INTRODUCTION
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus-8 (HHV-8), is the aetiological agent linked to Kaposi's sarcoma, primary effusion lymphoma and multicentric Castleman's disease [Reference Moore, Chang, Knipe, Griffin, Lamb, Martin and Straus1]. Since its initial discovery, KSHV infection has been reported with seroprevalence and risk factors exhibiting considerable variations across different geographical regions [Reference de Sanjose2, Reference Pfeiffer3].
Men who have sex with men (MSM) were first reported to be at high risk for developing Kaposi's sarcoma in the 1980s [4]. North America and Europe are regions with low KSHV seroprevalence in the general population but high prevalence of KSHV infection in the MSM population, especially in those co-infected with HIV [Reference Martin5–Reference Smith7]. It is now well established that MSM are at a higher risk for acquiring KSHV infection although the definite routes of KSHV transmission have yet to be established [Reference Bagni and Whitby8, Reference Dukers and Rezza9]. Based on previous reports, KSHV appears to be sexually transmitted in MSM, although the extent of sexual transmission in heterosexuals is less certain [Reference Engels10–Reference Dukers14].
In general, China is regarded as a region of low KSHV prevalence due to an apparent lack of Kaposi's sarcoma development, except for the Xinjiang Uygur Autonomous Region in the west of the country [Reference Fu15, Reference He16]. However, a seroprevalence study conducted in central China showed that KSHV prevalence was as high as 15·8% in a HIV-positive group [Reference Zhang17]. Although MSM have already been found to be a high-risk group for HIV and sexually transmitted infections (STIs) in China [18], there is no available information on KSHV infection in this population. It is estimated that approximately 18 million MSM reside in China. Given the large size of the MSM population and its potential impact on the HIV epidemic, this population will not only be at risk of increasing morbidity due to AIDS-related diseases in the near future, but could also be a potential source of transmission of KSHV infection to the heterosexual population, as seen in the early phases of the HIV epidemic.
To address this gap in the available literature, a cross-sectional study was performed to investigate the prevalence and correlates of KSHV infection in MSM in Taizhou, a rural area in eastern China where the HIV/AIDS epidemic is rapidly spreading in the MSM population.
MATERIAL AND METHODS
Study population
The present study was conducted in the MSM population in Taizhou prefecture, Zhejiang province in eastern China.
Given the difficulty of approaching the MSM population in rural areas in China, a combined sampling strategy using both venue-based sampling and snowball sampling was applied for subject recruitment. To be eligible, a study participant had to be a male who: (1) was aged ⩾18 years, (2) acknowledged ever having had sex with another man, and (3) provided written informed consent for participation. During the 1-year study period from December 2008 to December 2009, a total of 239 MSM were approached, of whom 208 (87·0%) agreed to participate in this study. This study was approved by the Institutional Review Board of Fudan University, Shanghai, China.
Data and sample collection
A structured questionnaire, aimed at obtaining information about socio-demographic characteristics and sexual behaviours, was developed from validated data collection instruments previously reported in the literature. Face-to-face interviews were administered by trained public health workers.
Venous blood was drawn and transferred to the laboratory within 2 h after collection. Plasma samples were coded with a unique identifier, and stored at −80 °C until testing.
Serological testing
HIV testing
All plasma samples were screened for HIV antibodies using an enzyme-linked immunosorbent assay (ELISA; Abbott Laboratories, USA), according to the manufacturer's instructions. Positive samples were confirmed by Western blotting (Genelabs Diagnostic, Singapore).
KSHV testing
Plasma samples were tested by immunofluoresence assay, as reported previously [Reference Minhas19]. Briefly, two KSHV serology tests were performed: first, BC-3 cells (KSHV-positive and Epstein–Barr virus-negative B cell line; American Type Culture Collection, USA), stimulated by tetradecanoyl phorbol acetate were fixed and permeabilized and used for monoclonal enhanced immunofluorescence assay. Second, Spodoptera frugiperda clone 9 expressing viral recombinant proteins, ORF73, ORF65 and ORF-K8·1, was used for testing. The procedure was similar to the BC-3 immunofluoresence assay. A sample was considered KSHV seropositive only if it was positive at a standard serum dilution of 1:40 with both the BC-3 and S. frugiperda assay. Each slide was read independently by two experienced laboratory workers.
Syphilis testing
Plasma samples were tested using a rapid plasma reagent test (Span Diagnostics Ltd, India), and results were confirmed by the Treponema pallidum haemagglutination test (TPHA; Syphagen TPHA, Biokit, Spain) for diagnosis of syphilis.
Other serological testing
All plasma samples were also tested with ELISA for the presence of IgG antibodies to hepatitis C virus (HCV) (Wantai Bio Co., China), IgG antibodies to herpes simplex virus-2 (HSV-2) (HerpeSelect ELISA kit, Focus Technologies, USA), and hepatitis B surface antigen (Wantai Bio Co.), according to the manufacturers' instructions.
All the above serological tests were performed by two experienced technicians, with duplicate negative, positive and blank controls being tested in parallel.
Statistical analysis
Original questionnaires and laboratory testing results were managed in EpiData3.0 (EpiData Association, Denmark), and transferred to a statistical analysis system (SAS Institute Inc., USA) database for further analyses. Demographic characteristics and risk behaviours were analysed using descriptive statistics, i.e. mean, median and interquartile range (IQR) for continuous variables, and proportions for categorical variables.
KSHV seroprevalence was computed using the normal approximation, and tabulated by sociodemographic characteristics of the study subjects, followed by Pearson's χ2 test to determine statistical significance. Initially, univariate logistic regression analysis was conducted, followed by multivariate logistic regression analysis to explore associations between sexual behaviours and KSHV seropositivity. Odds ratio (OR) and 95% confidence interval (CI) were used to determine whether a variable was associated with KSHV infection. Mann–Whitney U test was used to assess the difference of geometric mean titres (GMTs) of anti-KSHV IgG between the KSHV mono-infection group and the co-infection group. A P value ⩽0·05 was considered to be statistically significant. All statistical analyses were performed using the SAS System for Windows version 8.0 (SAS Institute Inc.).
RESULTS
Characteristic and KSHV seroprevalence in participants
A total of 208 MSM participated in this study. Sociodemographic characteristics of the participants are summarized in Table 1. Briefly, the median age of the participants was 26 years (IQR 23–31 years). About 78·4% of the participants were non-sex workers (referred to as ‘general MSM’) and the other 21·6% were male sex workers known as ‘money boys’ who provided commercial sex to other men. Approximately 48·1% of the study participants were self-identified homosexual men, 28·8% were bisexual and 23·1% were unsure of their sexual orientation.
Table 1. Sociodemographic characteristics, sexual orientation and KSHV infection in study participants

KSHV, Kaposi's sarcoma-associated herpesvirus; OR, Odds ratio; CI, confidence interval; MSM, men who have sex with men.
* Proportion.
† Prevalence of KSHV infection.
‡ Unadjusted ORs and 95% CIs obtained from univariate logistic regression analyses.
The overall seroprevalence of KSHV was 32·7% (68/208) in study participants. KSHV seroprevalence was significantly higher in those who were ever married compared to those who were never married, but the prevalence did not differ significantly based on other sociodemographic characteristics (Table 1).
Association of KSHV prevalence with sexual practices
Table 2 summarizes the prevalence of different sexual practices of the study participants and the association of KSHV prevalence with these practices. About 76·9% of study participants reported having had sex with women. The proportion reporting heterosexual behaviour was 67·6% in those never married, 97·0% in those ever married, 79·1% in ‘general MSM’ and 68·9% in ‘money boys’. Of those who had sex with women, 55·0% had heterosexual contact in the past month. However, only 38·6% had always used condoms whereas 39·7% had never used condoms during these heterosexual practices. About 92·7% (193/208) of study participants reported having had anal sex with a man, of whom 67·8% reported receptive anal sex and 77·9% reported insertive anal sex. About 89·4% of study participants reported having had anal sex with men in the past month. Of these, 41·9% had regularly used condoms.
Table 2. Sexual behaviours and their associations with KSHV infection in study participants

KSHV, Kaposi's sarcoma-associated herpesvirus; OR, Odds ratio; CI, confidence interval.
* Proportion.
† Seroprevalence of KSHV.
‡ OR and 95% CI adjusted for sociodemographic characteristics including MSM group category, age, and marital status as shown in Table 1.
About 93·1% of study participants reported having had oral sex with a man, 76·0% had receptive oral sex and 56·7% had insertive oral sex. About 91·3% of study participants reported having had oral sex with man in the past month. Of these, only 17·3% (33/190) had regularly used condoms.
Univariate analyses indicated that KSHV infection was significantly associated with anal sex with a man (Table 2). Those who had high frequency of anal sex with a man in the past month, did not use condoms for anal sex in the past month, and had ever had receptive anal sex with a man were more likely to be infected with KSHV. After adjusting for sociodemographic variables including MSM group category, age and marital status by multivariate logistic regression analyses, having ever had receptive anal sex with a man was still significantly associated with KSHV infection (OR 2·68, 95% CI 1·15–6·23). Whereas high frequency of anal sex with a man in the past month was associated with KSHV infection only at the marginal significance level (Table 2). KSHV infection was not significantly associated with sex with a woman and oral sex with a man (Table 2).
Other co-infections and association with KSHV infection
The overall seroprevalence in study participants was 4·3% for HIV, 16·3% for HBV (i.e. HBsAg), 1·0% for HCV, 18·3% for HSV-2 and 22·1% for syphilis (Table 3). The overall prevalence of infection with at least one of the five examined infectious agents was 61·5% for study participants, 57·1% (80/140) for KSHV-positive participants and 44·1% (30/68) for negative participants, but the difference was not significant (P = 0·078).
Table 3. Serostatus of HIV, HBsAg, HCV, syphilis and HSV-2 and their associations with KSHV infection in study participants

KSHV, Kaposi's sarcoma-associated herpesvirus; OR, Odds ratio; CI, confidence interval.
* Proportion or seroprevalence of HIV, HBsAg, HCV, HSV2 or syphilis, respectively.
† Seroprevalence of KSHV.
‡ OR and 95% CI adjusted for sociodemographic characteristics including MSM group category, age, and marital status as shown in Table 1.
Univariate logistic regression analyses indicated that both HSV-2 and syphilis infections were significantly associated with KSHV infection. Those who were infected with HSV-2 (OR 2·82, 95% CI 1·38–5·79) or syphilis (OR 3·29, 95% CI 1·67–6·48) were more likely to be infected with KSHV. Such associations were still significant after adjusting for potential confounders (Table 3), whereas, no statistically significant association between KSHV infection and HIV or HBV infections were detected.
To better explore how KSHV antibody titre was influenced by these co-infections, plasma from all KSHV-seropositive subjects was serially diluted and tested for anti-KSHV IgG antibody titre (Fig. 1). The GMT of the antibody was 613·3 (95% CI 366·7–860·0) for the KSHV-only infection group (n = 33) and 562·3 (95% CI 413·0–711·5) for the co-infection group (n = 35). The difference in the GMT of the antibody between the KSHV-only infection group and the co-infection groups was not significant (Mann–Whitney U = 488·5, P = 0·254).
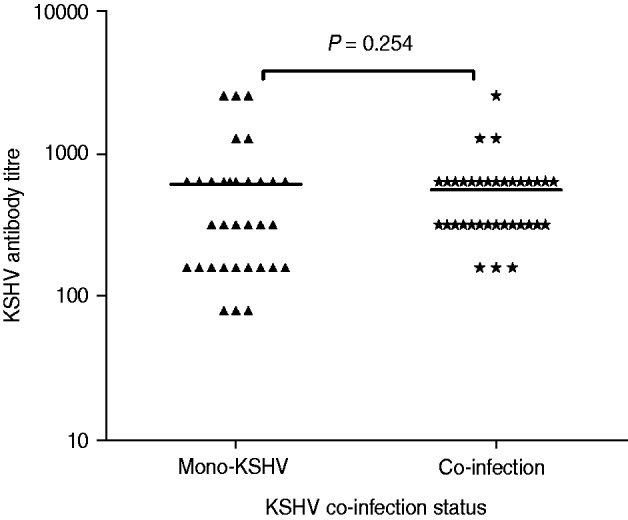
Fig. 1. Anti-Kaposi's sarcoma-associated herpesvirus (KSHV) IgG antibody titre in patients with KSHV mono-infection vs. patients with KSHV and other co-infections.
DISCUSSION
The epidemiology of KSHV infection depicting specific demographic pockets of endemicity has long been puzzling [Reference Bagni and Whitby8, Reference Dukers and Rezza9]. However, several studies have reported that MSM is a high-risk group for KSHV infection [Reference Guanira11, Reference Martro13, Reference Lodi20–Reference Giuliani23]. It is estimated that HIV infection in Chinese MSM has reached approximately 5% [24]. However, social stigma in China makes MSM very hard to reach, thus very little information about KSHV infection is available in this population. In addition, unlike in Western countries, most MSM in China are also bisexual, married and have families. Therefore, they are very likely to be a potential source of transmission of STIs to the heterosexual population. To the best of our knowledge, the present study is the first to report the seroprevalence and factors associated with KSHV infection in MSM in China.
The present study identified a high seroprevalence of KSHV infection in a sample of Chinese MSM, suggesting that MSM in China is also a high-risk group of KSHV infection, as documented by studies on MSM in other countries [Reference Guanira11, Reference Dukers14]. Furthermore, a substantial proportion of MSM reported heterosexual behaviours regardless of their stated sexual orientation, and a high KSHV infection rate in ever-married subjects was detected. This implies bisexually active men in China could potentially play a critical bridging role in spreading KSHV from their high-risk male sexual partners to low-risk female partners, which may explain a heterosexual transmission route of KSHV.
To further investigate the possible transmission mechanisms of KSHV in this population, we analysed the association between KSHV seropositivity and specific sexual practices. Receptive anal intercourse was consistently identified as a risky sexual practice for KSHV seropositivity. The association between KSHV infection and receptive anal intercourse supports the premise that KSHV is sexually transmitted in MSM [Reference Guanira11]. In contrast, oral sex practice was not found to be a risk factor for KSHV seropositivity in this study. It is, therefore, unlikely that oral genital sex and oral pharyngeal exposure to semen is a common mechanism of KSHV transmission in this MSM population. Our results are generally consistent with previous studies of KSHV in homosexual men in Western countries [Reference Guanira11, Reference O'Brien25, Reference Melbye26].
Co-infection with multiple infectious agents is a common phenomenon in the MSM population, and a high co-infection rate was detected in both KSHV positive and negative groups (57·1% vs. 44·1%, respectively). Moreover, no statistically significant difference in the GMT of antibody was obtained between KSHV mono-infected and co-infected individuals, suggesting that co-infections may not have a substantial effect on KSHV antibody titre. Interestingly, only two HCV-positive subjects, with none from the KSHV-positive group, were detected. HCV prevalence (0·9%) is quite low compared to syphilis prevalence in these MSM subjects. Although this low prevalence of HCV infection precluded further meaningful analysis of transmission routes associated with bloodborne mode, these data nevertheless indicate that MSM are at high risk for STIs but not for bloodborne infections. This supports our former study in a community with high risk of bloodborne infections, particularly HCV, demonstrating that KSHV and HCV might not share the same transmission routes in that population [Reference Zhang17]. Furthermore, HSV-2 and syphilis infections were found to be positively associated with KSHV seropositivity in this MSM population. Since both HSV-2 and syphilis are mainly sexually transmitted, these findings are consistent with data from previous studies, implying that HSV-2, syphilis and KSHV may share similar transmission routes [Reference Engels10, Reference Campbell27, Reference Nawar28].
Interestingly, HIV was not found to be associated with KSHV infection in this study. It is well known that Kaposi's sarcoma is linked to AIDS, and KSHV infection has been found to be strongly associated with HIV infection [Reference Ambinder29–Reference Sullivan31]. However, correlation between KSHV and HIV infection in study participants was not observed. A possible explanation is that HIV prevalence in MSM participants in most other studies was very high, whereas HIV prevalence in participants in the present study was relatively low. Nevertheless, our results suggest that KSHV infection and transmission may have already occurred prior to the introduction of HIV into this population. It is possible that sexual practices rather than HIV infection or associated immunosuppression may be risk factors associated with KSHV infection, at least in our study subjects. Given the close relationship between HIV and KSHV infections, it is plausible that a well-designed cohort will be needed to further investigate the association of HIV and KSHV infections in the MSM population in China.
There are two potential limitations in the current study. First, we did not compare KSHV seroprevalence between MSM and the general male population. Nevertheless, a systematic review showed that KSHV seroprevalence is generally low in males in eastern China [Reference Zhang32]. Second, a potential selection bias was introduced by using non-random venue-based and snowball sampling strategies for subject recruitment. Therefore, our study results may not be representative of the whole MSM population in China, which warrants large-scale study in the near future.
In conclusion, the present study is the first epidemiological study of KSHV infection in MSM in China, and provides evidence that KSHV is highly prevalent and very likely to be sexually transmitted, especially through receptive anal sex in this population. Given the high seroprevalence of KSHV in Chinese MSM and its potential for development of Kaposi's sarcoma, further large and longitudinal epidemiological studies, specifically designed to identify risk factors for KSHV in MSM in China will be of substantial public health interest.
ACKNOWLEDGEMENTS
We are grateful to all the participants for their cooperation which made this study possible. This study was supported by National Natural Science Foundation of China (grant no. 81161120407 to N.H.); the United States National Institutes of Health Fogarty International Centre (grant no. D43 TW001492, RO1 CA75903 and P30 GM103509 to C.W.), and Fundamental Research Funds for the Central Universities (10FX058).
DECLARATION OF INTEREST
None.






