It is now well recognised that the host response to tissue injury, whether it be due to surgery, trauma, burns or infection has a profound effect on organ function and metabolism(Reference Gabay and Kushner1). In the context of nutritional metabolism, this systemic host response has been previously referred to as the acute phase response and in the intensive care setting the systemic inflammatory response syndrome. Irrespective, whatever the tissue injury, the systemic inflammatory response is a stereotypical response that involves the metabolism of all the tissues and organs in the body and primarily reflects an innate immune response(Reference Gabay and Kushner1, Reference Hotamisligil2).
Assessment of the magnitude of the systemic inflammatory response in patients may be considered complex as all tissue and organs will display changes. However, acute phase proteins have been considered ideal since they are produced only by the liver in response to the production of pro-inflammatory cytokines at the site of tissue injury, especially IL-6(Reference Gabay and Kushner1). Of these acute phase proteins, C-reactive protein (CRP) is particularly useful as it is sensitive to tissue injury, well standardised and routinely clinically measured world-wide and reflects the magnitude of surgical injury(Reference Watt, Horgan and McMillan3).
Micronutrients such as trace elements and vitamins are important as enzyme cofactors in the metabolism of all cells. A typical micronutrient screen carried out by routine clinical laboratories in a variety of chronic disease states, such as gastrointestinal benign diseases, critical illness, morbid obesity and cancer, would include the essential trace elements including zinc, selenium and copper, fat soluble vitamins including A, D, E and K and the water soluble vitamins including B1, B2, B6 and vitamin C. Although in the past indirect measures of micronutrient status such as functional tests of enzyme activity have been used, most measurements of micronutrients are now carried out by direct measurement of the plasma concentrations of the micronutrient. In healthy subjects such measurements have been shown to be useful as plasma concentrations fall on deficiency and rise rapidly on supplementation and the magnitude of the change is associated with the degree of deficiency. However, the willingness to supplement patients with acute and chronic injury and where there is an apparent deficiency, has not, in the main, been subject to critical clinical research; in particular, the assessment of trace elements and vitamins in patients with acute and chronic activation of the systemic inflammatory response.
Almost two decades ago Galloway and co-workers(Reference Galloway, McMillan and Sattar4), in a systematic review, reported the profound effect of the systemic inflammatory response, as evidenced by CRP, on a variety of plasma micronutrient concentrations. For example, in major inflammation (CRP>80 mg/l) there was evidence that plasma zinc, selenium, vitamins A and B6 fell by approximately 40 % and plasma vitamin C and carotenoids lutein, lycopene, α- and β-carotene fell by approximately 80 %, independent of dietary supply. Other micronutrients such as erythrocyte measures of B1 and B2 were not perturbed by the systemic inflammatory response. However, there was insufficient data to quantify the magnitude of effect for the majority of micronutrients examined. Therefore, it was concluded that, in patients with acute or chronic diseases, plasma measurements of micronutrient concentrations should be carried out in conjunction with a measure of the inflammatory response.
The present systematic review examined the evidence, accumulated over the past two decades, of the impact of the systemic inflammatory response on plasma micronutrient status in acute and chronic tissue injury.
Systematic review
The present systematic review of the published literature was undertaken according to a pre-defined protocol described in the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols statement (Fig. 1). The primary outcome of interest of this systematic review was the relationship between plasma micronutrient concentrations and the systemic inflammatory response, as evidenced by CRP concentrations, in patients with acute and chronic tissue injury. Mild, moderate and major systemic inflammation was defined as CRP<10, 11–80 and >80 mg/l, respectively.
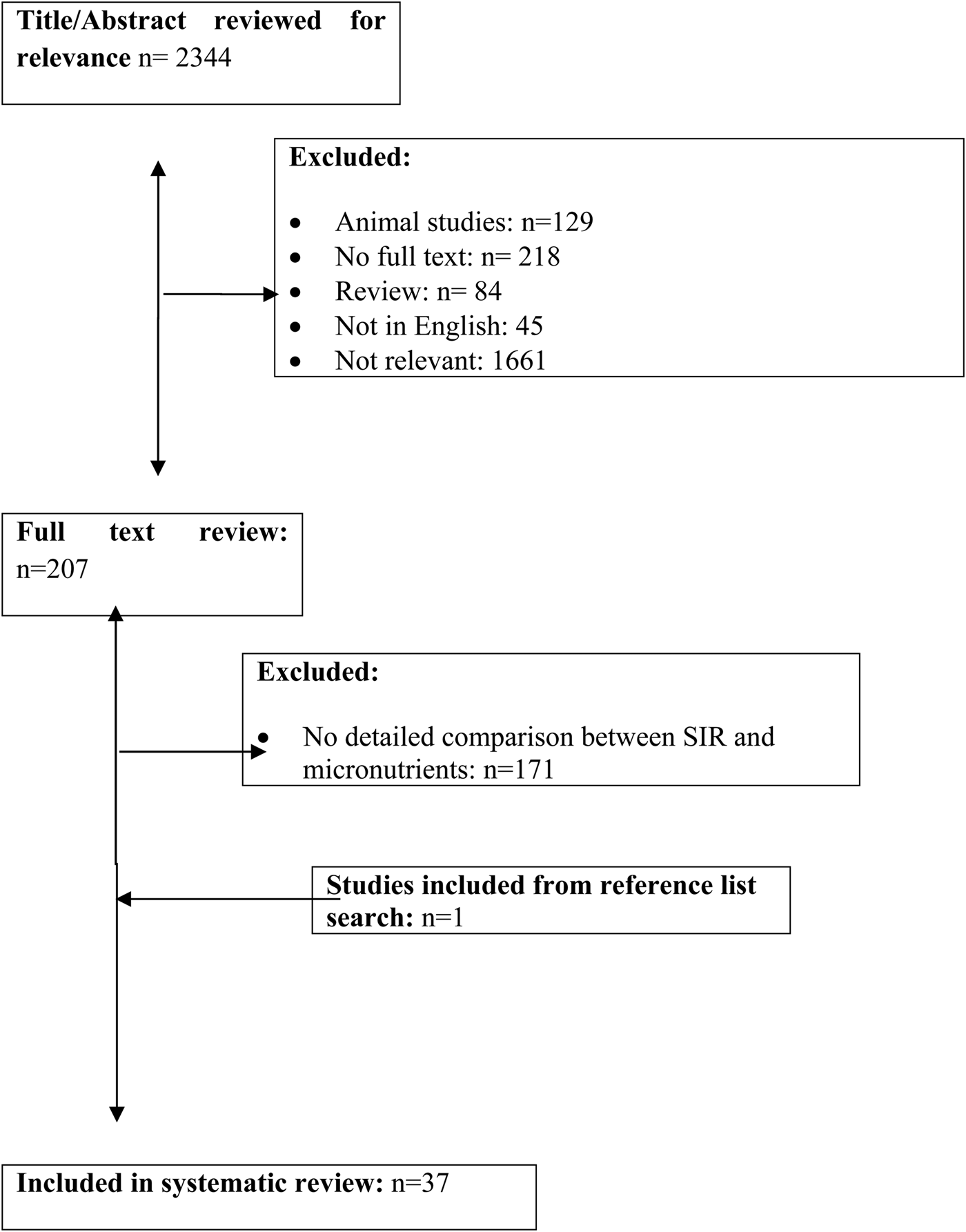
Fig. 1. A Preferred Reporting Items for Systematic review and Meta-Analysis Protocol flowchart demonstrating study selection process.
Studies were identified via a literature search of the electronic databases the US National Library of Medicine, the Excerpta Medica database and the Cochrane Database of Systematic Reviews between 1984 and 2018 using the following keywords: human, micronutrients, trace elements, vitamins, systemic inflammation, CRP, acute (surgical) and chronic tissue injury (last search update on 1 May 2018).
To be eligible for inclusion, studies had to meet the following criteria: (a) patients with micronutrient measurements; (b) patients with a measure of the systemic inflammatory response, specifically CRP; (c) patients with acute or chronic tissue injury; (d) published in English. Exclusion criteria included: (a) studies with no measure of micronutrients and CRP or data that could not be extracted from the manuscript; (b) available in abstract form only; (c) where there was significant haemodilution during surgery e.g. cardiopulmonary bypass.
On completion of the online search, the title and abstract of each identified study was examined for relevance. Where there were multiple publications from the same cohort the most recent paper was included. Full texts were obtained for all studies deemed potentially relevant. The bibliographies of all included articles were subsequently hand searched to identify any additional studies.
The literature search produced 2344 publications (Fig. 1). Of these plasma vitamin D, zinc and carotenoids were most commonly studied. In contrast, plasma vitamin K, vitamins B2 and B6 were least studied. In terms of acute injury (surgical) 222 abstracts and eighty-five full text articles were examined and thirteen studies (all prospective) were included in the review. In terms of chronic injury 2122 abstracts and 122 full text articles were examined and twenty-four studies (largely retrospective) were included in the review.
The study of the relationship between plasma micronutrient concentrations and the systemic inflammatory response following elective surgery is useful since patients are usually nutritionally replete and therefore any changes in micronutrient concentrations are likely to be the result of the systemic inflammatory response. The studies identified in the present systematic review are shown in Table 1.
Table 1. The relationship between the systemic inflammatory response and plasma micronutrient concentrations following elective surgery
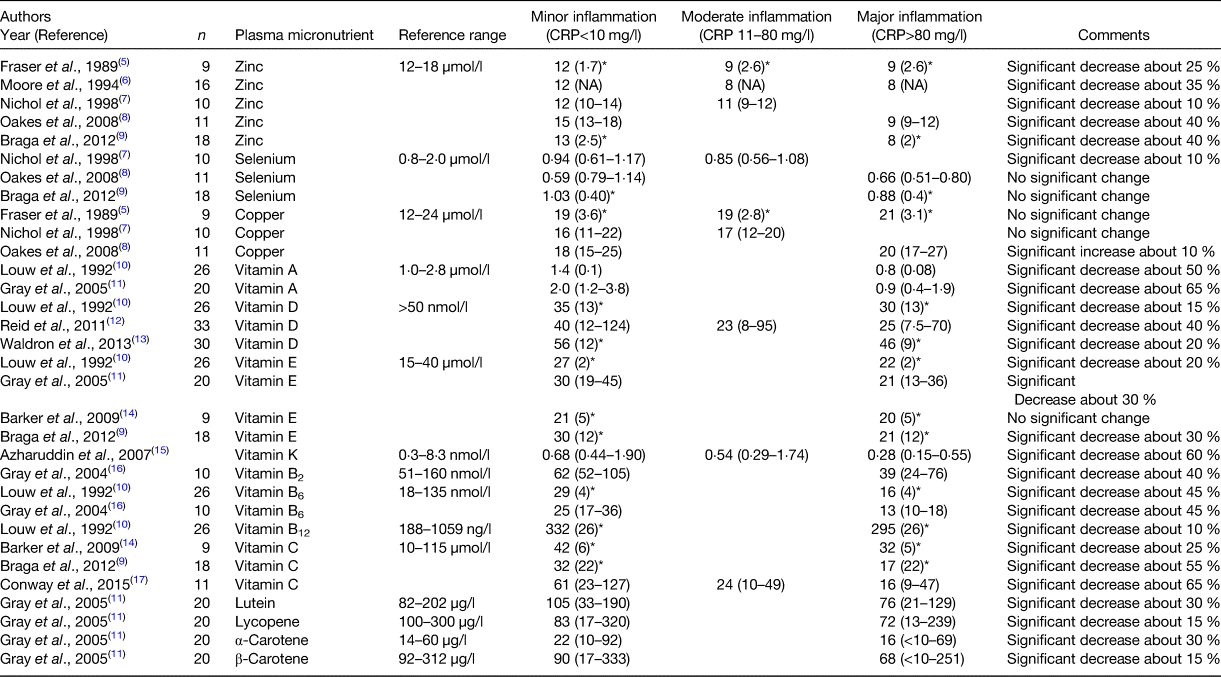
* Values are specified as mean and standard deviation; in all other instances values are median and range. CRP, C-reactive protein.
There were five independent studies that examined plasma zinc concentrations following elective surgery(Reference Fraser, Taggart and Fell5–Reference Braga, Bissolati and Rocchetti9). Zinc concentrations fell significantly with increasing CRP concentrations from minor to moderate to major inflammation by approximately 35 %. Minor inflammation was associated with median plasma zinc concentrations within the reference range in all five studies. Major inflammation was associated with median plasma zinc concentrations below the reference range in all four studies.
There were three independent studies that examined plasma selenium concentrations following elective surgery(Reference Nichol, Herdman and Sattar7–Reference Braga, Bissolati and Rocchetti9). Selenium concentrations fell significantly with increasing CRP concentrations from minor to moderate to major inflammation by approximately 10 % in one study(Reference Nichol, Herdman and Sattar7) and there was no significant change in two studies(Reference Oakes, Lyon and Duncan8, Reference Braga, Bissolati and Rocchetti9). Minor inflammation was associated with median plasma selenium concentrations within the reference range in two of three studies. Major inflammation was associated with median plasma selenium concentrations below the reference range in one of two studies.
There were three independent studies that examined plasma copper concentrations following elective surgery(Reference Fraser, Taggart and Fell5, Reference Nichol, Herdman and Sattar7, Reference Oakes, Lyon and Duncan8). Copper concentrations increased significantly with increasing CRP concentrations from minor to moderate to major inflammation by approximately 10 %(Reference Oakes, Lyon and Duncan8) and there was no change in two studies(Reference Fraser, Taggart and Fell5, Reference Nichol, Herdman and Sattar7). Minor and major inflammation was associated with median plasma copper concentrations within the reference range in all studies.
There were two independent studies that examined plasma vitamin A concentrations following elective surgery(Reference Louw, Werbeck and Louw10, Reference Gray, McMillan and Wilson11). Vitamin A concentrations fell significantly with increasing CRP concentrations from minor to moderate to major inflammation by approximately 55 %. Minor inflammation was associated with median plasma vitamin A concentrations within the reference range in both studies. Major inflammation was associated with median plasma vitamin A concentrations below the reference range in both studies.
There were three independent studies that examined plasma vitamin D concentrations following elective surgery(Reference Louw, Werbeck and Louw10, Reference Reid, Toole and Knox12, Reference Waldron, Ashby and Cornes13). Vitamin D concentrations fell significantly with increasing CRP concentrations from minor to moderate to major inflammation by approximately 25 %. Minor inflammation was associated with median plasma vitamin D concentrations below the reference range in two of three studies. Major inflammation was associated with median plasma vitamin D concentrations below the reference range in all three studies.
There were four independent studies that examined plasma vitamin E concentrations following elective surgery(Reference Braga, Bissolati and Rocchetti9, Reference Louw, Werbeck and Louw10, Reference Gray, McMillan and Wilson11, Reference Barker, Leonard and Trawick14). Vitamin E concentrations fell significantly with increasing CRP concentrations from minor to moderate to major inflammation by approximately 25 %. Minor inflammation was associated with median plasma vitamin E concentrations within the reference range in all four studies. Major inflammation was associated with median plasma vitamin E concentrations within the reference range in all four studies.
There was one study that examined plasma vitamin K concentrations following elective surgery(Reference Azharuddin, O'Reilly and Gray15). Vitamin K concentrations fell significantly with increasing CRP concentrations from minor to moderate to major inflammation by approximately 60 %. Minor inflammation was associated with median plasma vitamin K concentrations within the reference range. Major inflammation was associated with median plasma vitamin K concentrations below the reference range.
There was one study that examined plasma vitamin B2 concentrations following elective surgery(Reference Gray, McMillan and Wilson16). Vitamin B2 concentrations fell significantly with increasing CRP concentrations from minor to moderate to major inflammation by approximately 40 %. Minor inflammation was associated with median plasma vitamin B2 concentrations within the reference range. Major inflammation was associated with median plasma vitamin B2 concentrations below the reference range.
There were two independent studies that examined plasma vitamin B6 concentrations following elective surgery(Reference Louw, Werbeck and Louw10, Reference Gray, McMillan and Wilson16). Vitamin B6 concentrations fell significantly with increasing CRP concentrations from minor to moderate to major inflammation by approximately 45 %. Minor inflammation was associated with median plasma vitamin B6 concentrations within the reference range in both studies. Major inflammation was associated with median plasma vitamin B6 concentrations below the reference range in both studies.
There was one study that examined plasma vitamin B12 concentrations following elective surgery(Reference Louw, Werbeck and Louw10). Vitamin B12 concentrations fell significantly with increasing CRP concentrations from minor to moderate to major inflammation by approximately 10 %.
There were three independent studies that examined plasma vitamin C concentrations following elective surgery(Reference Braga, Bissolati and Rocchetti9, Reference Barker, Leonard and Trawick14, Reference Conway, Talwar and McMillan17). Vitamin C concentrations fell significantly with increasing CRP concentrations from minor to moderate to major inflammation by approximately 50 %. Minor inflammation was associated with median plasma vitamin C concentrations within the reference range in all three studies. Major inflammation was associated with median plasma vitamin C concentrations within the reference range in all three studies.
There was one study that examined plasma carotenoid concentrations following elective surgery(Reference Gray, McMillan and Wilson11). Carotenoid (lutein, lycopene, α- and β-carotene) fell significantly with increasing CRP concentrations from minor to moderate to major inflammation by approximately 15–30 %. Minor inflammation was associated with median plasma carotenoid concentrations within the reference range in three of the four carotenoids. Major inflammation was associated with median plasma carotenoid concentrations below the reference range in three of the four carotenoids.
The majority of elective surgical procedures carried out were orthopaedic for leg fractures(Reference Louw, Werbeck and Louw10) and knee or hip replacement(Reference Nichol, Herdman and Sattar7, Reference Oakes, Lyon and Duncan8, Reference Gray, McMillan and Wilson11–Reference Conway, Talwar and McMillan17). Other elective surgeries included cholecystectomy(Reference Fraser, Taggart and Fell5), hysterectomy(Reference Moore, Desborough and Powell6) and pancreaticoduodenectomy(Reference Braga, Bissolati and Rocchetti9).
The study of the relationship between plasma micronutrient concentrations and the systemic inflammatory response in chronic diseases is useful since this would show whether the magnitude of the effect was similar in both in acute injury and chronic diseases. The studies identified in the present systematic review are shown in Table 2.
Table 2. The relationship between the systemic inflammatory response and plasma micronutrient concentrations in chronic diseases
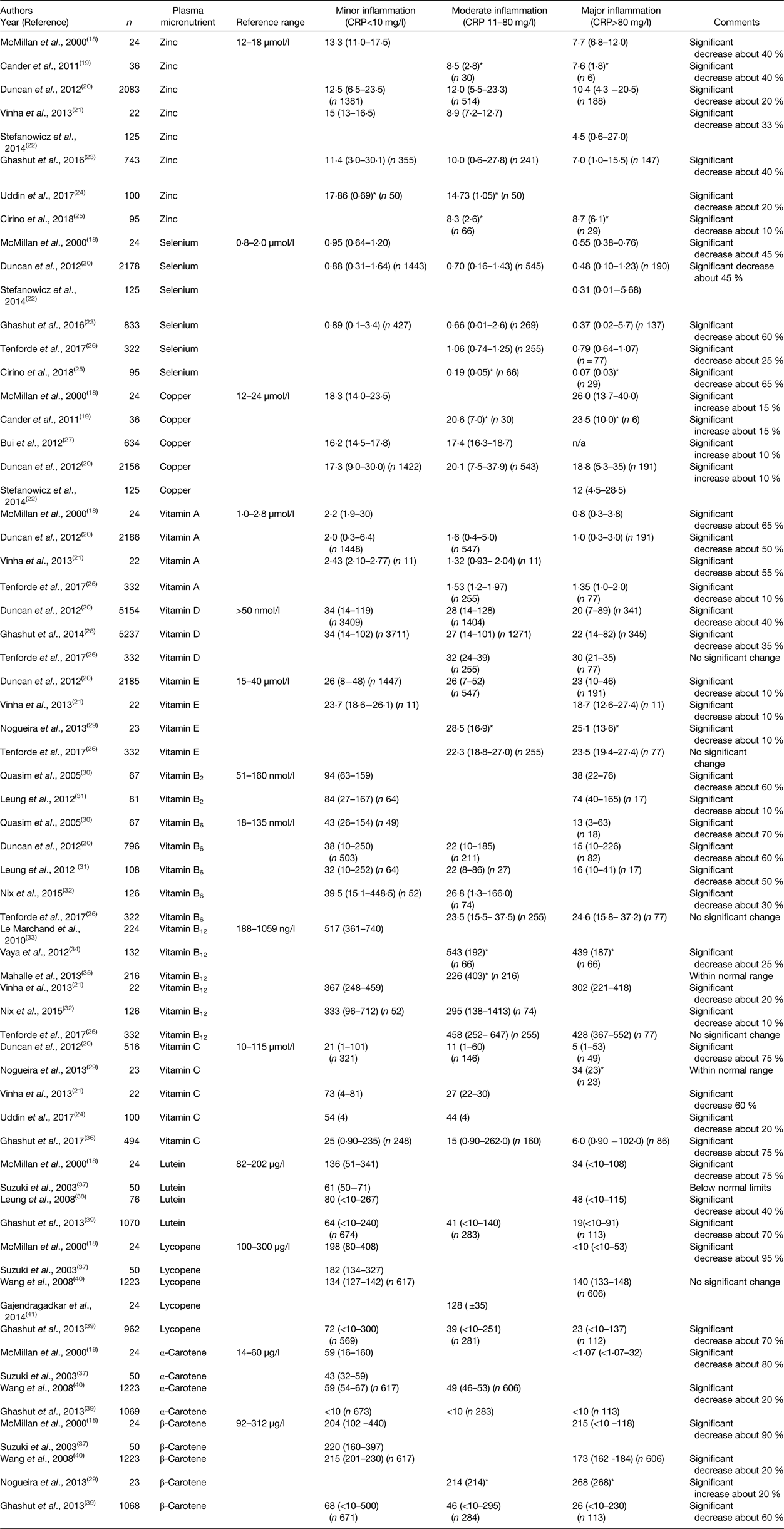
* Values are specified as mean and standard deviation; in all other instances values are median and range. CRP, C-reactive protein.
There were eight independent studies that examined plasma zinc concentrations in chronic diseases(Reference McMillan, Sattar and Talwar18–Reference Cirino Ruocco, Pacheco Cechinatti and Barbosa25). Zinc concentrations were significantly lower with increasing CRP concentrations from minor to moderate to major inflammation by approximately 25 %. Minor inflammation was associated with median plasma zinc concentrations within the reference range in four of the five studies. Major inflammation was associated with median plasma zinc concentrations below the reference range in six of six studies.
There were six independent studies that examined plasma selenium concentrations in chronic diseases(Reference McMillan, Sattar and Talwar18, Reference Duncan, Talwar and McMillan20, Reference Stefanowicz, Gashut and Talwar22, Reference Ghashut, McMillan and Kinsella23, Reference Cirino Ruocco, Pacheco Cechinatti and Barbosa25, Reference Tenforde, Yadav and Dowdy26). Selenium concentrations were significantly lower with increasing CRP concentrations from minor to moderate to major inflammation by approximately 45 %. Minor inflammation was associated with median plasma selenium concentrations within the reference range in three of the three studies. Major inflammation was associated with median plasma selenium concentrations below the reference range in six of the six studies.
There were five independent studies that examined plasma copper concentrations in chronic diseases(Reference McMillan, Sattar and Talwar18, Reference Cander, Dundar and Gul19, Reference Duncan, Talwar and McMillan20, Reference Stefanowicz, Gashut and Talwar22, Reference Bui, Stein and DiGirolamo27). Copper concentrations were significantly higher with increasing CRP concentrations from minor to moderate to major inflammation by approximately 10 %. Minor inflammation was associated with median plasma copper concentrations within the reference range in three out of the three studies. Major inflammation was also associated with median plasma copper concentrations within the reference range in three of the four studies.
There were four independent studies that examined plasma vitamin A concentrations in chronic diseases(Reference McMillan, Sattar and Talwar18, Reference Duncan, Talwar and McMillan20, Reference Vinha, Martinez and Vannucchi21, Reference Tenforde, Yadav and Dowdy26). Vitamin A concentrations were significantly lower with increasing CRP concentrations from minor to moderate to major inflammation by approximately 55 %. Minor inflammation was associated with median plasma vitamin A concentrations within the reference range in three of the three studies. Major inflammation was associated with median plasma vitamin A concentrations below the reference range in two of the three studies.
There were three independent studies that examined plasma vitamin D concentrations in chronic diseases(Reference Duncan, Talwar and McMillan20, Reference Tenforde, Yadav and Dowdy26, Reference Ghashut, Talwar and Kinsella28). In two studies vitamin D concentrations were significantly lower with increasing CRP concentrations from minor to moderate to major inflammation by approximately 35 % and one study reported no significant change. Minor inflammation was associated with median plasma vitamin D concentrations below the reference range in two of the two studies. Major inflammation was associated with median plasma zinc concentrations below the reference range in three of the three studies.
There were four independent studies that examined plasma vitamin E concentrations in chronic diseases(Reference Duncan, Talwar and McMillan20, Reference Vinha, Martinez and Vannucchi21, Reference Tenforde, Yadav and Dowdy26, Reference Nogueira, Borges and Lameu29). Vitamin E concentrations were significantly lower with increasing CRP concentrations from minor to moderate to major inflammation by approximately 10 %. Minor inflammation was associated with median plasma vitamin E concentrations within the reference range in two of the two studies. Major inflammation was associated with median plasma vitamin E concentrations within the reference range in four of the four studies.
No studies examined vitamin K in chronic diseases.
There were two studies that examined plasma vitamin B2 concentrations in chronic diseases(Reference Quasim, McMillan and Talwar30, Reference Leung, Roxburgh and Talwar31). Vitamin B2 concentrations were significantly lower with increasing CRP concentrations from minor to moderate to major inflammation by approximately 30 %. Minor inflammation was associated with median plasma vitamin B2 concentrations within the reference range in two of the two studies. Major inflammation was associated with median plasma vitamin B2 concentrations below the reference range in one of the two studies.
There were five independent studies that examined plasma vitamin B6 concentrations in chronic diseases(Reference Duncan, Talwar and McMillan20, Reference Tenforde, Yadav and Dowdy26, Reference Quasim, McMillan and Talwar30–Reference Nix, Zirwes and Bangert32). Vitamin B6 concentrations were significantly lower with increasing CRP concentrations from minor to moderate to major inflammation by approximately 60 %. Minor inflammation was associated with median plasma vitamin B6 concentrations within the reference range in four of the four studies. Major inflammation was associated with median plasma vitamin B2 concentrations below the reference range in three of the four studies.
There were six studies that examined plasma vitamin B12 concentrations in chronic diseases(Reference Vinha, Martinez and Vannucchi21, Reference Tenforde, Yadav and Dowdy26, Reference Nix, Zirwes and Bangert32–Reference Mahalle, Kulkarni and Garg35). Vitamin B12 concentrations were significantly lower with increasing CRP concentrations from minor to moderate to major inflammation by approximately 10 %. Minor inflammation was associated with median plasma vitamin B12 concentrations within the reference range in three of the three studies. Major inflammation was associated with median plasma vitamin B12 concentrations within the reference range in three of the three studies.
There were five independent studies that examined plasma vitamin C concentrations in chronic diseases(Reference Duncan, Talwar and McMillan20, Reference Vinha, Martinez and Vannucchi21, Reference Uddin, Hossain and Rahman24, Reference Nogueira, Borges and Lameu29, Reference Ghashut, McMillan and Kinsella36). Vitamin C concentrations were significantly lower with increasing CRP concentrations from minor to moderate to major inflammation by approximately 75 %. Minor inflammation was associated with median plasma vitamin C concentrations within the reference range in four of the four studies. Major inflammation was associated with median plasma vitamin C concentrations below the reference range in two of the three studies.
There were four studies that examined plasma lutein concentrations in chronic diseases(Reference McMillan, Sattar and Talwar18, Reference Suzuki, Ito and Ochiai37–Reference Ghashut, McMillan and Kinsella39). Lutein concentrations were significantly lower with increasing CRP concentrations from minor to moderate to major inflammation by approximately 40 %. Minor inflammation was associated with median plasma lutein concentrations below the reference range in three of the four studies. Major inflammation was associated with median plasma lutein concentrations below the reference range in three of the three studies.
There were five studies that examined plasma lycopene concentrations in chronic diseases(Reference McMillan, Sattar and Talwar18, Reference Suzuki, Ito and Ochiai37, Reference Ghashut, McMillan and Kinsella39–Reference Gajendragadkar, Hubsch and Mäki-Petäjä41). Lycopene concentrations were significantly lower with increasing CRP concentrations from minor to moderate to major inflammation by approximately 95 % in one study with no significant change in another study. Minor inflammation was associated with median plasma lycopene concentrations within the reference range in three of the four studies. Major inflammation was associated with median plasma lycopene concentrations below the reference range in two of the three studies.
There were four studies that examined plasma α-carotene concentrations in chronic diseases(Reference McMillan, Sattar and Talwar18, Reference Suzuki, Ito and Ochiai37, Reference Ghashut, McMillan and Kinsella39, Reference Wang, Gaziano and Norkus40). α-Carotene concentrations were significantly lower with increasing CRP concentrations from minor to moderate inflammation by approximately 20 %. Minor inflammation was associated with median plasma α-carotene concentrations within the reference range in three of the four studies. Major inflammation was associated with median plasma α-carotene concentrations below the reference range in two of the two studies.
There were five studies that examined plasma β-carotene in chronic diseases(Reference McMillan, Sattar and Talwar18, Reference Nogueira, Borges and Lameu29, Reference Suzuki, Ito and Ochiai37, Reference Ghashut, McMillan and Kinsella39, Reference Wang, Gaziano and Norkus40). β-Carotene concentrations were significantly lower with increasing CRP concentrations from minor to moderate to major inflammation by approximately 20 %. Minor inflammation was associated with median plasma β-carotene concentrations within the reference range in three of the four studies. Major inflammation was associated with median plasma β-carotene concentrations below the reference range in one of the four studies.
Discussion
The results of the present systematic review over the past two decades show that there was consistent evidence that direct measurements of most micronutrients in plasma are significantly perturbed by the presence of a systemic inflammatory response and the magnitude of perturbation is similar whether this is as a result of acute (surgical) or chronic tissue injury. For some micronutrients the perturbation is such that there is apparent deficiency in the majority of patients. Taken together it is clear that, in the presence of a systemic inflammatory response, plasma micronutrient concentrations are unlikely to be reliable measures of nutritional status.
In the present review CRP was used as an objective, reliable and routinely clinically available measure of the magnitude of systemic inflammatory response(Reference Gabay and Kushner1, Reference Watt, Horgan and McMillan3). Such a measure facilitates the interpretation and comparison of plasma micronutrient concentrations in the presence of a systemic inflammatory response whether due to an acute or chronic tissue injury. Therefore, CRP should be measured alongside plasma micronutrient concentrations where the disease state may result in a systemic inflammatory response.
In the present systematic review the magnitude of the decrease in plasma micronutrient concentrations associated with minor, moderate and major systemic inflammatory response varied from micronutrient to micronutrient. For example, compared with minor inflammation (CRP<10 mg/l), moderate inflammation (CRP 11–80 mg/l) was associated with a 10–20 % fall in micronutrient concentrations. In contrast, compared with minor inflammation (CRP<10 mg/l), major inflammation (CRP>80 mg/l) was associated with a 30–40 % fall in micronutrient concentrations. In particular, zinc, vitamins A, D, B6, C, lutein and lycopene were sensitive to major inflammation with falls in plasma concentrations of approximately 50–60 %. Therefore, such perturbation is particularly problematic since the majority of these micronutrient results obtained in the presence of a systemic inflammatory response could be considered deficient (i.e. below the normal reference range).
Therefore, one of the important implications of the present review is that when one encounters patients who have low plasma concentrations of micronutrients it is difficult to differentiate between a true and apparent deficiency. The present review would suggest that those patients with a low micronutrient concentration and minor inflammation are likely to be truly deficient. In the case of those patients with low plasma micronutrient concentration and major inflammation the deficiency may be apparent rather than true. In these patients a number of approaches could be used to determine whether there was real deficiency. First, serial measurements could be used to examine changes in plasma micronutrients relative to changes in CRP. Secondly, functional tests may be useful e.g. glutathione peroxidase in the case of selenium and transaminases in the case of vitamin B6. Thirdly, intracellular measurements could be carried out. There are now erythrocyte measures of zinc, selenium, vitamins E, B2 and B6 that appear to abrogate the acute effect of the systemic inflammatory response seen in plasma micronutrients(Reference Oakes, Lyon and Duncan8, Reference Gray, McMillan and Wilson16, Reference Ghashut, McMillan and Kinsella36, Reference Vasilaki, Leivaditi and Talwar42). Fourthly, potential correction of plasma micronutrients using CRP or other acute phase proteins and carrier proteins. This approach has been examined for zinc(Reference Ghashut, McMillan and Kinsella23), vitamin A(Reference Thurnham43, Reference Thurnham and Northrop-Clewes44), vitamin D(Reference Reid, Toole and Knox12, Reference Ghashut, Talwar and Kinsella28), vitamin C(Reference Ghashut, McMillan and Kinsella36) and carotenoids(Reference Ghashut, McMillan and Kinsella39). Recently, the case has been made for the use of IL-6, a pro-inflammatory cytokine, as a correction factor for iron, zinc and selenium(Reference MacDonell, Miller and Harper45). There are striking paralells between the present observations and that recently reported by the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anaemia project. They recommend adjustment of iron status and anaemia using measures of the systemic inflammatory response in a regression approach(Reference Namaste, Aaron and Varadhan46). In the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anaemia publications CRP and α-1 acid glycoprotein (acute phase proteins with varying half-life) were used but others have suggested that CRP and albumin may be a more useful combination in clinical practice(Reference McSorley, Talwar and McMillan47). However, since the correlation between the plasma micronutrient and the correction factors is often not strong in the presence of systemic inflammation there remains concern over the variability of such corrections.
The basis of the fall in most micronutrients in the plasma as part of the systemic inflammatory response irrespective of nutritional status is of increasing interest. Thurnham and Northrop-Clewes(Reference Thurnham and Northrop-Clewes44) stated ‘In people who are sick or exposed to trauma there is a systemic inflammatory response when the concentration dynamics of micronutrients between the tissues and the blood change and nutrient concentrations may no longer reflect status. There are several reasons for the changes; some are known whereas others are speculative. Because of the essential nature of micronutrients they must be conserved, shielded from pathogens and/or prevented from reacting with damaged tissue and exacerbating the disorder’. Also, it is now recognised that there is an intimate evolutionary link between immune and metabolic responses in all mammalian cells, so called immunometabolism. Simply, activation of innate immune cells will result in a metabolic response at the cellular, tissue and whole body levels and vice versa. This important immune/metabolic response link has been implicated in the insulin resistance associated with obesity and diabetes(Reference Hotamisligil2). With reference to micronutrients this may explain why raising plasma concentrations by hypersupplementation has proven to be problematic in human subjects with systemic inflammation e.g. vitamin D(Reference Meyer, Holvik and Lips48). It is also of interest that the use of nonsteroidal anti-inflammatory drugs was associated with increasing plasma micronutrient concentrations in cancer patients with a systemic inflammatory response(Reference McMillan, Sattar and Talwar18). Irrespective of the afore-mentioned, it is clear that there is increasing recognition of the inverse relationship of plasma micronutrient concentrations and inflammatory responses(Reference Raiten, Sakr Ashour and Ross49).
Finally, from the present review it is clear that there are falls in a variety of plasma micronutrients associated with major inflammation. This would suggest that such changes are part of a coordinated cellular, tissue and systemic response. If this were to prove to be the case then it would be unlikely that targeting specific micronutrients for supplementation will result in benefit to the patient with a systemic inflammatory response. It may be that this patient will derive more benefits from targeting the systemic inflammatory response.
In summary, the results of the present systematic review indicate that almost all plasma micronutrients fall as part of the systemic inflammatory response and the effect of major inflammation is similar whether this is as a result of acute (surgical) or chronic tissue injury. Therefore, in the presence of a systemic inflammatory response, plasma micronutrient concentrations are unlikely to be reliable measures of nutritional status.
Acknowledgements
The authors gratefully acknowledge the support and advice of clinical and scientific colleagues at Glasgow Royal Infirmary.
Financial Support
The work has been supported by funding from the University of Glasgow, Glasgow Royal Infirmary Endowment Funds, the Chief Scientist Office and Cancer Research, UK.
Conflict of Interest
None.
Authorship
The authors had joint responsibility for all aspects of preparation of this paper.





