Readers may remember the correspondence about the identification of studies for a review of volumetric magnetic resonance imaging (MRI) findings in schizophrenia (Reference LawrieLawrie & Abukmeil, 1998). Adams et al (Reference Adams, Thornley and Joy1998) suggested that a more comprehensive search strategy would have identified other relevant studies. Lawrie (Reference Lawrie1998) questioned whether this effort would alter the results of the review — particularly for a pre-specified region (the left amygdalo-hippocampus). We now report the outcome.
Clive Adams searched Medline between 1986 and June 1996 (inclusive) — the same period covered by the original investigation. A simple MRI search identified 142 studies in total. Employing Boolean logic and adding a refined schizophrenia search term (by using ‘and’) found 27 studies. Refining the MRI search term resulted in the location of 196 studies. Out of interest, EMBASE, a more comprehensive database covering 67% of 506 indexed psychiatry journals (v. 47% in Medline), was searched similarly and identified 289 potentially relevant studies. PsycLit, covering 73% of indexed psychiatry journals, was not searched. Stephen Lawrie examined every identified citation for volumetric MRI studies in patients with DSM-III-R (American Psychiatric Association, 1987) schizophrenia and healthy controls, giving raw data that could generate case-control differences (expressed as a percentage) for relevant brain regions.
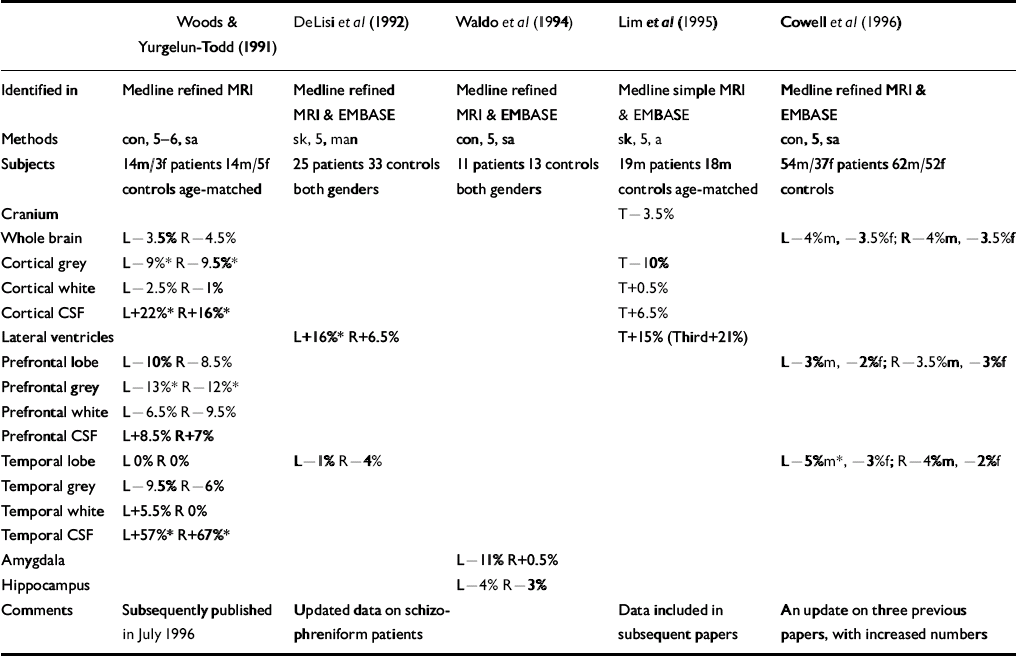
Five studies that should have been included in the original review were identified (see Table). One of these, Lim et al (Reference Lim, Beal and Harvey1995), should have been identified in the simple Medline search, and all should have been identified in the hand search of journals. Indeed, Lawrie & Abukmeil were aware of two of the studies but mistakenly excluded them for not giving relevant raw data (Reference Woods and Yurgelun-ToddWoods & Yurgelun-Todd, 1991) or for being published outside the time frame (Reference Cowell, Kostianovsky and GurCowell et al, 1996). It should be noted, however, that two of the studies (Reference DeLisi, Hoff and KushnerDeLisi et al, 1992; Reference Cowell, Kostianovsky and GurCowell et al, 1996) simply gave information on more subjects than in earlier papers which were included in the review, and another two included the data in subsequent papers.
Table Volumetric magnetic resonance imaging (MRI) studies in schizophrenia omitted by Lawrie & Abukmeil (Reference Lawrie and Abukmeil1998)
| Woods & Yurgelun-Todd (Reference Woods and Yurgelun-Todd1991) | DeLisi et al (Reference DeLisi, Hoff and Kushner1992) | Waldo et al (Reference Waldo, Cawthra and Adler1994) | Lim et al (Reference Lim, Beal and Harvey1995) | Cowell et al (Reference Cowell, Kostianovsky and Gur1996) | |
|---|---|---|---|---|---|
| Identified in | Medline refined MRI | Medline refined MRI & EMBASE | Medline refined MRI & EMBASE | Medline simple MRI & EMBASE | Medline refined MRI & EMBASE |
| Methods | con, 5-6, sa | sk, 5, man | con, 5, sa | sk, 5, a | con, 5, sa |
| Subjects | 14m/3f patients 14m/5f controls age-matched | 25 patients 33 controls both genders | 11 patients 13 controls both genders | 19m patients 18m controls age-matched | 54m/37f patients 62m/52f controls |
| Cranium | T-3.5% | ||||
| Whole brain | L-3.5% R-4.5% | L-4%m, ‒3.5%f; R-4%m, ‒3.5%f | |||
| Cortical grey | L-9%* R-9.5%* | T-10% | |||
| Cortical white | L-2.5% R-1% | T+0.5% | |||
| Cortical CSF | L+22%* R+16%* | T+6.5% | |||
| Lateral ventricles | L+16%* R+6.5% | T+15% (Third+21%) | |||
| Prefrontal lobe | L-10% R-8.5% | L-3%m, ‒2%f; R-3.5%m, ‒3%f | |||
| Prefrontal grey | L-13%* R-12%* | ||||
| Prefrontal white | L-6.5% R-9.5% | ||||
| Prefrontal CSF | L+8.5% R+7% | ||||
| Temporal lobe | L 0% R 0% | L-1% R-4% | L-5%m*, ‒3%f; R-4%m, ‒2%f | ||
| Temporal grey | L-9.5% R-6% | ||||
| Temporal white | L+5.5% R 0% | ||||
| Temporal CSF | L+57%* R+67%* | ||||
| Amygdala | L-11% R+0.5% | ||||
| Hippocampus | L-4% R-3% | ||||
| Comments | Subsequently published in July 1996 | Updated data on schizophreniform patients | Data included in subsequent papers | An update on three previous papers, with increased numbers |
Incorporating the figures from the Table into the calculations of median percentage differences between patients with schizophrenia and controls generally has little effect for most brain regions — probably as a consequence of the small amount of additional data gleaned for any particular region in specific subject groups. The only region in a subject group to have more than one additional datum was the left and right temporal lobes in both genders combined. The result for this region was also changed by more than any other cortical region, from ‒6% and ‒9.5% (left and right) to ‒3.5% and ‒7%. Similarly sized but opposite effects were found for the prefrontal lobes, rendering the revised median differences more compatible with those of the temporal lobes (-5.5% and ‒4%, respectively). The largest overall change was for the right lateral ventricle volume in both genders, the median difference being reduced from 36% to 23% in patients with schizophrenia. The pre-specified region of maximal interest (left amygdalo-hippocampus) was not altered — the only relevant data (Reference Waldo, Cawthra and AdlerWaldo et al, 1994) reporting these structures separately. One previous study had reported data this way, giving a new median estimate (between the two studies) of ‒11.5% and ‒9% (left and right) for the amygdala, and ‒6 and ‒4% for the hippocampus.
The grey/white segmentation data in Woods & Yurgelun-Todd (Reference Woods and Yurgelun-Todd1991), that only had two relevant previous studies, were the only data that actually altered the findings. Whereas prefrontal and temporal white matter was bilaterally increased before (Reference Lawrie and AbukmeilLawrie & Abukemil, 1998), such volume increases are only evident in the left temporal lobe after incorporating the new data and the other three regions are actually reduced in line with overall and grey matter reductions. However, the inclusion of one further study — in an updated review (Reference Lawrie, Johnstone, Humphries and LangLawrie, 1999) — re-instates the previous finding. Overall, therefore, the main conclusions of the review — that patients with schizophrenia have small reductions in whole brain volumes as well as greater reductions in medial temporal lobe structures — remain unaltered.
What has this exercise taught us? First, systematic reviewers can fail to include relevant articles through oversight, despite doing appropriate searches. Second, full reporting of comprehensive searches is desirable — as a general rule and because unidentified articles where there are few published papers are disproportionately important. Finally, readers with good memories will remember that we staked a bottle of Glenndronnach malt whisky on the outcome of our efforts. As there were exactly five additional articles identified (rather than more or fewer) we have declared an honourable draw.




eLetters
No eLetters have been published for this article.