INTRODUCTION
Community-acquired pneumonia (CAP) is a leading cause of morbidity and mortality in persons aged ⩾65 years [Reference Niederman and Ahmed1], with incidence and mortality rates increasing with age [Reference Loeb2]. An annual incidence of 10·4/1000 has been reported from Spain for the elderly [Reference Vila-Córcoles3], while in Finland, an annual incidence of 15/1000 in the 60–74 years age group has been reported, increasing to 34/1000 for those aged ⩾75 years [Reference Jokinen4]. Hospitalized cases comprise the greatest burden from pneumonia in terms of severity and health-care costs. This burden is likely to increase in Australia as the population ages.
Periodic review of disease epidemiology can direct management and preventive strategies. Data are currently lacking for many aspects of basic epidemiology for CAP in elderly Australians given data availability from only four small studies (<200 subjects) [Reference Wilson and Ferguson5–Reference Lim8], with none focusing on the elderly or examining risk factors.
As part of a case-cohort study examining effectiveness of influenza vaccine and 23-valent pneumococcal vaccine (23vPPV), we undertook a large study of CAP in elderly Australians. This paper describe for elderly in-patients: general epidemiology including seasonality, burden of disease, mortality, risk factors for CAP vs. non-CAP, and mortality for those with CAP. Secondary outcomes include description of common symptoms and signs associated with CAP, investigation types performed and use of antimicrobial agents.
METHODS
Study design
The case-cohort variant of the case-control design includes a comparison group (the cohort) randomly selected from the entire population from which cases are drawn. Cohort selection is therefore representative of the source population, and independent of case characteristics. Unlike a case-control study, where controls are all unaffected persons, cases are also eligible for inclusion in the cohort [Reference Wacholder9]. In addition, the risk ratio is estimated directly from the ratio of incidence proportions without requiring information from every cohort member.
Study subjects
The study sampling frame included everybody aged ⩾65 years admitted to two large teaching hospitals in Melbourne, Victoria from April 2000 to March 2002 inclusive, representing 11% of Victorian hospitalizations and 13% of pneumonia hospitalizations for this age group [10]. Subjects with ICD-10-AM codes J10-J18 (pneumonia including those due to influenza) in any of the 14 diagnostic code positions were considered to have pneumonia. Nosocomial pneumonia was excluded (diagnosis >48 h after admission) [Reference Smith11].
Random oversampling of cohort subjects (1·2 times the number of cases per month) allowed for exclusion of repeat admissions, and those also selected as cases. For subjects with multiple admissions in the same month, one episode was retained at random. For month-to-month repeat admissions, the first selected admission was retained. Subjects were excluded if non-Victorian residents, admitted for short-stay procedures (e.g. dialysis, chemotherapy: ICD-10-AM codes Z49.1, Z49.2 and Z51.1), transferred between hospitals with the same diagnosis, or if hospital records were subsequently unavailable.
For seasonality, burden of disease and mortality, ICD-10-coded pneumonia cases were compared with ICD-10-coded non-pneumonia subjects. Where risk factor analysis for CAP vs. non-CAP was conducted, as per a case-cohort design, the entire cohort was the comparison group. For secondary objectives, data were available only for subjects with clinical notation of pneumonia in hospital records. For inclusion in these analyses, subjects were required to also have ICD codes for pneumonia.
Data collection
Data on demographics, outcomes, comorbidities and other potential predictors of CAP were obtained by record review blinded to case status. Presence of comorbidity was defined as having at least one of: excessive alcohol intake, current tobacco smoking, history of pneumonia, aspiration, other respiratory disease, diabetes, immunosuppression, ischaemic heart disease, liver, renal, cerebrovascular or rheumatological disease.
Radiology reports for chest radiographs (CXRs) undertaken during routine management were reviewed by two research assistants. Pneumonia was defined as ‘lobar’ (opacity, lobar distribution only), ‘bronchopneumonia’ (opacity beyond one lobe plus terms similar to/including ‘patchy’ and/or ‘airspace’), ‘other’ (opacity consistent with pneumonia not previously classified) or ‘not pneumonia’. When more than one CXR was performed during an admission, the first abnormal report was reviewed blinded to other reports. When reports could not be confidently interpreted, two of the investigators (S.S. and D.C.) made the final assessment after first establishing high inter-operator agreement (kappa >95%).
Additional data obtained from hospital and laboratory records for secondary objectives included: (1) investigations performed, (2) use of antimicrobial agents, and (3) symptoms and signs usually associated with pneumonia [Reference Metlay12, Reference Neill13]. The latter included cough, sputum, pleuritic chest pain, fever ⩾37·5°C, shortness of breath, crackles, and aspiration (present/absent/not recorded).
The study was approved by the Human Research Ethics Committee, Melbourne Health (ref. 2000.022).
Statistical methods
Logistic regression was used to estimate risk ratios for factors predicting CAP or associated mortality. To adjust for unequal probabilities of recruitment due to multiple admissions, hospital of admission, and clustering due to the monthly selection process, models included month of discharge, hospital, and a weight equal to the inverse of the total number of admissions for each subject during the study period. Covariates were first assessed individually in logistic statements. Those with P values <0·20 were retained for backward stepwise multivariate analysis with the exception of variables with >15% missing data whose impact was examined by addition only to the final models. Stepwise elimination of variables was determined by removing the variable with the largest P value >0·05. The final model was determined when all remaining variables had a P value<0·05. Stata version 9.1 was used [14].
RESULTS
Study subjects
Of 4772 eligible study subjects, 1952 had first presentations with CAP and 2927 were first- presentation cohort subjects, including 107 also selected as cases (Fig. 1).

Fig. 1. Eligible first presentation cases of community-acquired pneumonia (CAP) and cohort subjects.
Basic epidemiology and disease burden
In total, 1952 cases of CAP represented 4% (1952/49 692) total admissions aged ⩾65 years for the study period in the two hospitals. A mean of 81 CAP admissions occurred per month (median 81, range 50–125), peaking in winter (June–August 588/1952, 30%) and spring (September–November 490/1952, 25%) (Fig. 2).

Fig. 2. Seasonality of first admissions with community-acquired pneumonia (CAP), April 2000 to March 2002.
Table 1 shows characteristics of in-patients with CAP. Their mean/median age was 78 years. Males comprised 58%. At least one comorbid condition (mean 2·5, range 0–8) was present in 98% (1833/1870) of cases.
Table 1. Characteristics of 1952Footnote * persons with first admissions for community-acquired pneumonia (CAP) and 2927 cohort subjects

* Variables with missing data from medical record review (no. of subjects): previous hospitalizations: 4 (0·2%), alcohol: 1027 (53%), smoking: 550 (28%), deaths during hospitalization: 6 (0·3%); all other variables had no missing data.
† A detailed description of variables is available on request.
‡ Percentage, unless labelled as a mean.
The mean length of stay (LOS) for cases was 9 days (median 6 days, range 0–100); significantly longer than for those without CAP (difference 3·8 days, 95% CI 3·2–4·3). For those with CAP, older subjects aged >79 years had a similar mean LOS to younger subjects (65–79 years; 9·0 vs. 8·9 days). Gender was not significantly associated with LOS, however, more than two comorbid conditions was associated with longer hospitalization (difference 1·1 days, 95% CI 0·2–2·1).
In-patients with CAP were admitted to intensive care units (ICUs) more often than those with non-CAP (278/1951, 14·2% vs. 291/2820, 10·3%; 95% CI difference 2·0–8·6). Younger patients (65–79 years) were much more likely to be admitted to an ICU than patients aged ⩾80 years (19·1%, 95% CI 16·7–21·4 vs. 8·0%, 95% CI 6·2–9·8; P<0·001). Neither gender nor presence of more than two comorbidities were associated with ICU admission.
Investigation
A CXR was performed for 1800/1861 (97%) subjects with clinical notation and ICD codes for pneumonia. Of these, 1736 (96%) had radiology reports available, and 1731 (96%) had pneumonia type recorded: bronchopneumonia for 617 (36%), lobar pneumonia for 610 (35%), other pneumonia for seven (<1%) and ‘not pneumonia’ for 497 (29%). Chest computerized tomography was performed in 64/1863 (3%) patients. The most common laboratory investigations performed were on blood (including culture, polymerase chain reaction, immunofluorescence: 1018/1863, 55%), sputum (713/1864, 38%) and urine (including urinary antigen: 310/1864, 17%). Less frequent were nasopharyngeal aspirate (28/1864, 2%), nose/throat swab (14/1864, 1%), and cerebrospinal fluid tap (8/1864, <1%). Excluding CXR, in-patients with CAP had a mean of 1·2 investigation types performed (range 0–6, median 1). Some had no additional investigations (520/1864, 28%), ⩾1 were performed in 1344 (72%) and ⩾2 in 641 (34%). The most commonly identified pathogens were: influenza A/B (41/1854 subjects, 2·2%), followed by Staphylococcus aureus (17/1854, 0·9%) and S. pneumoniae (11/1854, 0·6%).
Antimicrobial management
Of 1823/1854 (98%) subjects with data available on antibiotic use, 1755 (96%) received antibiotics. The most commonly prescribed were roxithromycin (380, 21%), ceftriaxone (363, 20%), amoxycillin/clavulanate (264, 14%), benzylpenicillin G (170, 9%), erythromycin (114, 6%) and amoxycillin (70, 4%).
Clinical features
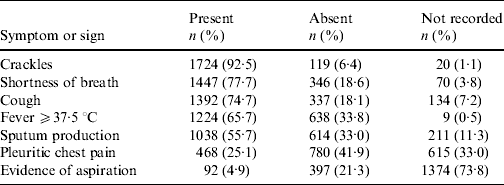
Clinical notation of pneumonia occurred for 1863/1952 (95%) in-patients with CAP. These subjects had a mean/median of 4/7 symptoms and signs of interest. Three or fewer were present in 627/1863 (34%) subjects and only four (0·2%) had all seven. Those most frequently recorded and present were crackles, shortness of breath, cough, fever ⩾37·5°C and sputum production (Table 2).
Table 2. Frequency of symptoms and signs extracted from medical records for 1863 subjects with clinical notation of pneumonia and ICD codes for pneumonia
Risk factors for CAP
The final multivariate model contained 14 independent predictors of CAP (Table 3). Subjects with pneumonia in the previous year, who spoke a first language other than English, or with a history of rheumatological disease were under-represented in the CAP group. Factors with no statistically significant association with CAP were vaccination status with influenza vaccine or 23vPPV, marital status, previous pneumonia (past year or 2–5 years), ischaemic heart disease, liver disease, cerebrovascular disease, prior hospitalizations (past year or 2–5 years), comorbidity, years since receiving 23vPPV, smoking habit or doctor visits (past 2–5 years).
Table 3. Predictors of community-acquired pneumonia for elderly in-patients, final multivariate model

* For example, nursing home, retirement village, hostel, lodge, etc.
† AIDS, cancer (excluding basal cell carcinoma and squamous cell carcinoma), chronic steroid Rx, HIV infection before development of AIDS, organ transplantation, dysgammaglobulinaemia, sickle cell disease, asplenia (functional or anatomical), nephrotic syndrome.
‡ Bronchitis, asthma, emphysema, other chronic obstructive airways disease (not pneumonia).
§ Rheumatoid arthritis, osteoarthritis, systemic lupus erythematosus, multiple connective tissue disease.
∥ Medical record documentation including ‘alcoholism’, ‘alcohol abuse’, ‘EtOH abuse’ or alcohol consumption described as in excess of 4 standard drinks/day for men and 2 standard drinks/day for women.
¶ ‘Excluded’ variable added only to final model following backward stepwise process.
Mortality and discharge location
Of 1946 in-patients with CAP and known discharge location, discharge to one's own home was most common (65%) followed by other hospitals (9%), nursing homes (5%) or hostel accommodation (2%). Prior to admission, 81% of subjects with CAP lived in their own home, 10% in nursing homes and 6% in hostels. Subjects with CAP were more likely to be discharged to a nursing home (difference 2·5%, 95% CI 1·3–3·7) or the same hospital (difference 1·1%, 95% CI 0·4–1·7), but less likely to be discharged to their own home (difference −16·8%, 95% CI −19·4 to −14·3) and more likely to die (difference 11·7%, 95% CI 10·0–13·6) compared to subjects without CAP.
For the 1952 subjects admitted with CAP, 16·3% died in hospital, 17·9% died within 30 days of admission, and 26·0% by the time of interview with next of kin (Table 4). Subjects with CAP had a significantly higher in-hospital mortality rate (difference 11·7%, 95% CI 10·0–13·6) and if they did die in hospital, a more rapid demise (difference −2·8 days, 95% CI −5·5 to −0·1) compared with those with non-CAP.
Table 4. Mortality for study subjects

CAP, Community-acquired pneumonia; ICU, intensive care unit.
* Includes deaths up until the time of interview with next of kin.
† Mortality in hospitalized subjects admitted to an ICU.
For those admitted to ICUs, mortality was significantly higher than the general hospital admission mortality rate (104/566, 18·4% vs. 447/4749, 9·4%), due to the higher mortality in those CAP in-patients admitted to ICUs (Table 4).
Of note, 68/277 (24%) of those with CAP admitted to ICUs were aged >79 years: 21 (31%) died in hospital and 28 (41%) by the time of next-of-kin interview. This was not significantly higher than for patients aged 65–79 years (difference −6·0%, CI −18·4 to 6·4 and −12·1%, 95% CI −25·3 to 1·2 respectively). Older patients with CAP and >2 comorbid conditions were not significantly more likely to be admitted to an ICU than those with fewer comorbid conditions (8·7% vs. 7·8%; difference −0·9%, 95% CI −4·6 to 2·9).
Risk factors for in-hospital mortality associated with CAP
The final model contained nine independent predictors of mortality. Increased risk was associated with ICU admission [risk ratio (RR) 2·68, 95% CI 1·90–3·76], renal disease (RR 1·78, 95% CI 1·24–2·56), living in one's own home (RR 1·68, 95% CI 1·21–2·35), history of immunosuppression (RR 1·58, 95% CI 1·16–2·14), increasing age (P<0·001) (RR 1·59 in the 75–59 years age group, increasing to 3·05 if ⩾85 years), multi-lobe CXR opacity (P<0·001; RR 3·0, 95% CI 2·1–4·3 vs. RR 1·4, 95% CI 0·97–2·1) and smoking (P<0·001). Current smokers were at greater risk than ex-smokers (RR 4·2, 95% CI 2·4–7·6 vs. RR 1·5, 95% CI 1·1–2·2). A reduction in risk of death was associated with influenza vaccination in the year prior to admission (RR 0·55, 95% CI 0·40–0·76) and history of other respiratory disease (RR 0·78, 95% CI 0·56–0·93).
DISCUSSION
Basic epidemiology
This study is the first to specifically examine CAP in elderly Australian in-patients and confirms a considerable disease burden, and seasonal pattern similar to other countries [Reference Marston15]. Hospitalizations with CAP represented 4% of all elderly admissions for the study hospitals, consistent with the other Australian estimate of 2% for all-aged adults [Reference Tsirgiotis and Ruffin16].
In-patients with CAP had a mean LOS of 9 days, consistent with previous studies (range 7–21 days) [Reference Niederman and Ahmed1, Reference Kaplan17, Reference Riquelme18]. This was on average 4 days longer than for those admitted with non-CAP, contrasting with Riquelme et al. who reported no difference [Reference Riquelme18]. Those with >2 comorbidities were hospitalized on average 1 day longer, while gender and older age had no significant impact on LOS. Almost all those with CAP had at least one comorbid condition; most frequently non-pneumonia respiratory disease, ischaemic heart disease, immunosuppression and diabetes; a pattern consistent with previous small Australian studies of all-age adults with CAP [Reference Wilson and Ferguson5, Reference Thompson7] and a large American cohort study [Reference Kaplan17].
The 14% ICU admission rate for CAP in-patients was consistent with previous studies (range 2–22%) [Reference Kaplan17, Reference Conte19]. ICU admission occurred significantly more often than for non-CAP admissions (difference 4%). Mean LOS for those with CAP admitted to ICUs was 15 days, similar to the United States (11 days) [Reference Kaplan17]. Younger elderly patients (<80 years) were much more likely to be admitted to ICUs than older patients (19% vs. 8%), consistent with Kaplan et al. (15% for those <90 years vs. 27%) [Reference Kaplan17].
Risk factors for CAP
This study provides the first Australian data on risk factors for CAP in the elderly. Antecedent non-pneumonia respiratory disease, pneumonia and aspiration were most strongly predictive of CAP vs. non-CAP, in addition to male gender, living in one's own home, history of diabetes, immunosuppression, renal disease, excessive alcohol intake, increasing age >75 years and numbers of doctor visits in the past year. A large cohort study from Finland reported similar findings for increasing age, immunosuppression, other lung disease and alcohol intake [Reference Koivula, Sten and Makela20]. In-patients experiencing pneumonia in the previous year were under-represented in the CAP group, perhaps due to increased contact with medical personnel or awareness of early symptoms and signs of pneumonia resulting in earlier intervention. The association between living in one's own home and admission for pneumonia may be related to isolation or fewer health service prompts, although there are no data in the scientific literature to support or refute this. It is unclear why having a first language other than English or a history of rheumatological disease might be negatively correlated with CAP.
Mortality and discharge location
Although two thirds of those admitted with CAP returned home (consistent with Conte et al. [Reference Conte19]) they were less likely to do so than those admitted with non-CAP, and more likely to be admitted to a nursing home or die. Most deaths occurred within 30 days of admission, as shown in North America [Reference Mortensen21]. Our 11% 30-day mortality rate is similar to previous small Australian studies in non-Aboriginal populations [Reference Padiglione6–Reference Lim8] and international studies [Reference Marston15, Reference Conte19, Reference Kaplan22].
Factors predictive of mortality in elderly persons admitted with CAP in this study concur with earlier studies, including history of renal disease [Reference Kaplan22], increasing age [Reference Wilson and Ferguson5, Reference Lim8, Reference Conte19, Reference Mortensen21], multi-lobe involvement on CXR [Reference Wilson and Ferguson5, Reference Riquelme18], admission to ICU [Reference Kaplan17] and immunosuppression [Reference Kaplan22]. This study also confirms a considerable risk reduction for mortality associated with antecedent influenza vaccination [Reference Rivetti23] and increased risk with current/previous smoking. The association between influenza vaccination and mortality is discussed in greater detail elsewhere [Reference Skull24]. As for admission with CAP, it is unclear why living in one's own home was independently predictive of in-hospital mortality.
Although those aged >80 years were less likely to be admitted to ICUs, the mortality rate for those admitted was not significantly higher than for younger ICU patients with CAP. While suggesting aggressive management in the very old is appropriate, these findings are also consistent with a selection bias favouring admission to ICUs for very old patients who are less severely ill. While no significant differences in comorbidity were found for older patients admitted vs. not admitted to ICUs, we did not assess differences in clinical severity. A future prospective study of clinical features in these two groups could answer this question.
Our data also indicate a risk reduction for death associated with antecedent non-pneumonia respiratory disease. Increased contact with respiratory specialists or greater awareness of clinical features of pneumonia might result in seeking earlier care. Similarly, health provider knowledge of the presence of another respiratory condition might prompt earlier intervention.
Clinical features
The most common clinical features noted for elderly persons admitted with CAP were similar to those described in previous studies [Reference Metlay12, Reference Neill13]. Of note, two thirds of subjects had three or fewer features present, consistent with the less distinct clinical presentation described for older patients [Reference Loeb25].
Investigations and antimicrobial management
Almost all patients with clinical notation and ICD codes for pneumonia had a CXR performed. About 30% had reports inconsistent with pneumonia, suggesting this remains an imperfect approach for retrospectively identifying elderly in-patients with pneumonia.
Although not designed to examine investigations, causative organisms or antimicrobial agent use in any detail, some general observations can be made from this study. Over one quarter of elderly persons admitted with CAP had no laboratory investigations performed, and of those who did, most had only one type (e.g. a blood test), indicating that an empirical approach to management is frequently used and many will not have a causative organism identified. However, as noted above, survival rates appear similar to those in countries such as the United States where investigation to determine a causative organism is more usual. Almost all patients with CAP received antibiotics during admission and these were consistent with current guidelines [26]. There were too few investigations identifying a causative organism to make any meaningful interpretation of those found.
Study limitations
Retrospective hospital record review meant that while some variables were well documented, others captured non-uniform practices of staff. While it is possible that some subjects did not have pneumonia, we have previously shown that ICD-10 codes are a valid tool for retrospective identification of hospitalized cases [Reference Skull27]. This study was conducted in hospitalized elderly persons and may not be generalizable to the wider elderly population, although estimates for vaccination coverage were similar to the general elderly Victorian community [Reference Andrews28].
Conclusions
This study of CAP provides the most comprehensive epidemiological dataset for elderly Australians to date, including the first on risk factors and associated mortality. It confirms that hospitalization with CAP in the elderly is common, frequently fatal and a considerable burden to the community. Investigation remains ad hoc and the approach to management empirical. A prospective study of pneumonia incorporating a systematic approach to investigation in Australia could make a valuable contribution to understanding causative organisms and better direct treatment options. The disparity in ICU admission rates between older and younger elderly patients raises the question of whether their similar ICU survival rates reflect preferential admission of older patients likely to have a good prognosis. Influenza vaccination should continue to be promoted in this population as it is associated with reduced mortality for those hospitalized with CAP. Further evidence is produced to advocate smoking cessation. For health practitioners, characteristics present at admission predict likelihood of CAP and risk of mortality. Greater awareness of risk factors such as aspiration and alcoholism could potentially lead to implementation of preventive strategies and improved outcomes.
ACKNOWLEDGEMENTS
This study was jointly funded by the Victorian Government Department of Human Services and the National Health and Medical Research Council (grant no. 146500). Graham Byrnes is supported by a National Health and Medical Research Council Capacity Building Grant in Population Health (251533). The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or preparation or approval of the report. This study was supported by research assistants responsible for data collection: Anne-Marie Woods, Carol Roberts, Caroline Watts and Joy Turner, with data entry by Thao Nguyen and Jason Zhu.
DECLARATION OF INTEREST
None.