an important evolutionary force. However, our current understanding of the effects of biotic interactions on evolution relies predominantly on a single taxonomic group, mollusks, and their predators. Drill hole frequencies on mollusk prey have classically been used as a proxy for predation intensity experienced by ancient species and communities (e.g., Reference Vermeij, Tevesz and McCallVermeij, 1983; Reference Kowalewski, Dulai and FursichKowalewski et al., 1998; Reference Kelley, Hansen, Kelley, Kowalewski and HansenKelley and Hansen, 2003; Reference Klompmaker, Kelley and ChattopadhyayKlompmaker et al., 2019), as they are readily identifiable and quantifiable. Several ecological and morphological trends in marine invertebrate groups have been interpreted as adaptations in response to the selective pressures of biotic interactions, most commonly trends observed during the Mesozoic Marine Revolution. These include but are not limited to increasing shell ribosity and spinosity (Reference Signor and BrettSignor and Brett, 1984; Reference Harper and SkeltonHarper and Skelton, 1993), increased prevalence of encrusting and cementing prey (Reference HautmannHautmann, 2004; Reference TackettTackett, 2016), increased mobility (Reference Aberhan, Kiessling and FürsichAberhan et al., 2006), and increased frequency and depth of infaunalization (Reference Buatois, Mángano and DesaiBuatois et al., 2022). If biotic interactions are an important cause of evolutionary change, we should also be able to identify patterns in interaction intensity and diversity in other groups, such as echinoids. Echinoids are important prey for several predatory groups in Recent marine ecosystems, including crabs, birds, fish, gastropods, and other echinoderms (see Reference Kowalewski and NebelsickKowalewski and Nebelsick, 2003; Reference Farrar, Graves and PetsiosFarrar et al., 2020 and references therein); however, evolutionary trends in response to predation have received relatively little attention (but see Reference KierKier 1974, Reference Kier1982; Reference Petsios, Portell and FarrarPetsios et al., 2021).
Predators and parasites of echinoids include gastropods (Reference MooreMoore 1956; Reference ChesherChesher, 1969; Reference Hughes and HughesHughes and Hughes, 1971; Reference HendlerHendler, 1977; Reference GladfelterGladfelter 1978; Reference SerafySerafy, 1979; Reference WarénWarén, 1980a; Reference WarénWarén, 1980b; Reference HughesHughes, 1981; Reference KierKier, 1981; Reference WarénWarén et al. 1983; Reference FujiokaFujioka, 1985; Reference Alekseev, Endelman and KaljoAlekseev and Endelman, 1989; Reference Levitan and GenoveseLevitan and Genovese, 1989; Reference Warén and MoolenbeekWarén and Moolenbeek, 1989; Reference MifsudWarén and Mifsud, 1990; Reference Warén and CrosslandWarén and Crossland, 1991; Reference Crossland, Alford and CollinsCrossland et al., 1991, Reference Crossland, Collins and Alford1993; Reference McClintock and MarionMcClintock and Marion, 1993; Reference Oliverio, Buzzurro and VillaOliverio et al., 1994; Reference Rinaldi and MalacologicoRinaldi and Malacologico, 1994; Reference Warén, Norris and TempladoWarén et al., 1994; Reference McClanahanMcClanahan, 1999; Reference Nebelsick and KowalewskiNebelsick and Kowalewski, 1999; Reference Ceranka and ZlotnikCeranka and Złotnik, 2003; Reference Vaïtilingon, Eeckhaut, Fourgon and JangouxVaïtilingon et al., 2004; Reference Złotnik and CerankaZłotnik and Ceranka, 2005a, Reference Złotnik and Cerankab; Reference Neumann and WisshakNeumann and Wisshak 2009; Reference Meadows, Fordyce and BaumillerMeadows et al., 2015), polychaetes (Reference Wisshak and NeumannWisshak and Neumann, 2006), crustaceans (Reference Tegner and LevinTegner and Levin, 1983; Reference SmithSmith, 1984; Reference Wirtz, de Melo and de GraveWirtz et al., 2009) including barnacles (Reference Madsen and WolffMadsen and Wolff, 1965; Reference Cross, Rose, Bruno, Guille and FeralCross and Rose, 1994; Reference Donovan, Jagt and NieuwenhuisDonovan et al., 2016) and copepods (Reference MargaraMargara, 1946; Reference RomanRoman, 1954), echinoderms (Reference Merrill and HobsonMerril and Hobson, 1970; Reference SerafySerafy, 1979), fish (Reference Borszcz and ZatońBorszcz and Zatoń, 2013; Reference Wilson, Borszcz and ZatońWilson et al., 2014) and other vertebrates, such as turtles, birds, and sea otters (see references in Reference SmithSmith, 1984; Reference Hendler, Miller, Pawson and KierHendler et al., 1995; and Reference Nebelsick, Carnevali, Bonasoro, Carnevali and FrancescoNebelsick et al., 1998) (Table 1). Herein, we review biogenic skeletal traces found in both fossil and Recent echinoids, and the ecological interactions that are known or are interpreted to have caused them. We focus on interactions in Recent ecosystems that produce traces that are likely to be recognized in the fossil record, namely traces left on parts of the echinoid that are more likely to enter the fossil record (such as the test and spines) and that are readily diagnostic as having been produced by specific types of ecological interactions. We briefly explore important symbiotic interactions on Recent echinoid populations that are unlikely to be preserved in the fossil record, so-called invisible interactions. Finally, we review taxonomic diversification trends across the Meso-Cenozoic in regular and “irregular” echinoids, along with some of their common predators and parasites.
Table 1 List of echinoid associates, ecology of interaction, and known traces
2 Predators
Multiple vertebrate and invertebrate groups prey on both infaunal and epifaunal echinoids. Given their vastly different life habits, regular, and irregular echinoids employ different antipredatory strategies, and yet several predator groups prey on both regular and irregular echinoids alike (Reference Nebelsick, Carnevali, Bonasoro, Carnevali and FrancescoNebelsick et al., 1998). Skeletal damage caused by these predators has varying degrees of likelihood of preservation and identification in the fossil record, dependent upon the extent of the damage, the lethality of it, and the setting in which the attack takes place (Reference Tyler, Dexter, Portell and KowalewskiTyler et al., 2018).
2.1 Whole-Test Crushing Predation
Crushing predation is differentiated herein from margin damage predation (see Section 2.2) in that crushing predation causes damage to all or most of the test, such that survival and recovery of the prey is not possible. Fish are a major group of predators of echinoids (Reference Kowalewski and NebelsickKowalewski and Nebelsick, 2003; Reference Nebelsick and MancosuNebelsick and Mancosu, 2022). Many groups of teleost fish prey on regular and irregular echinoids and utilize varying strategies leaving behind different traces of test damage. In Recent ecosystems, for example, the Mediterranean urchin Sphaerechinus granularis is preyed on by sparid fish resulting in large, gaping wounds from these lethal attacks. Scratch marks, spine damage, and semicircular indentations are visible at the edges of the wounds (Reference Sievers and NebelsickSievers and Nebelsick, 2018). Stingrays have been observed preying on the large spatangoid echinoid Meoma ventricosa in San Salvador, Bahamas, resulting in test damage and substantial plate loss on the oral side with no observed spine damage (Reference GrunGrun, 2016). Other vertebrates, such as turtles, seabirds, and marine mammals also prey upon echinoids (Reference Kowalewski and NebelsickKowalewski and Nebelsick, 2003). Turtles bite into the test and crush it to access the viscera, and Loggerhead turtles have been observed preying on the large spatangoid echinoid Meoma ventricosa (Reference ChesherChesher, 1969). Diverse predation strategies are used by birds, and these vary according to the bird species. Some peck holes in the tests whereas others carry the prey and drop it onto a hard surface to fracture the test. Sea otters are the most common marine mammals preying on echinoids, and they break the test, leaving no recognizable traces (Reference NebelsickNebelsick, 1999).
Crustaceans and asteroids are common invertebrate predators of echinoids (Reference NebelsickNebelsick, 1999). Spiny lobsters use their mandibles to crush the tests of small sea urchins, and they feed on larger individuals by piercing and opening the peristomal membrane to access the viscera. Some of the large spiny lobsters even consume small urchins entirely. For example, the lobster Homarus americanus has been observed cracking urchin tests into pieces and feeding on the viscera (Reference Hagen and MannHagen and Mann, 1992). Spider crabs and rock crabs prey on regular and irregular echinoids. They pierce the peristomal membrane and enlarge the opening by removing pieces of the test and finally feed on the interior (Reference Kowalewski and NebelsickKowalewski and Nebelsick, 2003). The great spider crab Hyas araneus has been observed attacking the sea urchin Strongylocentrotus in the northern polar waters, and an individual was recorded surviving more than 40 hours after extensive test damage (Reference Wisshak and NeumannWisshak and Neumann, 2020). In sand dollars, crustaceans can leave behind marginal traces because of nonlethal predation (Reference ZinsmeisterZinsmeister, 1980; Reference NebelsickNebelsick, 1999). Asteroids prey on echinoids, usually consuming the entire test (Reference Merrill and HobsonMerrill and Hobson, 1970; Reference Birkeland and ChiaBirkeland and Chia, 1971). The sea urchin Diadema antillarum has been observed to prey on other echinoid species by removing their spines and creating a puncture hole in the test (Reference QuinnQuinn, 1965), and echinoid spines have been found in the gut content of Eucidaris tribuloides (Reference SerafySerafy, 1979).
Whereas crushing predation by vertebrates has reduced probability of being recognized in the fossil record due to the near total destruction of the test, there have been a few instances where potential traces of this behavior have been identified. Reference Neumann and HampeNeumann and Hampe (2018) interpreted a series of aligned circular puncture holes on the oral surface of a single specimen of the Maastrichtian holasteroid Echinocorys ovata as having been produced by a sublethal bite, potentially from a mosasauroid. Reference Borszcz and ZatońBorszcz and Zatón (2013) described Jurassic fish regurgitates containing the disarticulated and etched plates and spines of cidaroids, representing some of the earliest direct evidence of fish predation on echinoids. Reference Wilson, Borszcz and ZatońWilson et al. (2014) described slightly younger evidence of fish predation, from paired indentations on rhabdocidaroid echinoid spines that are interpreted as fish bite marks.
2.2 Margin Damage
Triggerfish prey on both regular and irregular echinoids (Reference Frazer, Lindberg and StantonFrazer et al., 1991). When triggerfish attack regular urchins, they bite into the peristomal membrane and ingest the viscera (Reference Kowalewski and NebelsickKowalewski and Nebelsick, 2003). The gray triggerfish (Balistes capriscus) has been observed feeding on clypeasteroid echinoids in the Gulf of Mexico (Reference Frazer, Lindberg and StantonFrazer et al., 1991; Reference KurzKurz, 1995). The triggerfish uses a complex technique to capture and feed on its prey. It exposes the sand dollar with jets of water, after the test is exposed, it then grasps the sand dollar with its teeth, lifts it and drops it on the substrate until its oral side is facing upward. The fish will then attack by crushing the edges of the test with its jaws and feeding on the viscera. Successful attacks leave behind large wounds in the oral side, jagged wound edges, intraplate fragmentation, test abrasion, and parallel tooth marks on the test (Reference Frazer, Lindberg and StantonFrazer et al., 1991; Reference Kowalewski and NebelsickKowalewski and Nebelsick, 2003). Cuspate-shaped bite marks on the ambitus produced in successful triggerfish attacks look similar to nonlethal marginal traces; however, they record lethal attacks and the damage is not limited to the ambitus. Similar traces have been observed in clypeasteroids in the Red Sea (Reference NebelsickNebelsick, 2020 and references therein). Sublethal triggerfish attacks can be identified by apparent healing of the test along the damaged margin, though the irregular shape of the test outline persists in the surviving echinoid (Figure 1H and 1K). In this way, sublethal margin damage is akin to crab repair scars on mollusks and can be used as a proxy for unsuccessful predation.

Figure 1 Examples of biotic traces found on fossil and Recent echinoids. Fossil (A–C) and Recent predation traces (D–F) produced from cassid gastropod predation. Note the highly beveled drill hole morphology in (A), and the subcircular drill hole morphology in (C). Parasitic trace on fossil echinoid (G) and Recent equivalent (J) denoted by arrows, with multiple eulimids still present on Recent specimen (also denoted by an arrow). Fossil (H) and modern (K) crab predation traces; fossil (I) and Recent (L) tube worm traces; fossil octopus predation trace (M); and post-depositional biotic traces with gastrochaenid bivalve borings (N) and clionid sponge borings (O). Species as follows: Fernandezaster whisleri (A, UF-IP 114520); Rhyncholampas gouldii (B, UF-IP 128804; C, UF-IP 5782; G, UF-IP 128439; M, UF-IP 128988); Meoma ventricosa (D, UF-IZ uncatalogued); Clypeaster reticulatus (E, UF-IZ 431); Echinoneus cyclostomus (F, UF-IZ 2642); Encope tamiamiensis (H, UF-IP 13759); Oligopygus wetherbyi (I, UF-IP 47955; O, UF-IP 46714); Echinothrix calamaris (J, UF-IZ 2226); Encope michelini (K, UF-IZ 4939); Plococidaris verticillata (L, UF-IZ 11109); Clypeaster rosaceus (N, UF-IZ 125419). Scale bars 1 cm
Lethal or nonlethal test damage in clypeasteroid echinoids can be caused by both predation and hydrodynamics (Reference Weihe and GrayWeihe and Grey, 1968; Reference Lawrence and TanLawrence and Tan, 2001 and references therein). Traces of nonlethal marginal test damage can be potentially diagnosed as biological in origin because of nonrandom species selectivity and site selectivity on tests. Marginal traces suggesting nonlethal predation have been observed on fossil and live specimens. This is not surprising given that clypeasteroids are robust and can survive multiple predatory attacks (Reference Nebelsick and KowalewskiNebelsick et al., 1999). The prevalence of nonlethal predatory traces in the fossil record (Reference Nebelsick and KowalewskiNebelsick et al., 1999) is attributed to the structural integrity of the test being preserved when the damage is limited to the ambitus (Reference NebelsickNebelsick, 2020). Diverse predators that might produce nonlethal traces have been documented in Recent environments. These include predation by blue crabs (Callinectes sapidus) on Mellita (Reference Weihe and GrayWeihe and Grey, 1968), Cancer sp. and sheep crabs (Loxorhynchus grandis) on Dendraster excentricus (Reference Merrill and HobsonMerrill and Hobson, 1970), benthic fish on Leodia sexiesperforata (Reference CrozierCrozier, 1919), and spiny lobsters (Panulirus interruptus) on Dendraster excentricus (Reference MacGinitie and MacGinitieMacGinitie and MacGinitie, 1968). Nonlethal predatory traces are more common in large specimens, and it is suggested that damage to the ambitus in smaller individuals or juveniles might be lethal (Reference Lawrence and TanLawrence and Tan, 2001). According to Reference CrozierCrozier (1919), the presence of multiple bites on a single test may be due to the fragility of the ambitus of the test or its form, making it easier for predators to break off pieces. Test damage tends to be more frequent in the posterior side of clypeasteroid tests (Reference Weihe and GrayWeihe and Gray, 1968; Reference BorzoneBorzone, 1992, Reference Borzone1994; Reference Nebelsick, Kampfer, Guille, Féral and RouxNebelsick and Kampfer, 1994; Reference Sonnenholzner, Lawrence, Mooi and TelfordSonnenholzner and Lawrence, 1998; Reference Lawrence and TanLaurence and Tan, 2001). Higher exposure of the posterior region of the test when buried in the substrate might cause more frequent attacks on the posterior portion of the test (Reference CrozierCrozier, 1919).
2.3 Drilling Predation
Cassid gastropods (family Cassidae) are well-known echinoid-targeting specialist predators today and produce diagnostic drill hole traces on their prey, which can be used to quantify the intensity of this biotic interaction in the fossil record (Reference Hughes and HughesHughes and Hughes, 1971; Reference McNamara, Guille, Féral and RouxMcNamara, 1994; Reference Nebelsick, Carnevali, Bonasoro, Carnevali and FrancescoNebelsick et al., 1998; Reference Kowalewski and NebelsickKowalewski and Nebelsick, 2003; Figures 1A–F and 2). Predation pressure by cassids can be high, leading to considerable echinoid mortality with drilling frequencies of 95 percent in some Recent populations (Reference McClintock and MarionMcClintock and Marion, 1993; Reference Tyler, Dexter, Portell and KowalewskiTyler et al., 2018). In many respects, cassid drilling behavior on echinoids is mechanistically analogous to that of other gastropod predators, such as naticids and muricids on mollusks, in that they actively hunt their prey and use a combination of mechanical and chemical dissolution to weaken the skeletal structure and gain access to internal tissue (Reference Hughes and HughesHughes and Hughes, 1971). Though understudied with respect to mollusk-associated drill holes, the microstructure, morphology, and stereotypy of echinoid-associated drill holes has recently become the focus of renewed interest (e.g., Reference Grun, Sievers and NebelsickGrun et al., 2014; Reference Tyler, Dexter, Portell and KowalewskiTyler et al., 2018; Reference Farrar, Graves and PetsiosFarrar et al., 2020). Recent studies have additionally shown that the position of the drill hole relative to particular test structures (e.g., pore pairs, plate boundaries, tubercles) can dramatically alter the morphology of the drill hole (Reference Złotnik and CerankaZłotnik and Ceranka, 2005a; see also discussions in Reference Farrar, Graves and PetsiosFarrar et al., 2020). The resulting wider range of drill hole morphologies on echinoids relative to those found on mollusk prey have perhaps impeded wide-scale efforts quantifying drill hole occurrences to the same extent as have been done for mollusks (e.g., Reference Kelley, Hansen, Kelley, Kowalewski and HansenKelley et al., 2003; but see Reference Petsios, Portell and FarrarPetsios et al., 2021). Though other forms of trace-producing predation are known to occur on Recent or fossil echinoids (e.g., Reference Sievers, Friedrich and NebelsickSievers et al., 2014; Reference Wilson, Borszcz and ZatońWilson et al., 2014; Reference GrunGrun, 2016; Reference Sievers and NebelsickSievers and Nebelsick, 2018; see discussion above), drill holes remain the best proxy for consistent assessment of predation intensity across longer timescales.

Figure 2 Examples of traces interpreted as predatory cassid drill holes in fossil and Recent echinoids. (A) Cassiduloid echinoid Rhyncholampas evergladensis from the Pliocene of Florida (UF-IP 21420). (B) Spatangoid echinoid Fernandezaster whisleri from the Pliocene of Florida (UF-IP 114520). (C) Recent clypeasteroid echinoid Leodia sexiesperforata from the Bahamas (UF-IZ 18904). Arrows indicate drill holes. Adoral view left, oral view right. Scale bar 1 cm
3 Parasites and Other Symbionts
Parasitism is common in Recent ecosystems (Reference Poulin and PoulinPoulin, 2011; Reference LeungLeung, 2017) and is known to be a significant driver of co-evolutionary adaptive pressures in host and parasite species. The complex relationship between parasites and their potentially multiple hosts across different parasite life stages make unraveling the evolutionary history of this interaction difficult in most cases (Reference De Baets and LittlewoodDe Baets and Littlewood, 2015). Studying parasitism in the fossil record at macroevolutionary scales is further obstructed by the paucity of fossilized evidence of parasitism, either in the form of the parasite body fossil itself, or a trace fossil of parasitic activities associated with the host (Reference DonovanDonovan, 2015). Drilling, bioerosive, and gall-forming parasitism in Recent ecosystems which produce potentially diagnostic traces on echinoid hard parts (Reference JangouxJangoux, 1987), are among the most likely to be recognized in the fossil record and can give critical insights into the evolution of echinoid-targeting parasitism.
3.1 Parasites That Produce Holes
Oichnus-type circular to subcircular depressions or holes produced by echinoid parasites have been distinguished from drill holes produced by predators mostly based on their much smaller size but sometimes by the morphology of the trace (see discussions in Reference Farrar, Graves and PetsiosFarrar et al., 2020). However, though some detailed descriptions exist of traces produced by a known parasite observed in direct contact with the echinoid host (see Reference Warén and CrosslandWarén and Crossland, 1991), few studies of Recent echinoids document or illustrate the style of attachment and the presence, absence and/or detailed morphology of drill holes at the attachment site beyond noting the association (see Reference WarénWarén, 1980b for examples). The diversity of drill hole trace morphologies produced by parasites is, thus, likely severely under-represented in the literature, hindering diagnosis of the ecology of these traces in the fossil record.
3.1.1 Eulimid Parasites
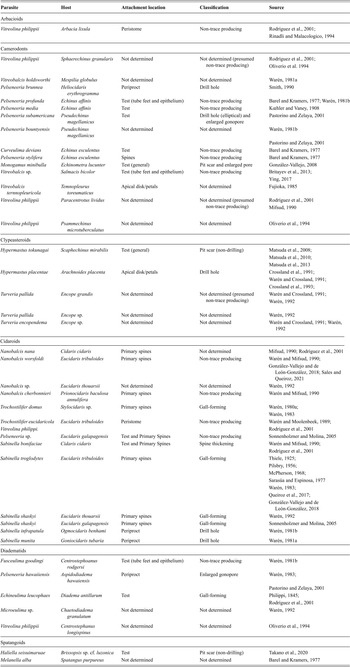
Ongoing efforts to clarify behaviors of trace-producing parasites focus on eulimid gastropods, the most common macroinvertebrate echinoid parasite (Reference WarénWarén, 1983; Figure 1J). Eulimid gastropods (family Eulimidae) are echinoderm specialized parasites (Reference Pearse, Cameron, Giese, Pearse and PearsePearse and Cameron, 1991) and are known to parasitize all five echinoderm classes: crinoids (Reference Schiaparelli, Ghirardo and BohnSchiaparelli et al., 2007; Reference Dgebuadze, Fedosov and KantorDgebuadze et al., 2012), asteroids (Reference ElderElder, 1979; Reference JanssenJanssen, 1985; Reference Salazar and Reyes BonillaSalazar and Reyes-Bonilla, 1998), holothurians (Reference WillWill, 2009; Reference González-Vallejo and Amador-CarrilloGonzález-Vallejo and Amador-Carrillo, 2021), ophiuroids (Reference WarénWarén, 1983; Reference Dgebuadze, Mekhova, Thanh and ZalotaDgebuadze et al., 2020), and echinoids (references in Table 1). Generally, eulimid genera are specific to hosts up to the level of order, though some occur on more distantly related hosts (e.g., the genus Pelseneeria occurs on cidaroids, diadematids, and camerodonts and the genus Vitreolina occurs on arbacioids, camerodonts, and diademids). Despite being one of the most diverse gastropod families with more than 100 genera and 1000 species (Reference Takano and KanoTakano and Kano, 2014; Reference Marshall and BouchetMarshall and Bouchet, 2015), the majority of species lack host information (Reference Warén and CrosslandWarén and Crossland, 1991) for various reasons related to the difficulty of collection, preservation, and systematic description of parasite-host association for zoological collections (e.g., Reference GeigerGeiger, 2016).
Eulimid parasites can be either endo- or ectoparasitic, with some highly specialized endoparasitic forms having lost a calcified shell completely (Reference WarénWarén, 1983). Ectoparasitic eulimids generally retain caenogastropod homologies, although many lack a radula or the proboscis (Reference WarénWarén, 1983). Despite this, eulimids have been observed producing drill hole traces in echinoid hosts, likely through some method of stereom dissolution, although the specific mechanisms at play are not well understood (Reference Warén and CrosslandWarén and Crossland, 1991). The “snout” of ectoparasitic eulimids consists of the proboscis (if present) and the specialized organ called the pseudopallium, which can be used to attach to the host and generally denudes the surrounding area of spines and may produce a circular pit scar. In Recent echinoids, ectoparasitic eulimids have been observed to attach to either the test (both in the interambulacral and ambulacral regions, Figure 3), peristomal membrane, periproct or periproctal membrane (including on the gonopores), and primary spines (Table 2). In the case of Thyca, female eulimids are known to use the pseudopallium to attach to hosts, which then aids the penetration of the proboscis through the exoskeleton, likely through chemical digestion of the stereom (Neumann and Wisshak, 2009 and references therein). This unique attachment strategy produces a relatively small, smooth-edged drill hole with an associated attachment halo trace, which had so far only been observed in asteroid-eulimid associations in present-day populations. Halo-bearing complete and incomplete drill hole traces in fossil echinoids have been postulated to represent a potentially extinct association of holasteroids and eulimids with similar attachment strategies observed in Thyca (Reference Neumann and WisshakNeumann and Wisshak, 2009). Eulimids associated with extant clypeasteroids can produce similarly small, circular, and smooth-edged drill hole traces lacking attachment haloes (Reference WarénWarén, 1981a). However, population surveys show that some eulimids attached to the test had penetrated the skeletal material (Reference Crossland, Alford and CollinsCrossland et al., 1991). Those that do penetrate the test where attached, specifically to the apical disk and petals, are presumably targeting the gonadal tissue (Reference Crossland, Alford and CollinsCrossland et al., 1991, Reference Crossland, Collins and Alford1993). In the limited cases, where details of the parasite-host biology are known (e.g., Reference Warén and CrosslandWarén and Crossland, 1991; Reference Crossland, Alford and CollinsCrossland et al., 1991), the parasite remains on the host for a few days, after which the host quickly heals the site of attachment and/or penetration. Whether or not non-trace producing associations like these still produce a skeletal trace or indentations that would be identifiable and diagnostic in the fossil record is a matter of further study. Thus, there are numerous hurdles to diagnosing and cataloging probable eulimid drill hole traces in the fossil record.

Figure 3 Micro-CT scans of two eulimid ectoparasites (Eulimidae indet., UF-IZ 463395) attached to Plococidaris sp. (UF-IZ 13593) at the (A–B) external interambulacral region of the echinoid host and (C) to the pore-pairs of the ambulacral region of the same echinoid. Scale bar 1 mm
Table 2 Eulimid parasites on echinoid and associated skeletal traces
Identification of trace fossils produced by eulimid parasitism is generally more challenging relative to cassid or fish predation. In the case of drilling eulimid parasitism, the drill hole may heal completely after the parasite abandons the host (Reference Warén and CrosslandWarén and Crossland, 1991) leaving no skeletal evidence of the association. Nevertheless, drill holes attributed to eulimid parasitism have been identified in the fossil record. Reference KierKier (1981) described multiple traces on the Early Cretaceous spatangoid Hemiaster elegans washitae. However, as pointed out by Reference Warén and CrosslandWarén (1991), these traces predate the first known fossil occurrence of the Eulimidae in the Late Cretaceous (Reference SohlSohl, 1964). The largest trace, which exhibits similar characteristics to Recent eulimid traces that form pit scars at the attachment site, likely formed syn-vivo based on the deformed growth of the ambulacral plates in the affected petal (Reference KierKier, 1981). It is likely that a hitherto unknown eulimid or eulimid ancestor may be responsible for the trace considering the relatively short time gap (17 to 41 Ma) between the Albian occurrence of this trace and the Campanian appearance of eulimids. The absence of eulimids during this interval may be the result of their poor fossil record in general, owing to their small size, thin shells, and lack of distinct shell characteristics that hinder preservation, collection, and identification. Reference Neumann and WisshakNeumann and Wisshak (2009) described several traces from Paleocene holasteroids that exhibit both penetrating and non-penetrating circular traces with conspicuous halos, which they likened to the attachment and penetration strategy of the Recent eulimid Thyca on their asteroid hosts. In some cases, the pit or attachment halo are far larger than any such Recent eulimid trace, confounding their interpretation. Additional fossil examples include those described by Reference Donovan, Jagt and DolsDonovan et al. (2010), Reference Donovan and JagtDonovan and Jagt (2013), and Reference Donovan, Jagt and LangeveldDonovan et al. (2018), which document several instances of non-penetrating pit scars on holasteroids from the Maastrichtian. In one case, a single specimen exhibited 170 pit scars that are assumed to have been formed syn-vivo (Reference Donovan, Jagt and LangeveldDonovan et al., 2018). Selectivity of the attachment sites near the ambulacra suggests that the trace maker was likely feeding on tissue associated with the tube feet. It is not clear that these traces were formed by eulimid parasites, but the non-penetrating pit scars and tube feet targeting behavior is known in Recent eulimids, though these behaviors are not known to occur simultaneously.
3.1.2 Muricid Parasites
A less common parasitic association involves the muricid gastropod Vexilla vexillum, which grazes on the epidermis of various echinoid hosts, including the echinometrid Colobocentrotus spp., and the camarodonts Echinometra mathaei and Tripneustes gratilla. Reference Vaïtilingon, Eeckhaut, Fourgon and JangouxVaïtilingon et al. (2004) described the life cycle and recruitment of the parasite and the impact of their feeding on the echinoid hosts. In T. gratilla, they observed that on smaller lesions, tube feet, spines, and pedicellaria regenerated after the parasite was removed, and did not result in alteration to the skeletal test. Larger lesions, however, were subject to secondary infestation and deteriorated to the point where the test was perforated, resulting in a skeletal trace. These traces were subcircular to irregular in outline, penetrated completely through the test, and led to host mortality. Trace fossils resembling this type of lesion have been documented in fossil echinoids (Reference Farrar, Graves and PetsiosFarrar et al., 2020).
3.1.3 Foraminifera Parasites
Foraminiferan parasitism has been postulated based on traces observed in fossils of the Maastrichtian holasteroid Echinocorys perconica. Reference Neumann and WisshakNeumann and Wisshak (2006) described shallow circular to subcircular indentations with an elevated rim on the outer margin of the oral side of the test, sometimes with a shallow central boss. These pits were thought to represent the outline of complete foraminifera tests, and the size and shape of these indentations has thus been used as a proxy for the size and shape of the parasite. Some pits have evidence of tubercle regeneration, indicating that this association was syn-vivo. The authors interpreted this interaction as similar to the modern-day behavior of the benthic foraminifera Hyrrokkin sarcophaga which is parasitic on shelly mollusks.
3.2 Parasites That Form Galls
Parasitic associations that result in the formation of a calcified gall on the test or spines of echinoids have been reported from Recent associations and less commonly from fossils.
3.2.1 Eulimid Galls
Some eulimid genera exhibit a unique parasitic strategy, whereby they form and occupy hollow gall domiciles on echinoid spines. The eulimid genera Sabinella and Trochostilifer form calcified galls on cidaroid spines in Recent ecosystems (Reference McPhersonMcPherson, 1968; Reference WarénWarén, 1980a; Reference Queiroz, Neves, Sales and JohnssonQueiroz et al., 2017; Reference González-Vallejo and León-GonzálezGonzález-Vallejo and de León-González, 2018). Spine galls have so far only been documented on cidaroids, which are especially vulnerable to epibiont attachment due to having exposed cortex without an epithelial layer in fully matured spines, unlike the spines of regular euechinoids (Reference EbertEbert, 1986). The eulimid parasites are commonly found within the gall cavity, and it is not uncommon to find both the larger female, smaller male, and egg pouches preserved in association (Figure 4). Both female and male Sabinella have been observed to attach to the floor of the gall cavity with their snout, producing circular indentations in the gall material (Reference González-Vallejo and León-GonzálezGonzález-Vallejo and de León-González, 2018). Eulimids found within fully formed galls exhibit fewer instances of shell breakage, suggesting that the gall serves as protection for the female-male pair (Reference WarénWarén, 1992).

Figure 4 Eulimid spine galls on Eucidaris. (A) Sabinella eulimid gastropod galls on Eucidaris tribuloides spines (LACM E.1985–240.7), preserved attached (arrow) to test with eulimid parasite brood in the gall cavity. (B) Close-up of spine in A. (C) X-ray slice of spine gall cavity on E. tribuloides with eulimid parasite. (D) Sabinella eulimid gastropod galls on Eucidaris thouarsii (LACM E.1949–89.11) spines (arrow). (E) Close-up of spine in D. Scale bar 1 cm
The gall material itself is calcified but exhibits malformed stereom that is less dense than healthy spine stereom (Figure 4D). The exact growth mechanism is unclear, but it is thought that the continued presence of the parasite triggers abnormal cell proliferation and growth, similar to the formation of a tumor (Reference Queiroz, Neves, Sales and JohnssonQueiroz et al., 2017). In galls from the parasite Sabinella, the initial gall forms as a small depression at the site of attachment that gradually grows until a cavity is formed (Reference WarénWarén, 1992). Feeding by the eulimid continues to erode the spine material, likely by corrosive substances delivered via the proboscis at the site of attachment (Reference Queiroz, Neves, Sales and JohnssonQueiroz et al., 2017). Although it has not been studied as extensively it can be assumed that similar mechanisms are at play in galls formed by the parasite Trochostilifer.
In the cases of eulimid-induced spine galls on cidaroids, the host may heal the affected spine (Reference WarénWarén, 1992), or even potentially shed it (Reference ProuhoProuho, 1887), though the full life cycle of galled spines has not been thoroughly documented. If the predominant method the host employs against galling parasites is to heal the spine, then this would reduce the frequency of galled spines being preserved as fossils. The opposite would apply if the hosts predominantly shed galled spines. Nevertheless, the gall is comprised of skeletal material, though malformed, and should have some likelihood of entering the fossil record. However, there are no known examples of fossil galled spines to date, despite targeted efforts by the authors. The present authors examined >700 cidaroid spines from Pliocene and Recent populations from California and Florida for evidence of galls and other symbiotic traces. In Florida, fossils of Eucidaris tribuloides are presumed to be the likely ancestors of the living E. tribuloides populations in the eastern Gulf of Mexico, as are the E. thouarsii fossil and Recent populations on the California coast. Recent populations exhibited extensive fouling by bryozoans, annelids, sponges, and barnacles, which has been documented previously (Reference Hopkins, Thompson, Walker, Davis, Heinzeller and NebelsickHopkins et al., 2004). Galled spines in various stages of development were also documented, though these were less common than spines with epibionts. Male and female specimens of Sabinella were present in some galls. Interestingly, spines with galls had no other epibionts present on those spines, despite extensive fouling of neighboring spines (Figure 4). In well-developed galls, the internal cavity was found to be encroaching on or in some cases completely replacing the healthy central medulla (core) of the spine, increasing the likelihood of breakage. No evidence of galls was found in either the California or Florida Pliocene spines examined. The Recent spines were more heavily fouled relative to their fossil counterparts, and there was evidence of encrustation by epibionts on some fossil spines (Figure 5), but this too was not as extensive as that in analogous Recent populations. There is also no known fossil occurrence of Sabinella in Pliocene assemblages in either region, suggesting that either the Pliocene echinoid populations may not have been experiencing parasitism pressure by this eulimid, which was either rare or absent, or possibly poorly preserved due to the small size and thin shell of Sabinella. This raises the possibility that the gall-forming association with Sabinella may have recently evolved, or at least recently intensified in both regions, possibly due to anthropogenic impacts. Though parasitism intensity is generally thought to increase in anthropogenically impacted populations (e.g., Reference Vidal-Martinez, Pech, Sures, Purucker and PoulinVidal-Martinez et al., 2010; Reference Huntley, Fürsich, Alberti, Hethke and LiuHuntley et al., 2014), Reference Sonnenholzner, Lafferty and LadahSonnenholzner et al. (2011) demonstrated that overfished food webs in the Galapagos region tend to have reduced eulimid density on echinoids, due to complex feedback between top predators, echinoid-targeting commensal crabs, and their eulimid prey. Alternatively, the preservation potential of galled spines may be severely reduced due to the gall compromising the structural integrity of the spine, both by causing the proliferation of malformed and less dense stereom, and by degrading the healthy spine core. In either case, additional exploration is needed to determine the likely cause of this discrepancy between fossil and Recent populations.

Figure 5 Eucidaris tribuloides and Eucidaris thourarsii distribution in North and South America. Large diamonds indicate Pliocene fossil spines surveyed for evidence of galling, while small diamonds indicate Recent occurrences as reported on GBIF.org. Pie charts show the number of Pliocene fossil spines that were non-altered, encrusted, or eroded. No galls were observed
3.2.2 Copepod Galls
Gall-forming copepods are known to parasitize deep-sea echinothurioid echinoids. Reference Anton, Stevenson and SchwabeAnton et al. (2013) described the gall-forming behavior of Pionodesmotes domhainfharraigeanus on Sperosoma grimaldii and Pionodesmotes phormosomae on Hygrosoma petersii. Other authors described the internal cysts formed by the same copepod, Pionodesmotes phormosomae, on echinothurioid Phormosoma uranus (Reference Bonnier, Albert and GuerneBonnier, 1898; Reference KoehlerKoeler, 1898; Reference SolovyevSolovyey, 1961; Reference BoucotBoucot, 1990). The galls in both cases are formed internally, in the ambulacral region of the test, indicating that the juvenile parasite uses the pore-pairs to gain entry to the test (Reference Anton, Stevenson and SchwabeAnton et al., 2013). The galls are comprised of skeletal material (Reference KoehlerKoehler, 1898), similar to galls associated with other parasites on echinoids. The combination of the galls being internal, as well as the relatively poor fossil record of echinothurioids in general, owing to their deep-sea habitat and thin, imbricated test, means that this type of association has a low probability of being preserved and found, and predictably, has not yet been documented from the fossil record. However, there have been numerous examples of external test cysts documented from fossil cidaroid and salenioid echinoids (Reference Radwańska and RadwańskiRadwańska and Radwański, 2005; Reference Radwanska and PoirotRadwańska and Poirot, 2010). These cysts were originally interpreted as forming due to trematode infestations by Reference Mehl, Mehl and HäckelMehl et al. (1991) but reinterpreted as copepod parasitism by Reference Radwańska and RadwańskiRadwańska and Radwański (2005) given the similarities to the modern-day internal cysts on echinothurioids. These external galls are calcified, bulbous, and highly elevated above the surface of the test, and, depending on the level of development of the cyst, resemble “halloween pumpkin masks” (sensu Reference Radwańska and RadwańskiRadwańska and Radwański, 2005). Imaged examples from Late Jurassic cidaroids Plegiocidaris coronata, Plegiocidaris crucifera, and Plegiocidaris monilifera, and the salenioids Hemicidaris intermedia and Acrosalenia spinosa depict an irregularly shaped bulbous mass, with irregularly spaced pores on the surface forming on the interambulacral region of the host (Reference Mehl, Mehl and HäckelMehl et al., 1991; Reference Radwańska and RadwańskiRadwańska and Radwański, 2005; Reference Radwanska and PoirotRadwańska and Poirot, 2010). This is likely an extinct parasite association, as these external copepod galls have not been documented from Recent echinoids.
Malformed spines have also been documented as the result of copepod parasitism in Recent echinothurioid echinoids. Reference StockStock (1968) described these galls as having the appearance of “bird nests glued against a stem,” with the copepod inhabiting an asymmetrical swelling halfway up the spine. These galls have been documented in the echinothurioid Calveriosoma gracile and Hygrosoma hoplacantha, caused by copepods Calvocheres globosus and Calvocheres engeli, respectively (Reference StockStock, 1968). Reference SimonelliSimonelli (1889), and Reference Radwańska and RadwańskiRadwańska and Radwański (2005) reported on similarly shaped cysts on a two cidaroid spines, one from the Jurassic and the other from the Miocene. The morphology of these cysts as described are unlike those documented from present-day eulimid domicile cysts, and so are tentatively described as copepod infestations, based on similarities to copepod-induced swelling on present-day echinothurioid spines and crinoid arm pinnules. These types of spine galls have yet to be documented in the fossil record on echinothurioids, which is unsurprising given the small and fragile nature of echinothurioid spines and their deep-sea habitat. Additionally, Reference StockStock (1968) described the gall material itself as calcified but loose, implying the malformed skeletal material of the affected spine is likely even more fragile still.
3.2.3 Barnacle Galls
Barnacles are common epizoans on echinoid hosts, typically benefiting from using the echinoid test or spines as a suitable substrate for settlement. A morphologically unique gall is formed on the test of the host during the symbiotic association of the stalked barnacle Rugilepas pearsei on the diadematids Echinothrix diadema and Diadema setosum (Reference Grygier and NeumannGrygier and Newman, 1991; Reference Yamamori and KatoYamamori and Kato, 2020). On Echinothrix diadema the galls are described as semi-open, occurring in the interambulacral oral area of the outer surface of the host (Reference Yamamori and KatoYamamori and Kato, 2020). In Diadema setosum, Reference Grygier and NeumannGrygier and Newman (1991) additionally described test scarring on the internal surface of the host’s test as a result of gall formation. In life, the barnacle is camouflaged in the same color as the epidermis of the host and surrounded by the toxin-bearing secondary spines of the host. After death, the gall appears as a raised crater-shaped calcified gall that is distinct from the rounded galls formed by copepods. The walls and base of the gall are thickened, and the center pit of the gall has puncture holes where the peduncular attachment appendages of the barnacle are anchored deeply into the host. A single gall may be occupied by two to four mating barnacles, the number of which can be determined by the number of anchor holes. The barnacle is a suspension feeder and does not feed on the echinoid host’s tissue, so this association is not clearly parasitic in nature (Reference Grygier and NeumannGrygier and Newman, 1991; Reference Yamamori and KatoYamamori and Kato, 2020). Though galls were common in the populations studied by Reference Yamamori and KatoYamamori and Kato (2020), with more than 9 percent of E. diadema individuals bearing galls, none have been described from the fossil record to date.
The pedunculate barnacle Microlepas diademae, on the other hand, is documented to attach not to the test but to the tips of primary spines of the host diademid Echinothrix calamaris (Reference Grygier and NeumannGrygier and Newman, 1991; Reference Grignard, Jangoux, David, Guille, Féral and RouxGrignard and Jangoux, 1994). Infested spines are noticeably shorter than surrounding healthy primary spines. The infested spines are club-shaped rather than needle-shaped and, like the test galls, exhibit puncture holes from the peduncular attachment appendages of the barnacle. Reference Grignard, Jangoux, David, Guille, Féral and RouxGrignard and Jangoux (1994) observed that infested spines were not able to regenerate or heal, even following the removal of the epibiont. This case of spine infestation is unique, as regular euechinoid spines are generally devoid of epibionts due to the epidermal layer that still surrounds fully grown spines, in contrast to cidaroids. To our knowledge, no diademid spines with clear barnacle infestation have been documented in the fossil record.
3.3 Encrusting and Bioerosive Associations
Encrusting epibionts are commonly present on both Recent and fossil echinoids (Reference Zamora, Mayoral, Vintaned, Bajo and EspílezZamora et al., 2008; Reference BorszczBorszcz, 2012), with a number of these interactions occurring syn-vivo. As noted above, an association must exhibit demonstrable reduction in the fitness of the host to be deemed parasitic; and for many Recent and fossil echinoid-symbiont associations, detrimental effects on the host have not been demonstrated conclusively. These associations, whether parasitic, commensal, or mutualistic, are prevalent in Recent echinoids, though few are likely to be recognized in the fossil record, except in instances of exceptional preservation.
3.3.1 Boring Associations
A commensal relationship between a boring polydorid polychaete of the Maastrichtian holasteroid Echinocorys ovata was interpreted by Reference Wisshak and NeumannWisshak and Neumann (2006) from linear to sinuous traces on the oral side of the test on a single specimen. Evidence of test healing suggests that this association is syn-vivo. An echinoid-polydorid association like this is not known from Recent ecosystems, so the nature of this association is inferred indirectly based on the commensalism observed between Recent polydorid polychaetes and mollusks.
3.3.2 Spine Fouling
The most common form of encrustation in echinoids is via spine fouling by various groups of epibionts. A large diversity of fouling organisms attaches to living cidaroid urchin spines, including bryozoans, annelids, sponges, foraminifera, mollusks, and barnacles (Reference Hopkins, Thompson, Walker, Davis, Heinzeller and NebelsickHopkins et al., 2004). Cidaroid spines serve as favorable habitats for settling sessile organisms, especially in deep-sea benthic environments where hardgrounds are scarce (Reference Hétérier, David, De Ridder and RigaudHétérier et al., 2008), increasing the local diversity where cidaroid echinoids are present. Reference Cerrano, Bertolino, Valisano, Bavestrello and CalcinaiCerrano et al. (2009) described the fouling behavior of demosponges Homaxinella balfourensis, Isodictya erinacea, Iophon unicorne, and Haliclona (Rhizoniera) dancoi on the cidaroid Ctenocidaris perrieri. Reference Hétérier, De Ridder, David, Rigaud, Heinzeller and NebelsickHétérier et al. (2004) compared symbionts between Ctenocidaris spinosa and Rhynchocidaris triplopora and found that the more rugous spines of C. spinosa support a higher diversity of epibionts, suggesting that spine microstructure in cidaroids is adaptive for encouraging fouling behaviors. The authors observed that the epibiont cohorts were taxonomically distinct between the two cidaroids, suggesting specialization on the part of the symbiont to specific species of hosts.
In the fossil record, direct evidence of spine encrustation is generally limited to biocalcifying organisms such as bryozoans, barnacles, and oysters, and is commonly present on fossil cidaroid echinoid spines. Less direct evidence of other types of encrusters, such as boring polychaetes, is usually preserved as evidence of bioerosion on the spines. In cases of exceptional preservation, uncommon epibionts are observed. Reference SchneiderSchneider (2003) described the association between the Paleozoic stem group echinoid Archaeocidaris brownwoodensis and the spiriferid brachiopod Crurithyris planoconvexa in addition to encrustation by fenestellid bryozoan fronds. It is interpreted as a commensal relationship, with the epibionts benefiting from protection offered by the large spines and the echinoids receiving minimal camouflaging benefit. Archaeocidarid spinosity may be an adaptation to support epibionts in this manner (Reference Schneider, Sprinkle and RyderSchneider, 2005), as in Recent cidaroid descendants (Reference Hétérier, De Ridder, David, Rigaud, Heinzeller and NebelsickHétérier et al., 2004).
3.3.3 Encrustation
A rare instance of encrustation directly onto the test of an echinoid was described by Reference QueirozQueiroz (2020), who observed a bryozoan colony of Schizoporella errata growing on the test of the camarodont Echinometra lucunter. The site of attachment was relatively large and was denuded of primary spines, secondary spines, and tube feet. When the colony was removed, the author observed an inflammatory response in the host at the attachment site, suggesting that this was a detrimental association for the host and could potentially be classified as parasitism. The test material under the site of attachment was not damaged, and the epidermis healed in a few days following the encrusters’ removal, indicating that this association did not produce a skeletal pathology on the host itself that could be identified in the fossil record. However, as the bryozoan is itself calcifying, there is potential to find fossil evidence of this association, as long as it can be distinguished from postmortem encrustation by bryozoans on the test of the echinoid.
4 Non-trace Producing Associations
Echinoid-associated biotic interactions that do not produce long-term pathologies on the skeletal material of the test or that induce traces on echinoid skeletal material that does not readily preserve in fossils are likely to be highly underestimated in the fossil record. Understanding the prevalence of so-called invisible interactions in present-day ecosystems is the first step for determining the degree to which these types of associations are underrepresented in the fossil record (see examples in Table 2).
Many present-day eulimid-echinoid associations can be classified as non-trace producing in this way, such as the parasitism by the eulimid Trochostilifer eucidaricola on the peristomal membrane of Eucidaris tribuloides (Reference Warén and MoolenbeekWarén and Moolenbeek, 1989), which is readily degraded post-mortem and rarely found in even otherwise articulated fossil cidaroids (Reference Donovan and GordonDonovan and Gordon, 1993). Reference González-Vallejo and León-GonzálezGonzález-Vallejo and de León-González (2018) described a short-term association between the eulimid parasite Nanobalcis worsfoldi and Eucidaris tribuloides, with the eulimid living around the base of the primary spines but not producing a spine gall like the co-occurring Sabinella troglodytes. Reference WarénWarén (1981b) described an association of Fusceulima goodingi on a diadematid echinoid that likely targeted tube-feet and epithelial tissue, while not producing skeletal traces on the echinoid. The eulimid Pelseneeria hawaiiensis penetrated the test of the echinoid host via the gonopore, which would be difficult to recognize in the fossil record unless special care is taken to look for enlargement of gonopores or pore pairs. The present authors additionally document an instance of a eulimid (indet.) parasitizing a specimen of Plococidaris sp. in both the ambulacral and interambulacral regions (Figure 3), with neither eulimid producing an identifiable trace. The smaller eulimid (Figure 3C) may, however, be accessing internal host tissue via the pore-pairs.
Copepods are common symbionts on Recent echinoids; and whereas parasitic associations can produce skeletal galling in some cases, the majority of cases are non-trace producing. Reference Venmathi Maran, Kim, Bratova and IvanenkoVenmathi Maran et al. (2017) described a non-trace producing symbiotic association between the poecilostomatoid copepods Mecomerinx ohtsukai and Clavisodalis toxopneusti on the camarodont Toxopneustes pileolus. The copepod Onychocheres alatus lives among the spines of Diadema antillarum (Reference Stock and GoodingStock and Gooding, 1986). Several species of the copepod genus Clavisodalis are described as living in the esophagus and jaw apparatus of several regular euechinoid taxa (Reference HumesHumes, 1980; Reference Dojiri and HumesDojiri and Humes, 1982). Whereas copepod-echinoid associations are numerous and common in Recent ecosystems, there is little hope of preserving evidence of these associations in the fossil record, outside of the handful of copepod genera that are known to produce galls.
Associations between the hesionid polychaete Oxydromus cf. angustifrons and the camarodont echinoids Salmacis sphaeroides and Temnopleurus toreumaticus are described by Reference Chim, Ong and TanChim et al. (2013) as commensal in nature. The polychaetes preferentially live near the peristomal membrane of the echinoid and do not appear to negatively impact the host. Unlike the probable polychaete association described by Reference Wisshak and NeumannWisshak and Neumann (2006) on fossil holasteroids, this association leaves no skeletal evidence of the host, and therefore cannot be directly studied in the fossil record.
Pea crabs (the brachyuran family Pinnotheridae) are obligatory symbionts of a wide range of host organisms including bivalves, crustaceans, echinoderms, polychaetes, tunicates, and fish (e.g., Reference SchmittSchmitt, 1973; Reference PowersPowers, 1977; Reference WilliamsWilliams, 1984; Reference Takeda, Tamura and WashioTakeda et al., 1997; Reference Thoma, Heard and VargasThoma et al., 2005, Reference Thoma, Heard and Felder2009; Reference Ahyong and NgAhyong and Ng, 2007). Echinoids are frequently infested by pea crabs (Figure 6), which utilize their host to seek shelter, food, and a protected habitat for reproduction (e.g., Reference Wirtz, de Melo and de GraveWirtz et al., 2009; Reference Wirtz, de Melo and de GraveWirtz and Grave, 2009). Interactions between crabs and their echinoid hosts have been interpreted as either “commensal” or “parasitic” (e.g., Reference Campos-GonzálezCampos-González, 1986; Reference Campos and GriffithCampos and Griffith, 1990; Reference Campos, de Campos and RamirezCampos et al., 1992; Reference Wirtz, de Melo and de GraveWirtz and Grave, 2009; Reference Guilherme, Brustolin and BuenoGuilherme et al., 2015). In the commensal interpretation, pea crabs do not harm the host but only feed on its fecal matter (Reference GlassellGlassell, 1935). In contrast, according to the parasitic interpretation, crabs reduce host fecundity (Reference De Bruyn, Rigaud, David and De RidderDe Bruyn et al., 2009) and feed on host tissues (e.g., Reference DexterDexter, 1977; Reference TelfordTelford, 1982; Reference De Bruyn, Rigaud, David and De RidderDe Bruyn et al., 2009; Reference Martinelli Filho, dos Santos and RibeiroMartinelli Filho et al., 2014). Interestingly, pea crabs appear to also be also able to prey on eulimid snails (Reference Sonnenholzner, Lafferty and LadahSonnenholzner et al., 2011) parasitizing their common host, suggesting that the infesting crabs may, at least occasionally, benefit their host. Regardless of the ecological interpretation of pinnerid–echinoid interactions, pea crab infestation is not known to result in any test or spine deformities or damage suggesting that symbiotic interactions with pea crabs are unlikely to be identifiable in the fossil record. However, to our knowledge, the ichnological consequences of pea crab infestation has not been investigated rigorously.

Figure 6 (A) Clypeaster subdepressus, infested by different crab species; Dissodactylus latus on the left and Clypeasterophilus stebbingi near the center. (B) Mellita tenuis infested by Dissodactylus mellitae from the Gulf of Mexico. Scale bar of echinoids = 1 cm. A closer look towards some commensal pea crabs (C) D. latus, (D) C. stebbingi, and (E) D. mellitae. Scale bar of crabs = 5 mm
5 Evolutionary Trends
Predation pressure is thought to have played a significant role in echinoid evolution and may have led to the mid-Mesozoic infaunalization of echinoids (Reference KierKier, 1982), which coincides with the diversification of several important groups of predators during the Mesozoic Marine Revolution (Figure 7) (Reference McRobertsMcRoberts, 2001). Infaunalization, therefore, may have been an evolutionary adaptation to escape increasing predation pressure (Reference KierKier, 1982). Although the infaunalization of irregular echinoids began during the Mesozoic Marine Revolution, their early morphology restricted burrowing to coarse grained sediments (Reference SmithSmith, 1984; Table 3). During the Early Jurassic, the infaunal life-mode was facilitated by the evolution of uniformly sized dorsal spines packed more densely together (Reference SmithSmith, 1984), which prevented suffocation in tightly packed sediment by creating a layer of water around the test (Reference SmithSmith, 1984). By the Eocene, clypeasteroid echinoids were able to burrow into fine-grained sediments (Reference SmithSmith, 1984). The globular shapes of some spatangoid tests may help support burrow walls preventing collapse, thus facilitating deep burrowing (Reference KanazawaKanazawa, 1992). In contrast, the flattened test shapes are associated with shallow burrowing (Reference KanazawaKanazawa, 1992), and may prevent the test from being disturbed by currents, and allow the test to be covered with sediment, acting as camouflage (Reference KierKier, 1974). The following morphological features are thought to be indicative of burrowing by various spatangoids and holasteroids (Reference SmithSmith, 1984): (1) the dense development of aboral tubercules that indicate aboral spines that prevented sediment from falling between the spines; (2) larger oval ambulacral pores in the adapical region of ambulacrum III versus ambital ambulacral pores that suggests the presence of tunnel-building tube feet; (3) sunken anterior ambulacrum with enlarged interambulacral tubercles adjacent that are used for constructing shafts; and (4) ambulacral pores that are larger than the adjacent tube-feet pores, indicating the presence of tunnel-building tube feet.

Figure 7 Mesozoic to Cenozoic trends in richness at the genus level of (A) epifaunal and infaunal echinoids, (B) echinoid-targeting drilling gastropods, and (C) crushing predators, as calculated from Paleontological Database occurrences.
Table 3 Evolutionary history of echinoid infaunalization (Reference SmithSmith 1984)
| Time period | Group | Morphology/life mode | Interpretation |
|---|---|---|---|
| Eocene | Clypeasteroids | Tiny but dense aboral spines, flattened test | Increased camouflage by covering flat test with sand (Reference KierKier, 1974) |
| Infaunal, fine sands | |||
| Cretaceous | Holasteroids and spatangoids | Tunnel-building tube feet, dorsal fascioles, and dense spatulate aboral spines | Fascioles developed to create a mucus gland to protect the test from sediment and to draw water in the burrow (Reference SmithSmith, 1984) |
| Infaunal, fine sediment | |||
| Middle Jurassic | Echinaceans | Additional and stronger oral tube feet, well developed phyllodes | Phyllodes allowed for stronger attachments in turbulent environments (Reference KierKier, 1974) |
| Shallow turbulent habitats | |||
| Early Jurassic | Holectypygoids and galeropygoids | Denser dorsal spines | Smaller spines allowed the test to be covered with sediment for camouflage (Reference KierKier, 1974) |
| Infaunal, coarse sediment | |||
| Irregular echinoids (pygasteroids) | Epifaunal | Not adapted for borrowing (Reference SmithSmith, 1984) | |
| Triassic | All regular echinoids | Protected habitats, deeper waters | Began to diversify for different habitats (Reference SmithSmith, 1984) |
| Epifaunal | |||
| Paleozoic | All regular echinoids | Epifaunal | Regular echinoids do not have the morphology for successful burrowing (Reference KierKier, 1974) |
| Firm substrate, offshore or protected habitats |
Irregular echinoids diversified rapidly during the Jurassic and Cretaceous, outpacing the diversity of “regular” echinoids by the Cretaceous (Figure 7). Drill holes, as proxies for predation pressure by cassid gastropods, increased in intensity much later in the Eocene (Reference Petsios, Portell and FarrarPetsios et al., 2021), suggesting that the evolutionary adaptations associated with infaunalization were likely not driven by cassid predation pressure, or at least not initially. Although the frequency of drill hole traces in mollusks has been classically used as a proxy for predation pressure (see discussion in Reference HarperHarper, 2016), in the case of echinoids, drilling predators may be less impactful on their prey’s population dynamics than other forms of predation, such as crushing from actinopterygian fish, crustaceans, and asteroids. Asteroids in particular experience rapid diversification earlier in the Mesozoic compared to fish and crustacean predators, suggesting that asteroid predation pressure may have played a role in the evolution of early infaunal echinoids (Figure 7). Nevertheless, estimating the intensity of crushing predation pressure in fossil communities remains challenging due to the difficulty of differentiating damage due to crushing versus abiotic or post-mortem breakage. Other methods, such as comparing temporal trends in taxonomic diversification and morphological change in predator and prey groups, can be employed instead.
6 Concluding Remarks
In modern oceans, the numerous biotic interactions that involve echinoids impact the fitness of individuals and health of echinoid communities. Such interactions undoubtedly impacted past populations as well; but without rigorous quantitative estimates of the intensity and frequency of biotic interactions in ancient ecosystems, we are unable to constrain to what extent these interactions have driven long-term ecological and evolutionary trends. Understanding the ecologies of the various biotic interactions which result in skeletal pathologies in living echinoids is key to successfully characterizing the numerous traces observed in fossil echinoids. As summarized here, a great diversity of predatory, parasitic, commensal, and mutualistic interactions is known from Recent ecosystems. Many of those interactions result in traces on echinoid skeletons that have been or have the potential to be successfully diagnosed in the fossil record. However, trace producing interactions are only a fraction of the total likely ecological associations impacting short term fitness and long-term adaptive evolution of echinoids. In such cases, indirect methods can be often employed to assess the roles of these invisible interactions. Taken together, these direct and indirect lines of evidence for the presence of biotic interactions involving echinoids can inform observed morphological, behavioral, and adaptive trends and quantify more rigorously ecological pressures in the evolutionary history of echinoids.
Acknowledgments
We thank Troy Dexter (Gerace Research Centre), Edward Stanley (FLMNH Digital Imaging Division), Gordon Hendler and Austin Hendy (both LANHM), John Slapcinsky, Sean Roberts (both FLMNH), Paul Larson and Corinne Fuchs (both FFWCC), and Nathan Wright (Baylor University) for either assistance in the field, collections, or specimen imaging. We also thank the numerous contributors and editors of the Paleobiology Database (paleobiodb.org) and the Global Biodiversity Information Facility (gbif.org). This work was supported by a National Science Foundation grant to Tyler and Kowalewski (EAR SGP-1630475 and EAR SGP-1630276).
Editor-in-Chief
Colin D. Sumrall
University of Tennessee
About the Series
The Elements of Paleontology series is a publishing collaboration between the Paleontological Society and Cambridge University Press. The series covers the full spectrum of topics in paleontology and paleobiology, and related topics in the Earth and life sciences of interest to students and researchers of paleontology.
The Paleontological Society is an international nonprofit organization devoted exclusively to the science of paleontology: invertebrate and vertebrate paleontology, micropaleontology, and paleobotany. The Society’s mission is to advance the study of the fossil record through scientific research, education, and advocacy. Its vision is to be a leading global advocate for understanding life’s history and evolution. The Society has several membership categories, including regular, amateur/avocational, student, and retired. Members, representing some 40 countries, include professional paleontologists, academicians, science editors, Earth science teachers, museum specialists, undergraduate and graduate students, postdoctoral scholars, and amateur/avocational paleontologists.