Diabetes mellitus (DM) is one of the most serious chronic diseases the incidence rate of which is increasing sharply globally. The number of people with DM has more than doubled globally over the past three decades( Reference Danaei, Finucane and Lu 1 ). There had been an estimated 285 million diabetics in 2010, 90 % of whom had type 2 diabetes mellitus (T2DM). Moreover, this number is projected to increase to 439 million by 2030, representing 7·7 % of the total adult population of the world aged 20–79 years( Reference Shaw, Sicree and Zimmet 2 ). DM (especially type 2) used to be the most common disease in developed countries. However, the greatest increase in prevalence is expected to occur in Asia and Africa. Perhaps, a ‘Western-style’ diet is the most important factor for the increase in the incidence of DM in developing countries( Reference Rathmann and Giani 3 ). In addition, there is no obvious cure for DM, except in very specific situations. The main management mechanism is keeping blood glucose concentrations as close to normal as possible, without causing hypoglycaemia. It has been demonstrated that the complications of diabetes are far less common and less severe in people who have well-managed blood sugar concentrations( 4 ). Glycaemic control can usually be accomplished with diet, exercise and use of appropriate medications( Reference Nathan, Cleary and Backlund 5 ). A number of efficacy trials have provided strong evidence for the prevention of T2DM among individuals with impaired glucose tolerance through lifestyle change programmes( Reference Li, Zhang and Wang 6 , Reference Ramachandran, Snehalatha and Mary 7 ). Therefore, exploring healthy diet and lifestyle to control blood glucose concentrations is critical for the prevention of T2DM.
Oats, which are considered to be unique among the cereals, belong to the Poaceae family and are known as ‘Jai’ or ‘Javi’ in the Indian subcontinent( Reference Sadiq Butt, Tahir-Nadeem and Khan 8 ). In the mid-1980s, oats were recognised as a healthy food helping prevent heart disease and then became more popular in human nutrition( Reference Haboubi, Taylor and Jones 9 ). The common oat (Avena sativa) is the most important crop among the cultivated oats. Oats are suitable for human consumption as oatmeal, rolled oats and other oat-enriched products. Recent studies in food and nutrition have revealed the importance of the various components of oats, such as dietary fibre, especially β-glucan, minerals and other nutrients( Reference Sadiq Butt, Tahir-Nadeem and Khan 8 ). Oats and oat-enriched products have been proven to control blood glucose concentrations and to be helpful in the treatment of diabetes. However, the results of clinical trials in human subjects that have investigated the effect of oat intake on glycaemic control and insulin sensitivity are inconsistent. Several studies have suggested that oats and oat-enriched diets can significantly decrease insulin responses and fasting and postprandial hyperglycaemia in overweight and type 2 diabetic subjects( Reference Battilana, Ornstein and Minehira 10 – Reference Beck, Tapsell and Batterham 15 ), which is mainly attributed to the markedly functional properties and enormous importance of β-glucan in human nutrition. β-Glucan is a kind of high-molecular weight polysaccharide exhibiting high viscosity at relatively low concentrations, which can reduce mixing of the food with digestive enzymes and delay gastric emptying. Increased viscosity also retards the absorption of glucose( Reference Braaten, Wood and Scott 16 , Reference Panahi, Ezatagha and Temelli 17 ). However, some studies have found that there are no diet-related effects on glycaemic control or insulinaemic responses to oat-enriched products( Reference Liljeberg, Granfeldt and Björck 18 – Reference Biorklund, Holm and Onning 21 ). Therefore, we conducted a meta-analysis of randomised controlled trials (RCT) to quantitatively assess whether oat intake has a beneficial effect on glycaemic control and insulin sensitivity and to make some suggestions regarding diabetes diet based on what we find.
Methods
Search strategy
A search for all the published RCT on the effect of oat intake on glycaemic control and insulin sensitivity was carried out independently by two authors (L. B. and X. C.). This systematic search was conducted in three databases – PubMed, ScienceDirect Online and The Cochrane Library – up to October 2013. The search strategy was implemented using the following key words: (‘oats’ OR ‘Avena sativa’ OR ‘oatmeal’ OR ‘oat bran’ OR ‘oat power’) AND (‘blood glucose’ OR ‘HbAlc’ OR ‘glycated hemoglobin’ OR ‘fasting plasma glucose’ OR ‘glycolated hemoglobin’ OR ‘FBG’ OR ‘insulin’). The search was restricted to studies in human subjects that were published in English.
Inclusion and exclusion criteria
The inclusion criteria of the meta-analysis were as follows: RCT conducted in human subjects with a parallel or crossover design; use of any type of oat-enriched product as the intervention product in the studies; use of data with available means and standard deviations, standard errors or 95 % CI as the endpoint values for glucose or insulin responses; inclusion of a control group; assessment of differences between the control and treatment groups in the studies based on the effect of oat-enriched product intake. Non-randomised studies and studies that were carried out in vitro were excluded.
Methodological assessment
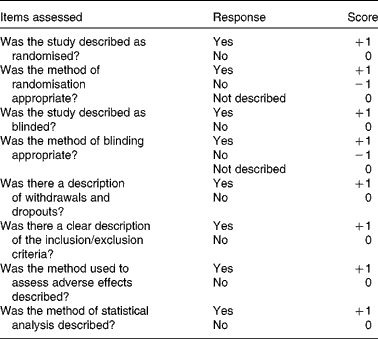
Methodological assessment was conducted using the modified Jadad scale( Reference Oremus, Wolfson and Perrault 22 ). It is an eight-item scale designed to assess randomisation, blinding, withdrawals and dropouts, inclusion and exclusion criteria, adverse effects and statistical analysis results (Table 1). The score for each article could range from 0 (lowest quality) to 8 (highest quality). The scores of 4 to 8 denote good to excellent quality and 0 to 3 poor to low quality. Critical appraisal was done by one investigator (L. B.) and was verified by another (M. X.).
Table 1 Modified Jadad scale with eight items
Study identification
The titles of all the articles that were retrieved were screened independently by two investigators (L. B. and X. C.). The abstract of any study that was potentially relevant to the meta-analysis was reviewed; full text was referred to when the information available from the abstract was inadequate. Discrepancies were resolved by consensus after discussion with a third investigator (Y. L.).
Data extraction
Detailed information from each article included in the meta-analysis was extracted independently by two investigators (X. C. and M. X.). Disagreements were resolved through discussion with a third investigator (Y. L.). Effective data from all trials including data available for the meta-analysis were collected. Details regarding the first author, publication year, study region, sample size, study design, type of intervention, dose and study duration and information on participants including age, sex, BMI and healthy status were extracted. Primary outcomes consisted of net changes in fasting glucose and insulin concentrations. Secondary outcomes included mean changes in HbA1c concentrations, homeostatic model assessment-insulin resistance (HOMA-IR) values and glucose AUC (GAUC; 0–120 min) values. All values were converted to mmol/l for glucose concentrations, pmol/l for insulin concentrations and min × mmol/l for GAUC values.
Statistical analysis
Net changes in glucose and insulin concentrations were calculated as the weighted mean differences and 95 % CI. Cochran's Q tests were used to assess statistical heterogeneity among the studies (P< 0·1)( Reference Whitehead 23 ). The I 2 statistic was also calculated and I 2>50 % was considered to indicate significant heterogeneity across the studies( Reference Higgins, Thompson and Deeks 24 ). If the overall pooled studies exhibited significant heterogeneity, a random-effects model was used. Otherwise, a fixed-effects model was used. Publication bias was examined using funnel plots and Egger's regression test( Reference Egger, Davey and Schneider 25 ) (significant at P< 0·1). Sensitivity analyses were carried out using subgroup analyses, which were used to examine the possible source of heterogeneity in these studies, including regions, healthy status, study design, β-glucan content in oats, intervention period and Jadad score. In addition, sensitivity analyses were also carried out using the Handbook for Systematic Review of Interventions of Cochrane software (version 5.0.2, The Cochrane Collaboration). Furthermore, dose–effect relationship was investigated using meta-regression analyses. The meta-analysis was carried out using Stata (version 12, StataCorp LP). P <0·05 was considered statistically significant.
Results
Literature search
In Fig. 1, a flow diagram of the strategy implemented for the selection of studies in the present meta-analysis is shown. A total of 569 articles were initially identified after the removal of duplicates, and after a careful review of the titles and abstracts, 524 articles were excluded as they were not relevant to the present meta-analysis. After full-text examination, fifteen articles that were eligible for inclusion in the meta-analysis were finally identified( Reference Tapola, Karvonen and Niskanen 12 , Reference Beck, Tapsell and Batterham 15 , Reference Panahi, Ezatagha and Temelli 17 , Reference McGeoch, Johnstone and Lobley 19 – Reference Biorklund, Holm and Onning 21 , Reference Saltzman, Das and Lichtenstein 26 – Reference Juvonen, Salmenkallio-Marttila and Lyly 34 ).
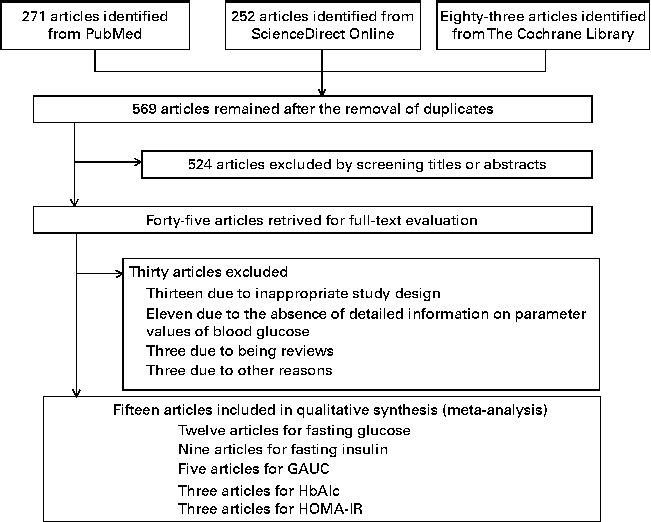
Fig. 1 Flow diagram for the selection of studies on the effect of oat intake on glycaemic control and insulin sensitivity in the present meta-analysis. GAUC, glucose AUC; HbA1c, glycated Hb; HOMA-IR, homeostatic model assessment-insulin resistance.
Study characteristics
The characteristics of the fifteen RCT included in the present meta-analysis are summarised in Table 2. The total number of participants in all the studies was 673. Among the articles identified, twelve were selected for reporting the effect of oat intake on fasting glucose concentrations, nine on fasting insulin concentrations, five on GAUC values, three on HbA1c concentrations, and three on HOMA-IR values. The β-glucan content in oats in the trials ranged from 3 to 10 mg/d (median 5 mg/d). The intervention period in studies reporting the effect of oat intake on glucose concentrations varied from 1 week to 16 weeks (median 8 weeks). Of the fifteen studies, five enrolled healthy individuals, while the remaining ten enrolled individuals with metabolic diseases (patients with T2DM in four trials, overweight individuals in three trials, individuals with high cholesterol concentrations in two trials and individuals with elevated blood pressure in one trial). A parallel design was used in ten studies and a cross-over design in five studies.
Table 2 Characteristics of the fifteen randomised controlled trials included in the present meta-analysis (Number of subjects, mean values and standard deviations)

M, male; F, female; NR, not reported.
Quality assessment was conducted according to the modified Jadad scale( Reference Oremus, Wolfson and Perrault 22 ). Of the fifteen studies, eight( Reference Beck, Tapsell and Batterham 15 , Reference Panahi, Ezatagha and Temelli 17 , Reference Cugnet-Anceau, Nazare and Biorklund 20 , Reference Biorklund, Holm and Onning 21 , Reference Davy, Davy and Ho 27 , Reference Maki, Galant and Samuel 29 , Reference Reyna-Villasmil, Bermudez-Pirela and Mengual-Moreno 31 – Reference Granfeldt, Nyberg and Bjorck 32 ) were classified as high-quality studies (Jadad score ≥ 4) and the remaining seven as low-quality studies (Jadad score < 4).
Effect of oat intake on fasting glucose concentrations
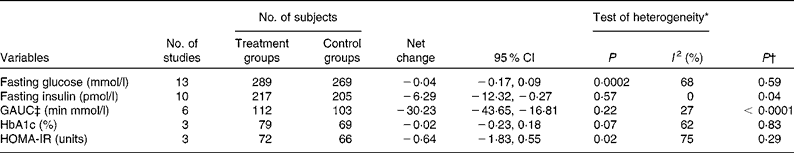
Data on the effect of oat intake on fasting glucose concentrations were reported by thirteen studies in twelve articles. Overall, no significant difference was observed in subjects who consumed oat-enriched products ( − 0·04 (95 % CI − 0·17, 0·09) mmol/l; P= 0·59; Table 3 and Fig. 2). High heterogeneity was observed among the studies (I Reference Shaw, Sicree and Zimmet 2 = 68 %). No significant publication bias was indicated by the funnel plot (Fig. 3) and Egger's test (P= 0·49). No dose–effect relationship between oat intake and fasting glucose concentrations was indicated by the meta-regression analyses (P= 0·91).
Table 3 Pooled effects of oat intake on glucose control and insulin sensitivity (Number of studies, number of subjects, net changes and 95 % confidence intervals)
GAUC, Glucose AUC; HbA1c, glycated Hb; HOMA-IR, homeostatic model assessment-insulin resistance.
* P and I 2 were used for heterogeneity assessment by Cochran's Q test, and P< 0·1 or I 2>50 % was considered to indicate significant heterogeneity across the studies.
† P for meta-analysis: P< 0·05 was considered statistically significant.
‡ 0–120 min.

Fig. 2 Results of the meta-analysis carried out to investigate the effect of oat intake on fasting glucose concentrations. The results were obtained using a random-effects model. There were two series in the study carried out by Granfeldt et al. ( Reference Granfeldt, Nyberg and Bjorck 32 ). WMD, weighted mean difference. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
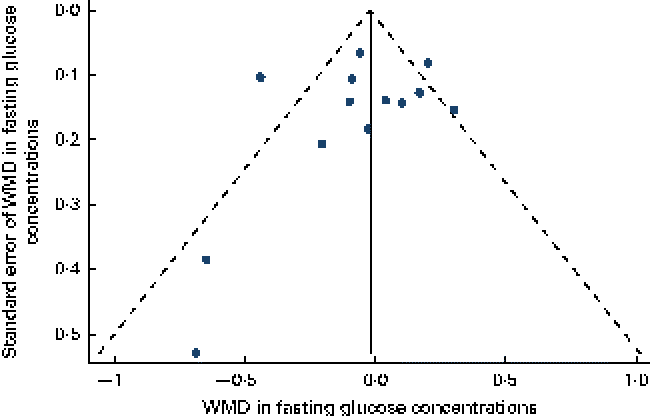
Fig. 3 Funnel plot with pseudo-95 % CI for the effect of oat intake on fasting glucose concentrations. WMD, weighted mean difference. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
Effect of oat intake on fasting insulin concentrations
Data on the effect of oat intake on fasting insulin concentrations were reported by ten studies in nine articles, and a significant reduction in fasting insulin concentrations was observed in subjects who consumed oats ( − 6·29 (95 % CI − 12·32, − 0·27) pmol/l; P= 0·04; Table 3 and Fig. 4) than in the control subjects. No significant heterogeneity was observed among the studies (I 2= 0). No publication bias was indicated by the funnel plot (Fig. 5) and Egger's test (P= 0·39). No dose–effect relationship between oat intake and fasting insulin concentrations (P= 0·58) was indicated by the meta-regression analyses.

Fig. 4 Results of the meta-analysis carried out to investigate the effect of oat intake on fasting insulin concentrations. The results were obtained using a fixed-effects model. There were two series in the study carried out by Granfeldt et al. ( Reference Granfeldt, Nyberg and Bjorck 32 ). WMD, weighted mean difference. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
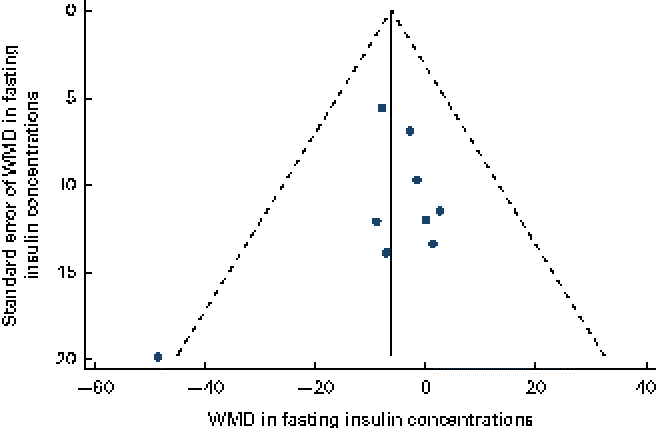
Fig. 5 Funnel plot with pseudo-95 % CI for the effect of oat intake on fasting insulin concentrations. WMD, weighted mean difference. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
Effect of oat intake on glucose AUC values, glycated Hb concentrations and homeostatic model assessment-insulin resistance values
Data on the effect of oat intake on GAUC values, HbA1c concentrations and HOMA-IR values were reported in five, three and three articles, respectively. As shown in Table 3, oat intake significantly decreased GAUC values ( − 30·23 (95 % CI − 43·65, − 16·81) min × mmol/l; P< 0·0001), but did not significantly affect HbA1c concentrations or HOMA-IR values (P= 0·83 and 0·29, respectively). Low heterogeneity was observed among the studies with regard to the effect of oat intake on GAUC values (I 2= 27 %), whereas high heterogeneity was observed with regard to the effect of oat intake on HbA1c concentrations and HOMA-IR values (I 2= 62 and 75 %, respectively). Funnel plots and Egger's test did not indicate any significant publication bias with regard to GAUC values, HbA1c concentrations and HOMA-IR values (Egger's test: P= 0·65, 0·15 and 0·15, respectively). No dose–effect relationship was found between oat intake and GAUC values (P= 0·06). A dose–effect analysis was not carried out for HbA1c concentrations or HOMA-IR values, as data on these two variables were reported by only a limited number of studies.
Sensitivity and subgroup analyses
As shown in Table 4, there was no substantial change in the effect of oat intake on fasting glucose and insulin concentrations by subgroup analyses. The results did not change on the exclusion of any individual study during the sensitivity analyses. The subgroup analyses of fasting glucose concentrations indicated that the overall outcome of fasting glucose responses was not affected by subgroups including regions, healthy status, study design, β-glucan content in oats, intervention period or Jadad score and no significant difference was found. Significant reductions in fasting insulin concentrations were observed after oat intake in the subgroups of both European countries and the USA. Significant reductions in fasting insulin concentrations were also observed in the subgroups of subjects who were healthy or had metabolic disease risk. Among the study design subgroups, subgroup analyses indicated that oat intake had a lowering effect on fasting insulin concentrations in subgroups with a parallel design, but that it did not affect fasting insulin concentrations in subgroups with a cross-over design. Significant reductions in fasting insulin concentrations were observed in subgroups that consumed oats at both high doses ( ≥ 5 g/d) and low doses ( < 5 g/d). In addition, when stratifying studies according to the intervention period, significant reductions in fasting insulin concentrations were observed in the subgroups of long-term studies ( ≥ 8 weeks), but not in those of short-term studies ( < 8 weeks). Finally, a significant reduction in fasting insulin concentrations was observed only in those studies with high Jadad scores.
Table 4 Results of the subgroup analyses of the effect of oat intake on fasting glucose and insulin concentrations (Number of studies, net changes and 95 % confidence intervals)

Discussion
In the present meta-analysis, we found that oat intake significantly lowered fasting insulin concentrations and GAUC values, but had no significant effect on fasting glucose concentrations, HbA1c concentrations and HOMA-IR values. Our assessment of the effect of oat intake on GAUC values, HbA1c concentrations and HOMA-IR values was limited due to the small number of studies available for analysis.
Consistent with what we found, a study has shown that oat intake significantly decreases insulin responses and postprandial plasma glucose responses in rats( Reference Zhang, Hu and Zhen 35 ). The effects are partly due to the ability of oat β-glucan to increase the viscosity of intestinal content and slow the digestion of carbohydrates and also delay the gastric emptying rate concomitantly, causing satiety for extended periods of time( Reference Hlebowicz 36 ). Moreover, β-glucan could be completely fermented by microflora in the large intestine, leading to the release of SCFA and then lowering of postprandial plasma glucose concentrations( Reference Battilana, Ornstein and Minehira 10 , Reference Behall, Scholfield and Hallfrisch 37 , Reference Drzikova, Dongowski and Gebhardt 38 ). Lv et al. ( Reference Lv, Song and Lee 39 ) demonstrated that dihydroavenanthramide D, which is the active component of oats, protects pancreatic β-cells from cytokine and streptozotocin toxicity and prevents the development of type 1 diabetes in streptozotocin-treated mice. In addition, oats have been demonstrated to possess antioxidant activity( Reference Ren, Yang and Niu 40 ) and enhance insulin signalling by changing the expression of glucose and lipid metabolism genes, which in turn improves insulin sensitivity( Reference Choi, Kim and Jung 41 ). Stratification according to the study design indicated that oat intake had a lowering effect on fasting insulin concentrations in subgroups with a parallel design, but had no effect in subgroups with a cross-over design. This difference could be due to the small number of participants in RCT with a cross-over design, four of which were also of the shortest duration among the included trials. Significant reductions in fasting insulin concentrations were observed in subgroups that consumed oats at both high doses ( ≥ 5 g/d) and low doses ( < 5 g/d), but notably oat intake at low doses was found to result in a slightly greater decrease in fasting insulin concentrations. The reason for this is unknown, as no study had reported an appropriate dose or dose–response relationship between oat intake and fasting insulin concentrations. We speculated that this might be due to an increase in carbohydrate content during high-dose oat intake or to the lowering of fasting insulin concentrations by the active component of oats. However, the results of the meta-regression analyses carried out indicated no significant dose–response relationship between oat intake and fasting insulin concentrations (P= 0·58). So, it is difficult to confirm the optimal dose for a dietary guideline to improve diabetic health and more in-depth studies on mechanisms through which oats lower fasting insulin concentrations are required. When studies were stratified according to the intervention period, significant reductions in fasting insulin concentrations were observed in the subgroups of long-term studies ( ≥ 8 weeks), but not in those of short-term studies ( < 8 weeks). We speculated that it might take a long period for oats to exert their biological effects. Finally, a significant reduction in fasting insulin concentrations was observed only in studies with high Jadad scores.
There has been conflicting evidence for the effect of oat intake on fasting glucose concentrations. A recent study has demonstrated oat products to significantly decrease fasting blood glucose and glycosylated serum protein concentrations in streptozotocin-induced diabetic mice( Reference Shen, Cai and Dong 42 ). Several studies in human subjects have also reported the same results( Reference Battilana, Ornstein and Minehira 10 – Reference Beck, Tapsell and Batterham 15 ). However, some studies have found oat-enriched products to have no effects on fasting blood glucose concentrations( Reference Liljeberg, Granfeldt and Björck 18 – Reference Biorklund, Holm and Onning 21 ). In the present meta-analysis, we found that there was a slight decrease in fasting glucose concentrations in subjects who consumed oat-enriched products, but the difference was not significant. This conclusion was consistent with that of a recently published meta-analysis that included 1250 participants from cohort studies, dose–response studies, and pre- and post-treatment studies and reported study designs (randomised, cross-over, parallel, etc.) with or without health conditions( Reference Tiwari and Cummins 43 ). However, the subgroup analyses carried out in the present meta-analysis indicated oat intake to slightly lower fasting glucose concentrations in long-term and high-quality RCT conducted in human subjects with a parallel design, which might be interesting candidates for future research.
The present meta-analysis has several limitations. The sample sizes of RCT investigating the effect of oat intake on glycaemic control and insulin sensitivity are relatively small and available studies reporting data on the effect of oat intake on HbA1c and HOMA-IR values are rather limited, which could have affected the results of the analysis. In addition, assessment of results for glucose control or insulin sensitivity was not the primary goal of most of the studies included in the present meta-analysis. Potential language bias could have occurred as articles that were not published in English were excluded.
Oats can be classified as hulled and naked oats. Naked oats, which are mainly produced in China, have a non-lignified husk, which becomes detached readily during harvesting. Naked oats have been shown to contain higher proportions of protein and oil and a lower content of lignin compared with hulled oats and there is a substantial variation in the contents of phytochemicals( Reference Shewry, Piironen and Lampi 44 ). No significant differences in β-glucan content between naked and hulled oats have been reported( Reference Shewry, Piironen and Lampi 44 , Reference Andersson, Lampi and Nyström 45 ). Naked oats have also been demonstrated to lead to metabolic control and CVD risk prevention in T2DM patients with the metabolic syndrome( Reference Ma, Gu and Zhang 46 ). However, the existing studies have focused on only hulled oats so far. Studies on naked oats are still limited and more studies are required to assess any beneficial effects.
In conclusion, the results of the meta-analysis indicated oat intake to significantly lower fasting insulin concentrations and GAUC values, but not to have any significant effect on fasting glucose concentrations, HbA1c concentrations and HOMA-IR values. The subgroup analyses indicated that additional long-term and high-quality RCT conducted in human subjects with a parallel design are required to further investigate the effect of oat intake on fasting glucose concentrations, which may provide evidence for the therapeutic potential of oats in diabetic patients or for preventing glucose dysregulation in those at a risk of DM.
The present work was supported by research grants from the National Natural Science Foundation of China (no. 81372995) and the Beijing Key Laboratory of Toxicological Research and Risk Assessment for Food Safety. The authors cordially thank Yuan Zhang, the Department of Clinical Epidemiology and Biostatistics, McMaster University, for helping analyse data.
Acknowledgements
The authors' contributions are as follows: L. B. and Y. L. conceived the research; L. B. and X. C. searched the databases and screened all the articles obtained according to the inclusion criteria and exclusion criteria; L. B. and M. X. conducted the critical appraisal; X. C. and M. X. extracted detailed information; L. B. analysed the data and wrote the draft of the article; X. C. contributed to the writing and reviewing of the article; L. B. was responsible for the critical revision of the manuscript.
None of the authors has any conflicts of interest to declare.











