Several studies have shown that increasing dietary NaCl intake from a low level (10 mmol/d) to a high level (350 mmol/d) causes fluid retention and extracellular volume (ECV) expansion(Reference Sagnella, Markandu and Buckley1, Reference Singer, Markandu and Buckley2). However, high NaCl intake is not necessarily accompanied by volume expansion. We have recently shown (Salty Life 1–5(Reference Heer, Baisch and Kropp3)) that high Na intake (400, 440, 550, 660 mmol/d) in humans may lead to Na storage accompanied by rising plasma volume (PV) but not by increased ECV or body mass (BM)(Reference Heer, Baisch and Kropp3, Reference Drummer, Hesse and Baisch4). ‘Salty Life’ is a set of experiments dealing with the effects of different levels of NaCl intake on body fluid regulation and Ca and bone turnover. Our results are consistent with those obtained by Palacios et al. (Reference Palacios, Wigertz and Martin5) in children (low NaCl intake, 57 mmol/d; high NaCl intake, 172 mmol/d) as well as by Titze et al. (Reference Titze, Krause and Hecht6, Reference Titze, Maillet and Lang7) in animal experiments (low-NaCl diet, 0·1 %; high-NaCl diet, 8 %).
Many studies have shown that high NaCl intake-induced increases in serum Na concentrations are accompanied by increased fluid retention(Reference Sagnella, Markandu and Buckley1, Reference Singer, Markandu and Buckley2). This is probably true when increasing NaCl intake from a low to high level. However, healthy male test subjects seem to have no further fluid retention when increasing NaCl intake from an average normal (200 mmol/d) to a much higher level (above 400 mmol/d).
In rat experiments with extremely high Na intake, Titze et al. (Reference Titze, Shakibaei and Schafflhuber8) have shown in the skin of rats that the expression of mRNA for certain enzymes that synthesise glycosaminoglycans (GAG) is increased with high Na intake. This might also be true in humans and could be a mechanism for developing water-free Na retention.
On the other hand, Sharma et al. (Reference Sharma, Cetto and Schorr9, Reference Sharma and Distler10) found in salt-sensitive humans an association between metabolic acidosis and genetic hypertension. Preliminary findings of increased renal acid excretion in their studies suggested that the perturbation of acid–base status may be the result of increased metabolic acid production.
The hypotheses of the present study (‘Salty Life 6’) are:
(1) Increasing NaCl ingestion from different onset levels, i.e. starting from a low to the average normal intake level of Germany (German Nutrition Society(11)) or increasing from this average normal level to a much higher level leads to different effects on metabolic water and electrolyte balance;
(2) Expression of mRNA for enzymes of GAG synthesis is increased during very high NaCl intake;
(3) Different levels of NaCl intake are accompanied by changes in acid–base balance in normotensive healthy humans.
Materials and methods
Subjects
Nine healthy male test subjects (aged 25·7 (sd 3·1) years; BM 71·5 (sd 4·0) kg; BMI: 21·9 (sd 3·1) kg/m2) participated in the present study in the metabolic ward at the DLR-Institute of Aerospace Medicine, Cologne, Germany. After approval by the Ethics Committee of the ‘Aerztekammer Nordrhein’, Duesseldorf, Germany, all volunteers gave their written informed consent before study entry. Temperature (24°C) and relative humidity (55 %) were controlled during the entire study in order to avoid any uncontrolled Na loss through the skin. Physical exercise, methylxanthine derivate intake and alcohol consumption were prohibited. The subjects were constantly monitored by a study nurse for their adherence to the study protocol.
Design and protocol
The study design is depicted in Fig. 1. Over 28 d, subjects were tested at four consecutive periods with different NaCl intake levels. The low NaCl intake period in the beginning was used for the adaptation of subjects to a balanced state. The low NaCl intake period at the end was used as a control period to exclude any time-related effect during the study. All subjects had a constant fluid intake of 40 ml/kg BM per d. Metabolic water (about 300 ml/d) resulting from the oxidation of nutrients was taken into account as additional water intake.

Fig. 1 Study design. Each bar represents the Na intake on the respective study day. Na intake went from low (0·7 mmol NaCl/kg body mass per d) NaCl intake in the adaptation period during the first 6 study days (□) to average normal NaCl intake (2·8 mmol NaCl/kg body mass per d) during the following 6 d (![]() ). The volunteers then received high NaCl intake (7·7 mmol NaCl/kg body mass per d) for 10 d (
). The volunteers then received high NaCl intake (7·7 mmol NaCl/kg body mass per d) for 10 d (![]() ) and went back to low NaCl intake (0·7 mmol NaCl/kg body mass per d) for the remaining 6 d (□).
) and went back to low NaCl intake (0·7 mmol NaCl/kg body mass per d) for the remaining 6 d (□).
The diet of the test subjects was tailored individually to their needs and prepared as described previously(Reference Heer, Baisch and Kropp3). Additionally, K intake was kept constant at 112 (SE 7) mmol/d and Ca intake at 25 mmol/d. The daily intake level of all other nutrients matched the German dietary recommended intake(12). All subjects consumed all of their meals at the scheduled times.
Sampling and analyses
Fasting blood samples (supine body position) were drawn in the morning at 07.00 hours, before breakfast, on days 4 and 6 of the first low NaCl intake period, on days 4 and 6 of the average normal NaCl intake period, on days 3, 8 and 10 of the high NaCl intake period, and on days 2, 4 and 6 of the second low NaCl intake period. Blood for analyses of arginine vasopressin, atrial natriuretic peptide (ANP), renin and aldosterone was collected in ice-chilled EDTA tubes. Blood for the analysis of serum electrolytes, osmolality, albumin, protein and creatinine concentrations was collected in tubes without any additives. For the analyses of packed cell volume and Hb, blood was drawn in EDTA tubes. Packed cell volume and Hb tubes were kept at room temperature and were immediately analysed by a Coulter Counter (model T660; Coulter Corp., Miami, FL, USA). Samples for hormone analysis were immediately separated by centrifugation, and plasma was stored at − 80°C until analysis. Commercially available RIA kits were used to measure arginine vasopressin (Mitsubishi Yuka, Tokyo, Japan), renin (IRMA; Nichols Institute Diagnostics, San Juan Capistrano, CA, USA) and aldosterone (MAIA; Adaltis, Italy). ANP analysis was done by RIA as previously described(Reference Drummer, Gerzer and Heer13).
On the last day of each study period, capillary blood was taken from the fingertip to analyse pH, bicarbonate and base excess by a blood gas analyser (ABL 5 Radiometer; Radiometer GmbH, Willich, Germany) immediately after drawing. All these measurements were done by the same technical assistant each time.
PV was determined by a dye-dilution method with Evans blue, according to the procedure of Johansen et al. (Reference Johansen, Foldager and Stadeager14). ECV was analysed by the sinistrin dilution method as described previously(Reference Heer, Baisch and Kropp3).
Daily 24 h urine was collected from 07.00 hours in the morning (emptying bladder) to 07.00 hours on the following morning. A sample of each 24 h urine pool was stored at − 20°C for analysis. Serum and urinary Na and K concentrations, as well as serum chloride concentrations, were analysed by an ion-selective electrode (Hitachi 704; Hitachi, Tokyo, Japan). Urinary chloride concentrations were analysed by an ion chromatographic method. Serum and urinary creatinine concentrations were analysed by an automatic system (Hitachi 704) according to the Jaffé method. Serum and urinary osmolality were analysed by freezing-point depression (Vogel osmometer type OM 801; Vogel, Giessen, Germany).
Daily and cumulative metabolic water, Na and K balances were calculated for each 24 h period from the respective intake and excretion data as described previously(Reference Heer, Baisch and Kropp3). Na balance was calculated by subtracting excretion via urine and skin from Na intake. The method used to analyse skin Na loss was identical to the one described earlier(Reference Heer, Baisch and Kropp3). Since changes in Na excretion via faeces are negligible (P = NS) with increasing NaCl intake(Reference Heer, Baisch and Kropp3), we did not include faecal Na loss into the metabolic Na balance calculation. K balance was calculated by subtracting K losses via urine, skin and faeces from daily K intake. Faecal losses were calculated by using K absorption rates determined in previous studies during different levels of NaCl intake (0·7 mmol/kg BM per d NaCl intake: K+ absorption rate = 85·3 %; 2·8 mmol/kg BM per d NaCl intake: K+ absorption rate = 86·1 %; 7·7 mmol/kg BM per d NaCl intake: K+ absorption rate = 88·7 %). For operational reasons, we analysed 24 h urinary chloride excretion only from the two last days of each study period from each individual test subject. Daily metabolic chloride balance of these 2 d of each study period was calculated by subtracting 24 h chloride excretion via urine from daily chloride intake.
BM was measured with a precision scale (Precision Scale BP2100S; sensitivity ± 5 g; Sartorius AG, Göttingen, Germany) every morning after voiding and before breakfast.
Blood pressure was measured each morning (06.45 hours) before the subjects got up, by an oscillometric method (BOSO-Medicus; Bosch+Sohn GmbH, Jungingen, Germany).
Skin biopsies (4 mm diameter) were taken from the subjects on day 5 of the first low NaCl intake period and on day 9 of the high NaCl intake period. The biopsies were taken from the peri-umbilical region after previous local skin anaesthesia with 1 ml Scandicain® (AstraZeneca, Plankstadt, Germany). Specimens were shock-frozen in liquid N2 and stored at − 80°C until analysis. For real-time PCR analysis, total RNA was extracted with RNeasy® Mini columns (Qiagen, Hilden, Germany). Skin slices (about 10–20 mg) were homogenised in 500 μl RLT buffer reagent with an Ultra Turrax (IKA® Werke GmbH & Co. KG, Staufen, Germany) for 30 s. After homogenisation, we added 950 μl water and 320 μg Proteinase K, incubated at 55°C for 10 min and then centrifuged at 12 000 rpm for 3 min. After 1 ml of 96 % ethanol was added, the solvent was transferred to the columns and eluted according to the standard protocol. First-strand cDNA was synthesised with TaqMan reverse transcription reagents (Applied Biosystems, Darmstadt, Germany) using random hexamers as primers. The final RNA concentration in the reaction mixture was adjusted to 0·1 ng/μl. Reactions without multiscribe RT were used as negative controls for genomic DNA contamination. RT products were diluted 1:1 with water before the PCR procedure was done. PCR was performed with an ABI PRISM 7000 Sequence Detector and SYBR Green reagents (Applied Biosystems) according to the manufacturer's instructions. We were interested in the expression of genes for GAG polymerisation key enzymes such as chondroitin synthase (dermatan sulfate elongation), xylosyl transferase (dermatan or heparan sulfate initiation) and EXT-like gene (EXTL, heparan sulfate elongation). All samples were run in duplicate. The relative amount of each mRNA of interest was normalised with respect to 18S rRNA. Dissociation curves were constructed to confirm the specificity of the PCR reaction.
Microdialysis was conducted on day 5 of the low NaCl intake period and on day 9 of the high NaCl intake period to analyse interstitial Na concentration. One microdialysis probe (CMA/60) was inserted into the abdominal subcutaneous adipose tissue and another one (CMA/70) into the dermis, both at the level of the umbilicus. Details of the microdialysis technique are described elsewhere(Reference Groth15–Reference Lonnroth17). Before the probe was inserted, a local anaesthetic (lidocaine) was applied in cream form. After probe insertion, tissue perfusion was started with a 5 % glucose solution (Serumwerk Bernburg AG) at a flow rate of 2 μl/min by using a microperfusion pump (CMA/102). The microdialysis probes and the microdialysis pump were from CMA Microdialysis AB (Solna, Sweden). A 60 min period was allowed for tissue recovery. Then, the perfusion rate was lowered to 0·3 μl/min to achieve almost complete Na recovery(Reference Stahle, Segersvard and Ungerstedt18) and dialysates were collected for 150 min.
Statistics
Data on subjects' characteristics are described as mean values and standard deviations. All results are presented as mean values with their standard errors. With the exception of metabolic balances, mean values of replicate measurements from each test subject of each study period were calculated and statistically compared by ANOVA (repeated-measures design) using the STATISTICA program (StatSoft, Inc., Tulsa, OK, USA). Cumulative metabolic balances were calculated per test subject per study period. Mean daily cumulative Na balance of the subjects per study period was statistically compared for the four study periods by ANOVA (repeated-measures design). For the relative number of copies of GAG enzyme mRNA expressed, the data were transformed to the logarithm and tested by ANOVA (repeated-measures design). For the latter analysis, data from one subject were taken out because no positive Na balances were seen. A significant effect of NaCl consumption was accepted when a significant influence of NaCl intake was evident (P < 0·05). Post hoc testing was done by Tukey's method using commercially available software (StatSoft, Inc.). P < 0·05 was taken as the minimum level of significance.
Results
Urinary Na excretion increased significantly with increasing Na intake (P < 0·0001) (Table 1). But, in both the average normal period and the high NaCl intake period, the amount of Na excreted was less than the amount consumed, leading to positive Na balances (Table 1) (P < 0·0001). While in the first low NaCl intake period in total − 105 (sem 23) mmol of Na were lost, during the average normal NaCl intake period, +193 (sem 13) mmol Na were retained, and in the high NaCl intake period, 244 (sem 77) mmol Na were retained (Fig. 2 (a)). When adding the positive Na balances of the average normal and high NaCl intake period, a total of +437 (sem 83) mmol Na were retained. However, in the second low NaCl intake period the cumulative Na balance was only − 157 (sem 31) mmol, resulting in a Na balance that was still positive.
Table 1 Urinary excretion over 24 h and mean daily metabolic balances during the different NaCl intake periods*
(Mean values with their standard errors)

BM, body mass; UNaV, urinary Na excretion; UKV, urinary K excretion; UClV, urinary Cl excretion.
* For Na and fluid, mean daily balances from each study period were calculated. For mean Cl− balance, data from the last 2 d of each study period were used.

Fig. 2 Cumulative metabolic Na balance (a) and fluid balance (b) per study period. The balances are derived from daily Na or fluid balances summed up for each study period: the two low NaCl intake study periods (□), the average normal NaCl intake period (![]() ) and the high NaCl intake study period (
) and the high NaCl intake study period (![]() ). Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the preceding period: ** P < 0·01, *** P < 0·001.
). Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the preceding period: ** P < 0·01, *** P < 0·001.
In the average normal NaCl intake period, in addition to the observed Na retention, BM (Fig. 3), PV and ECV (Fig. 4) increased significantly (BM, P < 0·001; PV, P < 0·001; ECV, P < 0·001). During the average normal NaCl intake period, the cumulative Na balance of +193 (sem 13) mmol, together with the increase in ECV and BM, suggests that the Na was stored in an osmotically active way. In contrast, with further Na retention in the high NaCl intake period, neither BM nor PV nor ECV (Figs. 3 and 4) increased further. This suggests that increasing NaCl intake from average normal to high NaCl intake led to water-free Na retention. During the second low NaCl intake period, both PV and ECV returned back to levels of the first low NaCl intake period (Fig. 4). Taking into account the serum Na concentration of 145 mmol/l (Table 2), the loss of 157 mmol Na in the second low NaCl intake period reflects a loss of ECV of 1·08 litres. This is in accord with our data for BM loss of 1·1 (sem 0·13) kg (Fig. 3) and loss in ECV of 1·4 (sem 0·4) litres (Fig. 4 (b)). Most of the Na that was retained in an osmotically active form (associated with fluid retention) was therefore excreted along with fluid when subjects went back to a low NaCl intake. However, some Na was still retained in the body in the absence of fluid retention.

Fig. 3 Body mass during the four periods at different NaCl intake levels: the two low NaCl intake study periods (□), the average normal NaCl intake period (![]() ) and the high NaCl intake study period (
) and the high NaCl intake study period (![]() ). Values are means, with standard errors represented by vertical bars. Increasing NaCl intake led to a significant increase in body mass. *** Mean value was significantly different from that of the preceding period (P < 0·001).
). Values are means, with standard errors represented by vertical bars. Increasing NaCl intake led to a significant increase in body mass. *** Mean value was significantly different from that of the preceding period (P < 0·001).

Fig. 4 Plasma volume (a) and extracellular volume (b) changes, analysed by dye dilution methods during the four periods at different NaCl intake levels: the two low NaCl intake study periods (□), the average normal NaCl intake period (![]() ) and the high NaCl intake study period (
) and the high NaCl intake study period (![]() ). Values are means, with standard errors represented by vertical bars. Increasing NaCl intake from low to normal NaCl intake led to both increases in plasma as well as extracellular volume. They, however, did not further increase during the larger rise in NaCl intake. Mean value was significantly different from that of the preceding period: *P < 0·05, **P < 0·01, ***P < 0·001.
). Values are means, with standard errors represented by vertical bars. Increasing NaCl intake from low to normal NaCl intake led to both increases in plasma as well as extracellular volume. They, however, did not further increase during the larger rise in NaCl intake. Mean value was significantly different from that of the preceding period: *P < 0·05, **P < 0·01, ***P < 0·001.
Table 2 Blood analytes during different NaCl intake study periods
(Mean values with their standard errors)

BM, body mass.
While dietary K intake was kept constant, average urinary K excretion rose significantly with increasing NaCl intake from the first low NaCl intake period to average normal intake (P = 0·0003; Table 1). The retention of osmotically active Na therefore seems to be accompanied by an exchange of K with Na, leading to an increase in urinary K excretion.
Calculation of cumulative K balance per study period revealed that +64 (sem 13) mmol K were retained in the first low NaCl intake period. During the average normal NaCl intake period, K was almost balanced (cumulative balance +17 (sem 10) mmol/d); during the high NaCl intake period, 54 (sem 11) mmol K were lost, and during the second low NaCl intake period, 40 (sem 13) mmol K were retained. The K loss in the high NaCl intake period did not match the Na retention of +244 (sem 77) mmol.
Mean Na retention during the average normal and high NaCl intake periods together was +437 (sem 85) mmol. Concurrently, mean BM (Fig. 4) increased by 1·0 (sem 0·1) kg from low to average normal NaCl intake and by 0·5 (sem 0·2) kg from average normal to high NaCl intake (comparing the last day of the respective study periods). Taking into account a mean serum Na concentration of 145 mmol/l (Table 2), a total of 218 mmol (1·5 litres × 145 mmol/l) of Na should have been retained during the average normal plus the high NaCl intake periods, if only osmotically active Na had been retained. This suggests that the Na retention in the average normal intake period (analysed cumulative Na balance 193 (sem 19) mmol) was fully osmotically active. Additionally, during the high NaCl intake period, 244 (sem 77) mmol Na were retained while 54 (sem 11) mmol K were lost, indicating that 54 mmol Na might have been exchanged by K. The remaining part, about 78 % of the retained Na, may have been stored without K exchange and without fluid retention.
Changes in expression (relative number of copies) of mRNA for chondroitin sulfate synthase 3, EXTL2 (a heparan sulfate polymerisation enzyme), xylosyltransferase 1 and hyaluronidase are shown in Table 3. Concurrently with the rise in NaCl intake, we found a significant rise in expression of the genes for EXTL2 and hyaluronidase. The mean expression of mRNA for xylosyltransferase 1 and chondroitin sulfate synthase 3 was not significantly increased during the high NaCl intake period.
Table 3 Expression (relative number of copies) of mRNA for enzymes that synthesise glycosaminoglycans in skin biopsies during low and high NaCl intake
(Mean values with their standard errors)

BM, body mass.
Increased expression of the gene for EXTL2 was accompanied by significant decreases in pH (P < 0·01), bicarbonate (P < 0·001) and base excess (P < 0·001) levels in capillary blood (Fig. 5).

Fig. 5 pH (a) and bicarbonate levels (b) in capillary blood during the four periods at different NaCl intake levels: the two low NaCl intake study periods (□), the average normal NaCl intake period (![]() ) and the high NaCl intake study period (
) and the high NaCl intake study period (![]() ). Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the preceding period: * P < 0·05, *** P < 0·001.
). Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the preceding period: * P < 0·05, *** P < 0·001.
In the dermis, dialysate Na concentration was 109·5 (sem 6·7) mmol/l after low NaCl intake and 109·0 (sem 3·9) mmol/l after high NaCl intake (NS, low v. high). In adipose tissue, dialysate Na concentration was 115·7 (sem 7·5) mmol/l after low NaCl intake and 108·7 (sem 9·9) mmol/l after high NaCl intake (NS, low v. high). Total dialysate volume did not differ from total perfusate volume.
Urine flow and metabolic fluid balance changed significantly with the NaCl intake level (Table 1). Urine flow decreased significantly (P = 0·0002) when NaCl intake increased from low to average normal intake levels, suggesting that fluid was retained along with Na. However, when NaCl intake increased from average normal to high intake levels, urine flow increased back to the level of the first low NaCl intake period and stayed at that level.
The patterns of change in urinary chloride excretion and urine osmolality were similar to that of urinary Na excretion (Table 1). Mean daily metabolic chloride balance, however, was not affected by different NaCl intake levels (Table 1; P = 0·500).
Serum Na, K and chloride concentrations as well as serum osmolality changes are shown in Table 2. Serum Na (P = 0·043) and chloride (P = 0·0001) increased with changes in NaCl intake (Table 2). Post hoc testing revealed a significant difference in serum Na concentrations only between the first low NaCl intake and the high NaCl intake periods (P = 0·027).
Plasma ANP concentration increased significantly with changing NaCl intake (ANP concentration, P < 0·0001; total ANP, P < 0·001). However, plasma ANP concentration decreased when decreasing NaCl intake from high to low level (ANP concentration, P = 0·0003; total ANP, P = 0·0002) (Table 2).
Mean values of mean morning blood pressure measured were 101·2 (sem 1·8), 98·1 (sem 2·3), 99·4 (sem 2·5) and 100·9 (sem 1·8) mmHg from the first to the fourth study period, respectively, and did not show any significant differences.
Discussion
In the present study we hypothesised that the onset level of NaCl intake may influence the physiological regulation of Na balance. Indeed, starting from a low NaCl intake level led, as described in the literature(Reference Sagnella, Markandu and Buckley1, Reference Singer, Markandu and Buckley2), to Na retention accompanied by fluid retention. In contrast, when NaCl intake was increased from the average normal to a much higher level, Na was also retained, but without fluid retention. The negative cumulative K balance could compensate only 22 % of the retained Na, meaning that 78 % of the retained Na was stored in an osmotically inactive form. Concomitantly, Na retention without K exchange or fluid retention led to increased mRNA expression of GAG-synthesising enzymes and to a low-grade metabolic acidosis.
Second, we examined whether water-free stored Na is released when the Na load is decreased again. The results of the present study showed that this was not the case. When the NaCl intake was reduced again, a certain amount of Na was excreted, but this did not at all compensate for the osmotically inactive Na storage. Therefore, Na seems to have been bound in an osmotically inactive form somewhere in the body.
The high NaCl intake levels we chose for the present study are both high (average normal intake, about 200 mol NaCl/d (2·8 mmol/kg BM per d); high, about 550 mmol/d (7·7 mmol/kg BM per d)) compared with the dietary reference intakes(19). Currently, the dietary reference intakes for Na are 65 mmol/d(19). The average normal NaCl intake level in the present study lies fairly in the range of the average Na intake of an age-matched German male population in the past years (109 mmol/d(20) to 249 mmol/d(21)). The high NaCl intake level reflects on the one hand the actual intake of some human populations on Earth, for example, populations in Northern Japan(19) and on the other hand also the intake of individuals all over the world who have particular eating habits. We chose these dietary NaCl intakes in order to examine differences in physiological effects when intake started from different onset levels.
Based on our data, an alternative model regarding the regulation of Na balance is proposed in Fig. 6. Fig. 6 (a) depicts the traditional view of volume regulation when starting from a previous low NaCl intake. Increasing Na intake from low to average normal intake levels leads to positive cumulative Na balance accompanied by PV rise and an increase in ECV, indicating an isotonic increase in the intravascular and interstitial volumes. This could be described as osmotically active Na retention. In this case, the renin–angiotensin system is very much suppressed.
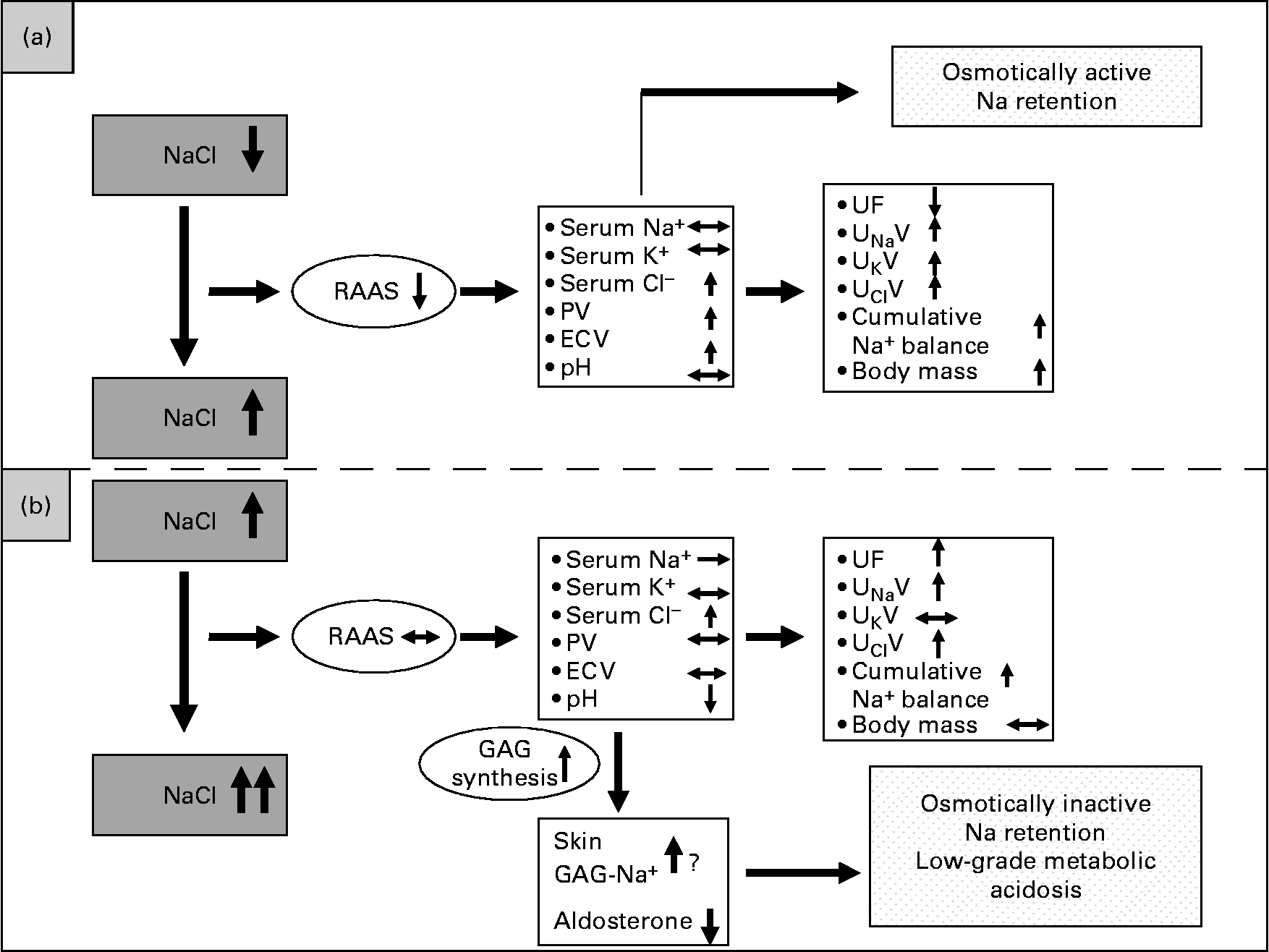
Fig. 6 Model of Na regulation dependent on different levels of NaCl intake. (a) Traditional view of Na and fluid retention when increasing NaCl intake from a low to a high level leading to osmotically active Na retention. (b) Alternative view of Na and fluid retention when increasing NaCl intake from an average-normal to a much higher level. The renin–angiotensin–aldosterone system (RAAS) can not be further suppressed and urinary Na excretion can not further be increased. In this case, a ‘scavenger’ mechanism has to be initiated in order keep serum Na concentration in its range. PV, plasma volume; ECV, extracellular volume; UF, urine flow; UNaV, urinary Na excretion; UKV, urinary K excretion; UClV, urinary Cl excretion; GAG, glycosaminoglycan.
Fig. 6 (b) depicts an alternative view of volume regulation when starting from an already high NaCl intake level like the average–normal NaCl intake. Then, after a further increase in NaCl intake, the cumulative metabolic Na balance is also positive, but without a further rise in plasma or ECV. This is in line with our previous findings(Reference Heer, Baisch and Kropp3) that increasing NaCl intake from an already high NaCl intake level led to Na retention without fluid retention. One could argue that there is a kinetic effect, meaning that the positive Na balances get higher over time. However, in the present study the positive Na balances were varying and could even get slightly negative on some days of the high NaCl intake phase. We therefore do not see a kinetic effect of osmotically inactive Na storage. We rather postulate that in this case a supplementary mechanism exists that prevents an increase in serum Na concentration and keeps serum osmolality within a safe range. This might be achieved by the osmotically inactive Na storage induced by increased synthesis of GAG, which then may bind Na, as already shown in animal studies(Reference Titze, Shakibaei and Schafflhuber8, Reference Titze, Lang and Ilies22) and corroborated by the data presented here. If Na were bound by GAG, interstitial Na concentration should be unchanged, a presumption confirmed by the present results. If GAG functioned as an ion exchanger, H might be released and Na bound. On the other hand, it was shown by Kessler et al. (Reference Kessler, Leibhammer and Laue23) and Siffert & Dusing(Reference Siffert and Dusing24) that acute saline infusion causes activation of the Na+/H+ exchanger and affects acid–base balance. Both may increase H concentration, leading to a pH reduction, stressing the buffering system and leading to a low-grade metabolic acidosis, as also corroborated by our data.
Recently, Seeliger et al. (Reference Seeliger, Ladwig and Reinhardt25) and Nguyen & Kurtz(Reference Nguyen and Kurtz26) have questioned if these positive Na balances lead to osmotically inactive Na retention. Seeliger et al. (Reference Seeliger, Ladwig and Reinhardt25) postulated that Na is stored in the intracellular space and exchanged with K. If the latter were true, the almost identical amount of positive cumulative Na balance in the high Na intake period would have had to be excreted as K. However, in the present study, the cumulative K balance, although negative in the high NaCl intake period, could account for only 22 % of the stored Na. Therefore, we conclude that 78 % of the stored Na was retained in an osmotically inactive form.
The mRNA expression patterns in our human experiment partially corroborate the findings from experiments in rats with extreme Na intake(Reference Schafflhuber, Volpi and Dahlmann27), suggesting that the negative GAG charge density may also play a role in osmotically inactive Na retention in humans and might potentially function as an ion-exchanger as proposed by Farber et al. (Reference Farber, Schubert and Schuster28). However, as we cannot present data on the skin GAG content and the negative skin GAG charge density from this experiment, our current data only provide indirect evidence that GAG in the extracellular matrix may be involved in Na storage in humans. Nevertheless, these results warrant further studies to examine the underlying mechanism and the role of the extracellular matrix charge density in retention of osmotically inactive Na.
In summary, the traditional view of Na regulation is applicable when dietary NaCl intake is increased from a low to a high level. When NaCl intake is increased from average normal intake levels, which are already high, to even higher levels, it seems that the underlying hormonal regulation cannot sufficiently increase Na excretion. Other regulating mechanisms must be activated, i.e. GAG synthesis, to store Na in order to maintain a constant serum Na level. In view of the need to conserve Na, the ability to store it seems to be a very valuable mechanism in a life-threatening situation, that is, when no dietary Na is available. In light of the over-consumption of Na in the Western world today, the questions remain, how much Na can be stored, and do genetic polymorphisms exist that may lead to the inability to store osmotically inactive Na, a condition that might underlie the pathophysiology of particular diseases.
Acknowledgements
Parts of the results were presented at the Experimental Biology meeting 2005, San Diego, CA, USA(Reference Heer, Frings and Baecker29).
We thank the test subjects for their excellent compliance during all the study periods. We are also grateful to Paul Kuklinski and his team (DLR-Aeromedical Center, Cologne, Germany) for the medical screening of the test subjects. Sincere thanks are also given to Heidi Bonnist and Henning Soll (DLR-Psychological Division, Hamburg, Germany) for the thorough psychological screening of the test subjects. We are also very grateful to Martin Vejvoda (DLR-Flight physiology division) and Guido Petrat (DLR-Biomedical Science Support Center) for their support in managing the research ward as well as the physiology laboratory. We greatly appreciate the efforts of Gabriele Kraus and Jette Hjorth-Mueller for their biological samples analysis. We also thank Jane Krauhs for technical review of the manuscript.
The study was supported by the Directorate of Space Programs at DLR, as well as a postgraduate grant from the Wernher von Braun Foundation, Germany. No author has a conflict of interest. M. H. was responsible for the study design, evaluation of data and manuscript preparation. P. F.-M. was responsible for conducting the main study and supported data evaluation. J. T. conducted all skin biopsies and GAG analyses and analysed those data. M. B. performed the microdialysis measurements and respective data analyses and took part in manuscript writing. S. F. conducted all the measurements and analyses on evaporative fluid as well as skin Na and K loss. N. B. supervised the hormonal analyses and took part in data evaluation. L. B. did all fluid compartment measurements, and supported the manuscript preparation and statistical analyses.











