Background
Hearing impairment affects 11 million people in the UK.1 Most have acquired hearing impairment, which predominantly affects older people.1 A minority experience pre-lingual deafness, with onset of severe or profound hearing impairment before speech development. There is evidence that people with hearing impairment have elevated rates of common mental illness (depressive and anxiety disorders),Reference Contrera, Betz, Deal, Choi, Ayonayon and Harris2–Reference Kim, Lee, Kim, Kim and Kim5 but not all studies have found this association.Reference Cimarolli, Jopp, Boerner and Minahan6,Reference Stam, Smit, Twisk, Lemke, Smits and Festen7 Variable sample sizes, outcome measures and study populations probably explain these conflicting findings.Reference Cimarolli, Jopp, Boerner and Minahan6,Reference West8 Severe visual impairment affects more than 2 million people in the UK and like hearing impairment, predominantly occurs in older people.Reference McManus and Lord9 The prevalence of depression among visually impaired adults has been reported to be higher than in comparable populations without visual impairment in most studies,Reference Han, Lee, Jung and Park4,Reference Cosh, Von Hanno, Helmer, Bertelsen, Delcourt and Schirmer10 although one prospective cohort study found that a deterioration in near vision did not increase the risk after controlling for confounders.Reference Carrière, Delcourt, Daien, Pérès, Féart and Berr11
Understanding of the mechanisms behind possible associations between sensory impairment and common mental illness is limited.Reference Hsu, Hsu, Wen, Lin, Tsai and Su12,Reference Simning, Fox, Barnett, Sorensen and Conwell13 Being unable to hear conversations, or see facial expressions, can present significant obstacles to accessing social support and connection. People with sensory impairment have reported experiencing barriers to going out, reduced friendship networks and higher rates of loneliness and social isolation.1,Reference Stam, Smit, Twisk, Lemke, Smits and Festen7,Reference McManus and Lord9 Reduced social support has been associated with depression and anxiety.Reference West8 Social functioning can be determined by social opportunities and is one way of describing interactions with other people, the environment and the community.
Barriers to access to mental healthcare have been described; 15–30% of pre-lingually deaf people have reported avoiding booking appointments.Reference Levine14,Reference Fellinger, Holzinger and Pollard15 Cited reasons include reliance on telephone booking and lack of funding for sign language interpreters.Reference Levine14,Reference Fellinger, Holzinger and Pollard15 A large US study found that people with acquired hearing impairment also experienced barriers to accessing treatment.Reference Pandhi, Schumacher, Barnett and Smith16 Similarly, people with visual impairment can experience problems because of providers giving only written information, or inadequate transport provision for appointments.Reference Sharts-Hopko, Smeltzer, Ott, Zimmerman and Duffin17 We hypothesised that because of difficulties accessing treatment, people with sensory impairment might live with common mental illness for longer; that is have a larger ‘treatment gap’.
It is important to understand whether people with sensory impairment are at increased risk of depression and anxiety, and if so, what mediates this, in order to inform strategies for identifying and intervening to ameliorate these symptoms, and mental health service planning. This is the first national survey comparing prevalence of symptoms, diagnoses and treatments for common mental disorders in people with and without sensory impairment; and to explore social functioning as a possible mediator of the relationship between sensory impairment and mental illness.
Aims
We tested our primary hypotheses that (a) people with sensory impairments have more symptoms of common mental disorder than people without; and (b) after adjusting for level of symptoms, they are less likely to have received a formal diagnosis or treatment. Our secondary hypothesis was that the association between sensory impairment and common mental illness is partly accounted for by reduced social functioning.
Method
We analysed cross-sectional data from the 2014 Adult Psychiatric Morbidity Survey (APMS).
Participants and procedures
The 2014 APMS is a sample of residents aged 16 and over living in private households in England.Reference McManus, Bebbington, Jenkins and Brugha18 Sampling was multistage and designed to produce a sample representative of the national population. At stage 1 postcode sectors were stratified before being chosen at random. Households were then randomly selected from within each chosen postcode sector. Finally, one individual from each selected household was randomly identified to participate. Data are weighted to account for selection probability and non-response. Our study is based on phase 1 data, which was collected by lay interviewers. Permission to use the data in our study was granted by NHS Digital. Further details on the APMS methodology and sampling strategy are available elsewhere.Reference McManus, Bebbington, Jenkins and Brugha18
Measures
Outcome measures
The Clinical Interview Schedule-Revised (CIS-R) is a measure of common mental disorders that corresponds to the ICD-10, which is highly reliable and valid.Reference Lewis, Pelosi, Araya and Dunn19 The total score, which ranges from 0 to 57, indicates the level of common mental illness symptoms. The CIS-R was used to assess for symptoms of depression, anxiety, phobias and obsessive–compulsive disorder. The total CIS-R score was our primary outcome. In addition, a standard cut-off of 12 and above was used to analyse presence or absence of clinically significant symptoms of common mental illness as a binary variable.Reference McManus, Bebbington, Jenkins and Brugha18
A secondary outcome was being in receipt of a psychiatric diagnosis or treatment within the past year. APMS participants were shown cards listing diagnoses, psychotropic medications and psychological conditions. They were asked, ‘Did a doctor, psychiatrist or other professional tell you that you had [any of this list of conditions]?’ to decide whether a psychiatric diagnosis had been received; they were also asked whether they had been prescribed any of the psychotropic medications listed on cards shown to them. Interviewers checked the packaging of medications to ensure correct identification. To decide if participants had received psychological therapy, they were shown a card listing psychological therapies and asked; ‘Are you currently having any counselling or therapy listed on this card for a mental, nervous or emotional problem?’
Exposure variables
Participants were asked whether they had hearing impairment or wore a hearing aid; those that gave an affirmative response were recorded as having a hearing impairment. Presence of distance vision impairment was determined by asking participants whether they had any difficulty seeing a face across the room even with visual aids; and presence of near vision impairment by asking whether they had difficulty reading a newspaper even with visual aids. Participants gave their answers as a degree of difficulty (no difficulty, mild difficulty, moderate difficulty, severe difficulty, cannot do). We analysed this data as binary (having difficulty of any severity or not).
Potential confounders measured
We recorded socioeconomic, health and health behaviour covariates that might be linked to the outcome (gender, ethnicity, age in 10-year brackets, employment type, history of significant physical health condition, household composition, marital status, verbal IQ as measured by the National Adult Reading Test,Reference Nelson and Willison20 history of smoking and Alcohol Use Disorders Identification Test (AUDIT)Reference Saunders, Aasland, Babor, de la Puente and Grant21 score). A physical health condition that could confound results was coded as present if the participant reported having had cancer, stroke, high blood pressure, heart attack or angina, diabetes, epilepsy, migraine or frequent headaches, dementia or Alzheimer's or another nervous system disorder since the age of 16.
Potential mediators measured
The Social Functioning Questionnaire (SFQ) is a validated measure of perceived social functioning.Reference Tyrer, Nur, Crawford, Karlsen, MacLean and Rao22 It is an eight-item scale with a score range from 0 to 24, with higher scores indicating lower social functioning. Topics covered include feelings of loneliness, stress, relationships and enjoyment of leisure time.
Statistical analysis
All analyses were performed using Stata version 14.2.23 Where participants answered ‘don't know’ or ‘not applicable’ or refused to answer a question, this data was treated as missing. The weighting used in the original APMS survey design was preserved in our study to keep the sample as representative as possible of the national population, when the full sample was used. We report weighted percentages but unweighted absolute numbers.
We assessed the association between presence of sensory impairment and CIS-R score using a series of regression models. We used two outcome variables: total CIS-R score and whether the CIS-R score was 12 or above. The three exposure variables were presence of hearing impairment, presence of near vision impairment and presence of distance vision impairment. Influence of sensory impairment on total CIS-R score was assessed using linear regression. The odds of having a CIS-R score of 12 or above for people with sensory impairment relative to those without were calculated using logistic regression, 95% CIs are presented throughout. We also ran sensitivity analyses including only participants aged under 65 or 65 and above, to see whether there was a different relationship between sensory impairment on CIS-R score in older and younger people.
To assess the odds of receiving treatment for people with sensory impairment relative to those without, we conducted logistic regressions adjusting for total CIS-R score. We assessed three self-reported outcome variables: receipt of a psychiatric diagnosis, receipt of medical treatment and receipt of psychological treatment. We used the same three exposure variables as in our previous models. We ran a sensitivity analysis including only people who had a CIS-R score typically considered high enough to warrant treatment (18 or greater).
To test for mediation, we checked whether putative mediators were associated with both the exposure variables and outcome variable (CIS-R score); an essential criterion for mediation. We then employed structural equation modelling using the sem command in Stata.23 We estimated the degree of mediation by dividing the coefficient of the adjusted indirect effect of sensory impairment on CIS-R score (via the potential mediator) by the adjusted coefficient of the total effect of sensory impairment on CIS-R score.
All of the above analyses were carried out in stages: as univariate models based on the analytic sample, and as multivariate models to adjust for potential confounders. We excluded socioeconomic covariates from regression models if they were not theoretically plausible confounders or did not show evidence of association with both sensory impairment and CIS-R score and effect on the relationship between these variables.
Results
In total, 7546 people participated in the APMS 2014 survey and completed the CIS-R. The overall response rate was 57%. There were 7488 people who had given information on all relevant exposures, primary outcomes and confounding variables and could be included as the analytic sample. The mean CIS-R score in this sample was 5.52 (s.d. = 7.67). There were 1220 (15.7%) people who had a clinically significant score of 12 or above. A total of 1311 (14.7%) people reported hearing impairment, 441 (5.1%) people reported distance vision impairment and 872 (10.4%) reported near vision impairment.
Tables 1 and 2 show the characteristics of the analytic sample. Of note, people with sensory impairment were more likely to be aged over 65, divorced or widowed, to live in social housing and to be economically inactive than people without. They were also more likely to have physical comorbidities and to smoke.
Table 1 Relationship of characteristics of analytic sample to hearing impairmenta
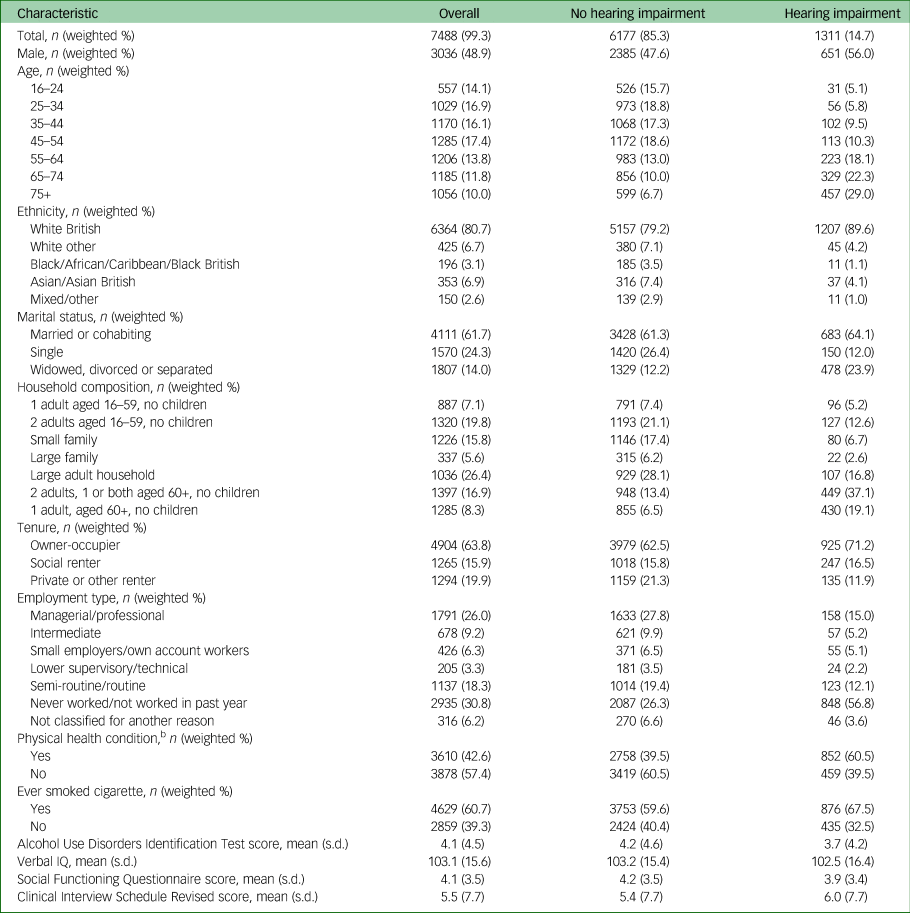
n, number in group (unweighted); weighted %, percentage of group weighted.
a. This is the analytic sample for the primary analyses.
b. Physical health condition was coded as present if the participant reported having had cancer, stroke, high blood pressure, heart attack or angina, diabetes, epilepsy, migraine or frequent headaches, dementia or Alzheimer's or another nervous system disorders since the age of 16.
Table 2 Relationship of characteristics of analytic sample to visual impairmenta
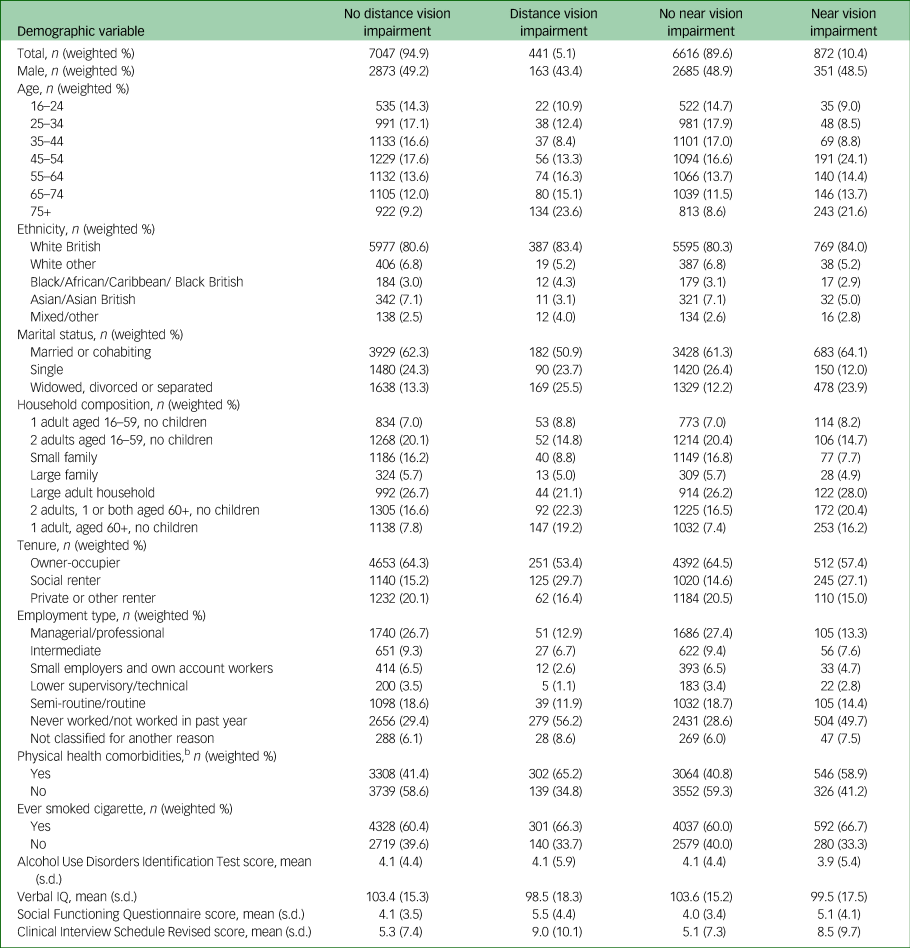
n, number in group (unweighted); weighted %, percentage of group weighted.
a. This is the analytic sample for the primary analyses.
b. Physical health condition was coded as present if the participant reported having had cancer, stroke, high blood pressure, heart attack or angina, diabetes, epilepsy, migraine or frequent headaches, dementia or Alzheimer's or another nervous system disorders since the age of 16.
Association of anxiety and depressive symptoms with sensory impairments
Results are presented in Tables 3 and 4. Analyses were all adjusted for age, gender, ethnicity, type of employment, ever having smoked and history of significant physical health condition.Reference Garin, Olaya, Lara, Moneta, Miret and Ayuso-Mateos24 Adjusted mean CIS-R score was 1.86 points higher in hearing-impaired compared with non-hearing-impaired people (95% CI 1.30–2.42, P<0.001). People reporting distance and near vision impairments had higher adjusted mean CIS-R scores compared with those not reporting these impairments; by 3.61 points (95% CI 2.58–4.63, P<0.001) and 2.74 points (95% CI 2.12–3.37, P<0.001), respectively.
Table 3 Influence of sensory impairment on total Clinical Interview Schedule Revised (CIS-R) score
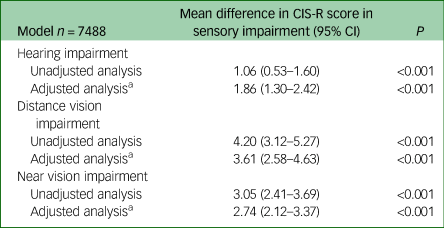
a. Adjusted for age, gender, ethnicity, employment type, history of significant physical health condition and ever having smoked.
Table 4 Odds of scoring ≥12 on clinical interview schedule revised (CIS-R) in people with sensory impairment relative to those without sensory impairment
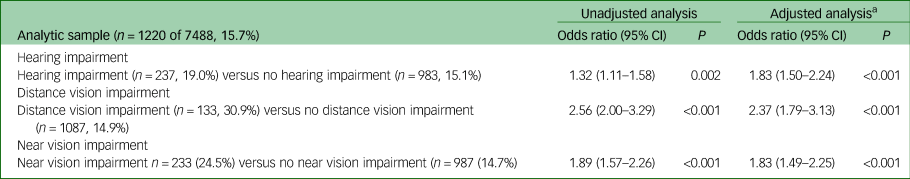
n, number in group scoring ≥12 on CIS-R; %, percentage of group scoring ≥12 on CIS-R weighted.
a. Adjusted for age, gender, ethnicity, employment type, history of significant physical health condition and ever having smoked.
The odds of having a clinically significant CIS-R score (12 or more) were higher in all groups with sensory impairment, with the odds being over twofold for distance vision impairment (adjusted odds ratio (AOR) = 2.37, 95% CI 1.79–3.13, P<0.001). For hearing impairment, the odds ratio was 1.83 (95% CI 1.50–2.24, P<0.001) and for near vision impairment it was 1.83 (95% CI 1.49–2.25, P<0.001). Unadjusted results were similar, although the relationship between hearing impairment and CIS-R score was negatively confounded by age.
Stratification by age
Upon analysing older and younger participants separately, we found that the mean difference in CIS-R score between those with and without sensory impairment was greater in people aged under 65. Following adjustment, it was 2.43 points (95% CI 1.72–3.15, P<0.001) in hearing impairment, 4.05 points (95% CI 3.00–5.10, P<0.001) in distance vision impairment and 3.95 points (95% CI 3.20–4.69, P<0.001) in near vision impairment. By comparison for people aged 65 and over, the mean CIS-R score difference was 0.85 points (95% CI 0.37–1.32, P<0.001) in hearing impairment, 2.65 (95% CI 1.90–3.40, P<0.001) in distance vision impairment and 2.30 points (95% CI 1.72–2.89, P<0.001) points in near vision impairment.
Odds of receiving treatment
Table 5 shows the results of these analyses. People with each of the three types of sensory impairment had increased odds of having received a diagnosis of mental illness relative to people without, following adjustment for CIS-R score, age, gender, ethnicity, employment type, history of significant physical condition and ever having smoked. There was no evidence that people with sensory impairment were any more or less likely to have received psychological therapy than people without. There was weak evidence that people with hearing impairment were more likely to have received medication, but no evidence of a difference in visual impairment following adjustment. A sensitivity analysis restricted to participants with a CIS-R score of 18 or above found no statistical evidence of a difference in rates of reported receipt of diagnosis, medication or psychotherapy in people with any kind of sensory impairment following adjustment (see supplementary Table 1; available at https://doi.org/10.1192/bjo.2019.81)
Table 5 Odds of receiving clinical intervention for people with sensory impairment relative to those without sensory impairment
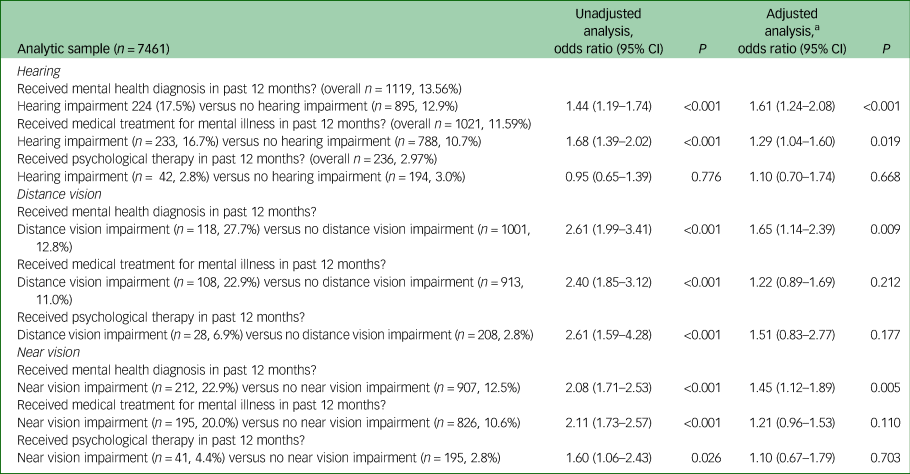
n, number in group receiving intervention; %, weighted percentage of group receiving intervention.
a. Adjusted for age, gender, ethnicity, employment type, history of significant physical health condition, ever having smoked and total CIS-R score.
Mediation
Table 6 shows the different pathways in the mediation analysis for SFQ score. Following adjustment, SFQ score was strongly associated with CIS-R score, hearing impairment and both distance and near vision impairment, and therefore met essential criteria for mediation. For hearing impairment, distance vision impairment and near vision impairment 47.3% (95% CI 27.3–67.3%, P<0.001), 54.9% (97% CI 40.8–69.0%, P<0.001) and 50.3% (95% CI 39.5–61.1%, P<0.001), respectively of the association between sensory impairment and CIS-R score appeared to be mediated by social functioning.
Table 6 Mediation analysis: assessment of mediation of association of sensory impairment with Clinical Interview Schedule Revised (CIS-R) score by Social Functioning Questionnaire (SFQ) scorea
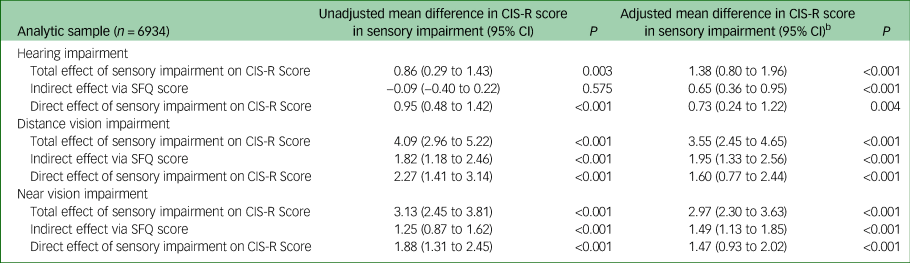
a. Total effect is the combined estimated direct effect of sensory impairment on CIS-R score + indirect effect mediated by SFQ score; the indirect effect is the estimated effect of sensory impairment on CIS-R score via effect on SFQ score; and the direct effect is the estimated direct effect of sensory impairment on CIS-R score (not via SQF score). Estimated degree of mediation was obtained by dividing the indirect effect (mean difference) by the total effect (mean difference).
b. Adjusted for age, gender, ethnicity, employment type, physical health condition and ever having smoked.
Discussion
We found evidence that the level of common mental illness symptomatology is higher, on average, in people with sensory impairment than people without. The odds of having a clinically significant level of symptoms was also increased, especially in distance vision impairment where they were over twice as high. This may explain why one cohort study found an association with common mental illness for distance but not near vision.Reference Carrière, Delcourt, Daien, Pérès, Féart and Berr11 We found that 19.0% of people with hearing impairment, 30.9% with visual impairment and 24.5% with near vision impairment had a clinically significant CIS-R score. These findings are in keeping with multiple previous studies, which have found that both hearing and visual impairment are associated with depressive and anxiety symptomatology.Reference Han, Lee, Jung and Park4,Reference Simning, Fox, Barnett, Sorensen and Conwell13,Reference Huang, Dong, Lu, Yue and Liu25–Reference Bernabei, Morini, Moretti, Marchiori, Ferrari and Dalmonte28 Our finding that the difference in CIS-R score is greater in younger people might reflect the fact that younger people with sensory impairment are more likely to be different to their peers in this regard, and to miss out on opportunities throughout life.Reference Du Feu and Fergusson29
It appears from our results that the association between all types of sensory impairment and common mental illness might be partially accounted for by self-perceived social functioning. This finding is consistent with research showing that older adults with hearing impairment are more likely to withdraw from social contactReference Rutherford, Brewster, Golub, Kim and Roose30 and that social isolation causing depression may be on the pathway from hearing impairment to cognitive decline according to cross-sectional findings.Reference Dawes, Emsley, Cruickshanks, Moore, Fortnum and Edmondson-Jones31 There is recent evidence that communication ability might determine a sense of social self-efficacy that mediates the pathway from hearing impairment to depression.Reference Palmer, Carder, White, Saunders, Woo and Graville32 There is also evidence linking visual impairment to loneliness.Reference Hodge and Eccles33 To our knowledge, ours is the first study to test social functioning as a mediator of the association between sensory impairment and common mental illness in a national survey sample.
The finding that people with sensory impairment were more likely to have received a diagnosis for common mental illness than people without runs counter to our second hypothesis. This is also unexpected in light of existing literature; it has previously been estimated that only 2% of deaf adults ever receive mental healthcare,Reference Leigh and Pollard34 and more than a quarter with hearing loss do not understand their diagnosis following a general practitioner appointment.1 Our finding may indicate that people with sensory impairment are more likely to be known to health professionals through the sensory impairment itself, leading to common mental illness being detected. There was, however, no strong evidence that they are more likely to receive treatment for common mental illness following its detection. It may be that milder mental illness that merits a ‘watch and wait’ strategy is being detected in people with sensory impairment. This is supported by the fact that in our descriptive sensitivity analysis, people with sensory impairment and a CIS-R score of 18 or more were no less likely to receive treatment (supplementary Table 1). It may, however, be the case that people with sensory impairment and milder common mental illness are receiving a diagnosis but not treatment even when it is appropriate.
Strengths and limitations
Strengths of our study include the use of a large nationally representative sample, separation of near and distance visual impairment and the assessment of multiple potential confounders and mediators of the relationship between sensory impairment and common mental illness.
There are also a number of limitations. First, causation cannot be inferred since the APMS data are cross-sectional and there is no means to determine the temporality of the data. This is particularly relevant to the mediation analysis. Although we have assumed that depression and anxiety do not directly cause sensory impairment, it could be that they reduce motivation to address medical causes of sensory impairment early on and facilitate its development. Stress and mental illness may even predispose to sensory impairment.Reference Zheng, Bokman, Lam, Christ, Swenor and West35–Reference Lin, Lin, Liu, Weng, Lin and Lin37 Social isolation might also reduce opportunity to obtain help for sensory impairment. Hence, reverse causation cannot be ruled out in our study, and a longitudinal analysis is needed to confirm our findings.
The CIS-R, despite being a valid measure, gives an indication of symptomatology only and cannot be as accurate as a professional diagnostic interview in ascertaining the presence of common mental illness. Measurement of the exposure was by self-report only, although self-reported sensory impairment has been shown to be a reasonably accurate measure.Reference Sindhusake, Mitchell, Smith, Golding, Newall and Hartley38,Reference Whillans and Nazroo39 Measurement of having received a diagnosis and treatment was also limited to self-report data. Some potential confounders were not measured, such as tinnitus.Reference Matthews40 This might have led to an overestimation of the association.
The analyses exploring the treatment gap may have included too few participants in each group to have the power to detect a clinically significant effect. This is one possible reason that we found people with sensory impairment to be more likely to have received a diagnosis of mental illness but not treatment. Including people with hearing aids might have selected proportionately more people who are better able to seek healthcare into the exposed group, reducing the likelihood of finding a treatment gap. The data we have used in assessing receipt of diagnosis and treatment may also be prone to recall bias, as it depended on participants recounting events over the past year.Reference McManus, Bebbington, Jenkins and Brugha18 Some of the participants would have been living with dementia; they may have had particular difficulties recalling diagnosis and treatment, but are at increased risk of experiencing anxiety and depression.Reference Jorm41 This would be expected to artificially inflate the treatment gap for people with sensory impairment; however, this was not seen in our study.
The APMS only covers those living in private households and excludes groups such as prisoners and people in nursing homes. The older population who live in communal or institutional settings are more likely to have worse mental health and dementia than older people who live in private households.Reference McManus, Bebbington, Jenkins and Brugha18,Reference Livingston, Barber, Marston, Rapaport, Livingston and Cousins42 As a large proportion of the population with sensory impairment are older this may have led to a slight underestimation of the association between sensory impairment and CIS-R score. It has, however, been estimated that those not living in private households represent less than 2% of the UK population.Reference McManus, Bebbington, Jenkins and Brugha18
It is also possible that people with sensory impairment may have been more prone to exclusion from the APMS than the general population. This may have led to an underestimate of the effect size if the most socially disadvantaged people with sensory impairment could not participate. We did not distinguish between degrees of impairment, whether impairment was well-corrected with aids or recently acquired and life-long impairments. The results between these groups could be different.Reference Du Feu and Fergusson29
Implications
Clinicians working with people with sensory impairments need to be alert to the possibility of comorbid mental illness so that timely interventions can be provided where appropriate. This is especially true in younger adults, for whom the association appears to be more pronounced. Treatment for clinically significant mental illness should be offered when it is detected, regardless of comorbidities, particularly given our finding that people with sensory impairment might be more likely to receive a diagnosis. Depression and anxiety should not be seen as inevitable or untreatable consequences of sensory impairment. Integrating mental and physical healthcare in sensory-impairment specific interventions has been shown to be effective in reducing the incidence of depressive disorders in a low vision clinic.Reference Rovner, Casten, Hegel, Massof, Leiby and Ho43 There is also evidence that auditory rehabilitation helps people with hearing impairment to adapt to this condition.Reference Blazer and Tucci44 Our findings also highlight the importance of preventing and correcting sensory impairment, which is often possible, to preserve psychological well-being.Reference Rishi, Rishi, Maitray, Agarwal, Nair and Gopalakrishnan45 Finally, clinicians should be aware of specialist mental health services for culturally deaf individuals.
It appears that further research into the mediators of common mental illness in people with sensory impairment is also required. Social functioning may be a potentially modifiable target for the prevention of mental illness in people with sensory impairment. Sensory impairment severe enough to interfere with social functioning in particular should alert clinicians to the need to consider the impact on the patient's mental health.
Funding
Supported by the University College London Hospitals National Institute of Health Research Biomedical Research Centre.
Acknowledgements
We would like to thank NHS Digital for granting us permission to use the APMS data.
Data availability
The data that support the findings of this study are available from NHS Digital. Restrictions apply to the availability of these data.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2019.81.









eLetters
No eLetters have been published for this article.