Introduction
Efforts to protect threatened bird species are often limited by the absence of basic knowledge of their biology. One of the main management aims for these species is to maintain or increase the survival and reproductive success of individuals in their populations (Gosling and Sutherland Reference Gosling and Sutherland2004). Therefore, it is essential to know the reproductive parameters and factors that may affect their reproductive success (Sutherland Reference Sutherland2000).
The Yellow Cardinal, Gubernatrix cristata, is a passerine endemic to southern South America, which at present is categorised as ‘Endangered’ (BirdLife International 2013). It is the only representative of the monotypic genus Gubernatrix and it is included in the group of tanagers, together with other genera of granivorous birds such as Diuca, Paroaria and Lophospingus, within the Family Thraupidae (Barker et al. Reference Barker, Burns, Klicka, Lanyon and Lovette2013).
In the past, this species was widely distributed in thorny deciduous shrubland forests of central Argentina (Espinal region), most of Uruguay and part of southern Brazil (Ridgely and Tudor Reference Ridgely and Tudor1994; Figure 1A). However, for over a century there has been a continuous extraction of individuals, mainly males, for use as cage birds (Pessino and Tittarelli Reference Pessino and Tittarelli2006, BirdLife International 2013). This, as well as the conversion of their forest habitat to cattle pasture, has caused a marked decline in range and population size. Yellow Cardinals are very rare and categorised as locally endangered in Brazil (Fontana et al. Reference Fontana, Bencke and Reis2003, Machado et al. Reference Machado, Drummond and Paglia2008) and population size is probably less than 300 individuals in Uruguay (Gabriel Rocha pers. comm.). In Argentina, its distribution is discontinuous, with the main populations in the provinces of Corrientes, La Pampa and Rio Negro (Collar et al. Reference Collar, Gonzaga, Krabbe, Madroño Nieto, Naranjo, Parker and Wege1992, BirdLife International 2013; Figure 1B). Another threat described for this species is hybridisation with Common Diuca Finch Diuca diuca (Bertonatti and López Guerra 1997, BirdLife International 2013). At present, the total population is estimated to number 1,500–3,000 individuals (BirdLife International 2013).
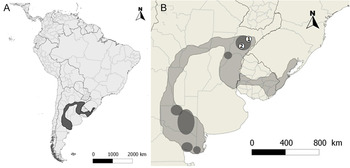
Figure 1. (A) Map showing the historic distribution of Yellow Cardinals (grey area) in South America. (B) Detail showing the main remaining populations in Argentina (darker areas within the historic distribution) and the study sites (1 = 'Reserva Rincón del Socorro', 2 = 'Estancia La Paz').
There is almost no information about the main reproductive parameters of this species (i.e. timing of breeding, clutch size, hatching success, chick survival) and factors that may affect its reproductive success. The only available information comes from De la Peña (1981, 1987), who described the nest and provided egg measurements. Besides this, there is an old report of Yellow Cardinals as hosts of the Shiny Cowbird Molothrus bonariensis (Friedmann Reference Friedmann1934), but there have been no further studies analysing the impact of brood parasitism on Yellow Cardinal reproductive success.
Parasitism by Shiny Cowbirds could decrease the reproductive success of Yellow Cardinals in several ways. Female Shiny Cowbirds puncture eggs when they visit host nests (Massoni and Reboreda Reference Massoni and Reboreda2002, Peer Reference Peer2006), which increases the probability of nest abandonment (Massoni and Reboreda Reference Massoni and Reboreda1998, Astié and Reboreda Reference Astié and Reboreda2006). In addition, parasitism may decrease hatching success and survival of host chicks (Fraga Reference Fraga1978, Astié and Reboreda Reference Astié and Reboreda2006, Tuero et al. Reference Tuero, Fiorini and Reboreda2007). The entire range of Yellow Cardinals is within the distribution of Shiny Cowbirds. Moreover, in the few areas where Yellow Cardinals are still present, Shiny Cowbirds have increased their population as a result of habitat transformation and changes in agricultural practices (i.e. cattle feedlots). Therefore, cowbird parasitism could be an important threat for this species.
Also, it has been reported that botfly larvae (of genus Philornis, Diptera) can be a serious threat to bird species (Fessl and Tebbich Reference Fessl and Tebbich2002). These larvae live subcutaneously on altricial nestlings, feeding on serous fluids, tissue debris, and blood cells (Uhazy and Arendt Reference Uhazy and Arendt1986, Texeira Reference Texeira, Guimarães and Papavero1999) and reducing chick growth and survival (Dudaniec and Kleindorfer Reference Dudaniec and Kleindorfer2006, Rabuffetti and Reboreda Reference Rabuffetti and Reboreda2007, Quiroga and Reboreda Reference Quiroga and Reboreda2012). Although there are no previous records of botfly parasitism in Yellow Cardinals, its presence has been described for other passerines present in the cardinal's distribution (Antoniazzi et al. Reference Antoniazzi, Manzoli, Rohrmann, Saravia, Silvestri and Beldomenico2010, Quiroga et al. Reference Quiroga, Reboreda and Beltzer2012).
In this study we present new data on the breeding biology of a Yellow Cardinal population at Corrientes province, north-east Argentina. We report the main reproductive parameters, examine factors that influence nest survival, and determine if parasitism by Shiny Cowbirds and botflies negatively affects the reproductive success of Yellow Cardinals.
Methods
Study sites
The study was conducted at two sites in the Province of Corrientes, north-east Argentina (Figure 1B), during the breeding seasons (early October–late December) of 2011 and 2012. Both sites are separated by approximately 100 km. Site one is located at 'Reserva Rincón del Socorro' (28°32´S, 57°10´W) on the margin of Iberá wetlands. The reserve is a mix of temporarily flooded open pasturelands, wooded savannas, hydrophilic forests and wetlands. Site two is located at 'Estancia La Paz' (29°20´S, 58°26´W) near the town of Mercedes. This site is mostly characterised by open pasturelands with patches of thorny deciduous shrubland forest dominated by Prosopis affinis.
Data collection
Using mist nets, we captured 16 males and 14 females during the breeding seasons of 2011 and 2012. We determined their mass with a 60 g Pesola scale (± 0.5 g). To follow the pairs and their reproductive attempts we banded them on the tarsus with a numbered aluminium ring and a combination of two coloured plastic rings. We searched for nests in areas of wooded savannah dominated by short (< 8 m in height) leguminous trees of the genera Prosopis and Acacia (Tressens et al. Reference Tressens, Vanni, López, Arbo and Tressens2002). We found nests by systematically searching potential nesting sites and observing the behaviour of territorial pairs following standardised techniques described in Martin and Geupel Reference Martin and Geupel1993 to minimise disturbance to the brood. During the study period we found a total of 46 nests; 21 nests corresponding to 16 different pairs during 2011, and 25 nests corresponding to 18 different pairs during 2012. We found 13 nests (28%) during construction, 5 (11%) during laying, 18 (39%) during incubation, and 8 (22%) after hatching.
We visited nests every 1–4 days until the chicks fledged or the nest failed. During visits we recorded the number of eggs and chicks and whether the eggs had punctures produced by Shiny Cowbirds, or the chicks had larvae of botflies. We marked eggs with waterproof ink, measured their length and width with a calliper (± 0.1 mm) and determined their mass with a 10 g Pesola scale (± 0.1 g). After hatching, we marked chicks on the tarsus with waterproof ink and weighed them with a 30 g Pesola scale (± 0.2 g). We ringed chicks when they were 8–10 days of age. We did not manipulate chicks after that age to avoid premature fledging.
Data analysis
We estimated clutch size from a sample of nests found during construction and laying and that completed laying (n = 17). We considered that a clutch was complete when the nest presented a constant number of eggs for at least two consecutive visits. Since eggs that belong to the same clutch are not statistically independent, we first averaged the length and width of the eggs and then used clutch averages to obtain reported values. We estimated the incubation period as the time elapsed since the laying of the last egg and the hatching of the last chick in clutches where all eggs hatched. We estimated frequency of parasitism as the proportion of nests with Shiny Cowbird eggs and intensity of parasitism as the average number of parasite eggs in parasitized nests. For this estimation we considered nests found during construction, laying and incubation that completed laying (n = 33). We estimated the prevalence of botfly parasitism as the proportion of nests at which the chicks were infested with botfly larvae. For this estimation we only considered nests that survived until chicks were five days of age (n = 18), as most botfly parasitism occurred before this age (Rabuffetti and Reboreda Reference Rabuffetti and Reboreda2007, Segura and Reboreda Reference Segura and Reboreda2011). We did not determine intensity of botfly parasitism to minimise nestling manipulation and length of nest visits.
We estimated hatching success as the number of hatchlings divided by the number of eggs at the time of hatching and chick survival as the number of fledglings divided by the number of hatchlings. To estimate hatching success and chick survival we only considered nests found during construction, laying or incubation that hatched and fledged chicks, respectively. We considered a nest deserted if the eggs or chicks were cold and no parental activity was observed near the nest during the visit, and predated if nest contents disappeared between consecutive visits and there was no parental activity near the nest.
We modelled daily nest survival rates (DSR) provided by software MARK 6.2 (White and Burnham Reference White and Burnham1999) incorporating hypothesised effects of variables that can affect nest survival and evaluated the support for each model using an information-theoretic approach (Burnham and Anderson Reference Burnham and Anderson2002). We estimated DSR at the egg stage (laying and incubation) and at the nestling stage (after the first chick hatched). For nests observed during both stages we truncated the observation period on the last day of the first stage (see below), and then initiated it on that day for the following stage (Dinsmore and Dinsmore Reference Dinsmore and Dinsmore2007). The observation period was truncated on the date of hatching of the first host chick (egg stage), and the date of departure of the fledglings (nestling stage) for nests that successfully completed each stage. When the exact date of fledging was unknown, the observation period was truncated at the average age of fledging (14 days, see below). An assumption in estimating DSR in MARK is that nest fates are independent (Dinsmore et al. Reference Dinsmore, White and Knopf2002). For this reason, we excluded re-nesting attempts from the analysis.
We estimated DSR at the egg stage from 20 nesting attempts for which we had information for the covariates. In this sample, we were able to determine clutch-initiation dates directly in nine nests found during construction and egg-laying. For nests found during incubation that survived until hatching (n = 6), we assigned clutch-initiation dates by backdating from hatching dates. For nests that were found and that failed during incubation (n = 5), we estimated clutch-initiation dates by assuming that the observed period was halfway between the end of laying and hatching.
We selected a priori two covariates that might influence nesting success at the egg stage: time of breeding = date of the breeding season at which the nest started (day 1 = October 1) and brood parasitism by Shiny Cowbirds. We ran the constant model including only the intercept and then we ran models that allow DSR to vary with time of breeding and brood parasitism. We also fitted an additive model including both variables.
To analyse factors influencing nest survival during the nestling stage we ran models including as covariates the time of breeding and parasitism by Philornis. We did not include brood parasitism because Shiny Cowbird chicks hatched in only three nests. For this analysis, we used a subset of 20 first nesting attempts that reached the nestling stage and from which we had information on parasitism by Philornis.
We obtained daily survival estimates from the logistic-regression equation of the best supported model. For the egg stage, the survival probability was the product of DSR over 15 days, which is the maximum length of this stage (i.e. modal clutch size of three eggs, start of incubation with the laying of the penultimate egg and 13 days of incubation). For the nestling stage, since the null model was the most supported and it assumes that DSR does not vary with time, we elevated the DSR of day 1 to 14, which is the number of days the chicks remain in the nest based on our field observations, see Results).
Statistical analysis
We analysed sexual differences in body mass with a Student's t-test and differences in hatching success and chick survival between parasitized and unparasitized nests with Mann Whitney U Tests. We performed these statistical tests using STATISTICA 7.0 (StatSoft Inc. 2004) with alpha set at 0.05. We compared the different candidate models of nest survival with the simplest null-hypothesis model of constant survival (S(.) in MARK notation). We selected MARK default options of sin for the constant survival model, and logit link function for models using covariates (Dinsmore et al. Reference Dinsmore, White and Knopf2002). We evaluated support for competing models within the candidate model set based on the Akaike Information Criterion (AIC) corrected for small sample sizes (AICc, Burnham and Anderson Reference Burnham and Anderson2002). We considered the model with the lowest AICc value as the best fit to the data. Values reported are means ± SE.
Results
Nest building, laying and incubation
We did not detect sexual differences in body mass (males: 47.3 ± 1.5 g, range 42–62 g, n = 15; females: 47.4 ± 0.9, range 39.5–53 g, n = 14, t = 2.0, P = 0.97). Earliest nesting attempts started during the first half of October and latest in mid- December, with most attempts (31%) occurring during the second half of November (Figure 2). Renesting occurred in 5/16 pairs during 2011 and in 6/18 pairs in 2012 (nine pairs with one re-nesting attempt, one with two, and one with three). The time elapsed between nesting attempts was 12.4 ± 1.3 days (n = 14, range 7–26 days). We did not observe renesting attempts in pairs that fledged young (n = 5 in 2011 and n = 6 in 2012).

Figure 2. Percentage of nests initiated through the breeding season by Yellow Cardinals, on the basis of 46 nests followed through the breeding seasons 2011 and 2012 at 'Reserva Rincón del Socorro' and 'Estancia La Paz', Corrientes Province, Argentina. The grey part of the bar indicates first nesting attempts and the white part, re-nesting attempts.
The nest was a semi-spherical cup of 15.5 ± 0.65 cm external diameter, 10.5 ± 0.46 cm in height, 8.3 ± 0.27cm internal diameter and 5.9 ± 0.36 cm deep (n = 25 nests). Nests had an external layer of twigs with thorns and lichens and an internal layer of finer branches, horse hair, compacted plant material, grass, seeds and nylon thread. Mean height above ground was on average 2.1 ± 0.11 m (n = 34 nests, range 1.1–4.1 m). Nests were built mainly in Prosopis affinis (31/41) and Acacia cavens (6/41).
Eggs had a bluish-green background colour with black spots. In most eggs, the spots were distributed over the entire surface, but sometimes spots were concentrated around the blunt egg pole. Eggs were 24.8 ± 0.28 mm in length (n = 17 clutches), 17.9 ± 0.12 mm in width (n = 17 clutches) and the mass was 3.3 ± 0.16 g (n = 12 clutches). Females laid eggs every 24 h and clutch size was 3 ± 0.12 (range 2–4, n = 17). Incubation was performed exclusively by the female, started with the laying of the penultimate egg and lasted 12.5 ± 1.7 days (n = 13). We did not observe egg losses during incubation in unparasitized nests (n = 21).
Hatching and chick growth
Hatching was asynchronous and in most cases the first two eggs (in clutches of three) hatched synchronously and the last egg hatched one day later, but we also observed cases in which the three eggs hatched on consecutive days. Recently hatched chicks had orange skin with dense and long (10–15 mm) grey down on the head, back, and underparts. The mouth was red with light yellow flanges. Chick body mass at hatching was 3 ± 0.25 g (n = 6 nests and 14 chicks). Both parents fed the offspring and chicks remained in the nest for 14 days.
We observed brood reduction in 85% (6/7) of nests that fledged chicks. On average, successful nests fledged 1.6 ± 0.2 chicks and chick survival was 0.67 ± 0.10 (n = 7).
Shiny Cowbird and bot fly parasitism
The frequency of Shiny Cowbird parasitism was 33% (11/33 nests) and intensity of parasitism was 1.09 ± 0.09 eggs per parasitized nest (range 1–2, n = 11). Parasite eggs were 25.8 ± 0.30 mm in length, 21.0 ± 0.12 mm in width and weighed 5.3 ± 0.10 g (n = 5). Egg punctures in parasitized nests reduced clutch size from 3.0 ± 0.19 to 1.0. ± 0.45 eggs (Wilcoxon test: z = 2.4, P = 0.02, n = 11) and as a result of egg punctures 54% of parasitized nests were abandoned. Hatching success did not differ between unparasitized and parasitized nests (unparasitized: 0.77 ± 0.08, n = 13; parasitized: 0.68 ± 0.11, n = 5; Mann Whitney U test, z = 0.74, P = 0.46). Because all nests in which Shiny Cowbird chicks hatched were depredated (n = 3), we could not evaluate whether the presence of a Shiny Cowbird chick reduced the survival of Yellow Cardinal chicks.
Prevalence of botfly parasitism was 22% (4/18 nests). The earliest nest with botflies was found on November 4 and the latest on November 18. Only two 2 of the four nests parasitized with botflies fledged chicks. Chick survival in nests with botflies was 0.25 ± 0.32 (n = 4) while in nests without botflies it was 0.78 ± 0.11 (n = 5) (Mann Whitney U test z = 1.84, P = 0.07).
Nest survival
The best model for explaining nest survival during the egg stage incorporated the additive effects of time of breeding and brood parasitism (ΔAICc >ΔAICc of null model) (Table 1). The covariates time of breeding and brood parasitism had negative slopes (βTime = -0.074 ± 0.03 and βParasitism = -1.45 ± 0.77) indicating a decrease in nest survival in association with Shiny Cowbird parasitism and as the breeding season progressed (Figure 3). As regards nest survival during the nestling stage, models including the effects of time of breeding and parasitism by Philornis received no support, as the best model explaining our data was the one including the intercept that assumes a constant DSR (β=2.98, lower confidence interval = 2.40, upper confidence interval = 3.56 and w = 1).
Table 1. Support for models predicting daily survival rates (DSR) in eggs stage (laying and incubation) of Yellow Cardinal nests during the breeding seasons 2011 and 2012 in Corrientes province, Argentina (n = 20 first nesting attempts). Covariates included in the models are: “brood parasitism” = brood parasitism by Shiny Cowbirds and “time of breeding” = date of the breeding season at which the nest started. Models are ranked according to second-order Akaike Information Criterion corrected for small samples (AICc) values. K indicates the number of parameters of the model; ΔAICc the difference between the AICc value for the current model and the model with the lowest AICc and w the model Akaike weight, a measure of each model’s relative support within the set of candidate models. S(.) is the general model that assumes a constant DSR among nests and over time.
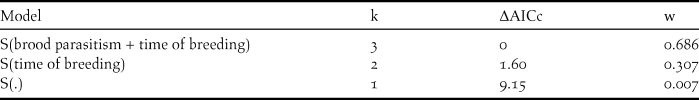

Figure 3. Daily survival rates (DSR) during the eggs stage (laying and incubation) of Yellow Cardinal nests unparasitized (white circles) and parasitized by Shiny Cowbirds (black circles) as a function of time of breeding (date of the breeding season at which the nest started, 1 = October 1). Lines above and below circles indicate the standard error of DSR (dashed = unparasitized, continuous = parasitized).
Cumulative probabilities of surviving along the nesting cycle for an unparasitized nest at the beginning of the breeding season (October 1) was 0.497 while the probability of surviving the whole nesting cycle for a parasitized nest initiated at the time most nesting attempts occurred (November 20) was 0.214.
Discussion
This study provides the first complete description of the main reproductive parameters and factors affecting the reproductive success of endangered Yellow Cardinals. We show that in our study population in north-east Argentina, nest survival decreases as the breeding season advances and as a result of brood parasitism by Shiny Cowbirds. We also show that Yellow Cardinals are parasitized by botflies and that botfly parasitism reduces the survival of cardinal chicks. These two factors had not been previously considered as potential threats to this species.
Shiny Cowbirds are extremely generalist brood parasites that use more than 260 hosts (Lowther Reference Lowther2013). They are prevalent throughout South America where they have expanded their range as a result of habitat transformation (Ortega Reference Ortega1998). In addition, during the last century they have invaded the Caribbean (Cruz et al. Reference Cruz, Wiley, Nakamura, Post and Wood1989) and at present they are invading North America (Post et al. Reference Post, Cruz and McNair1993, Kluza Reference Kluza1998, Post and Sykes Reference Post and Sykes2011). There are several reports of this species parasitizing threatened passerines like the ‘Critically Endangered’ Pale-headed Brush-finch Atlapetes pallidiceps (Oppel et al. Reference Oppel, Schaefer, Schmidt and Schröder2004), the ‘Endangered’ Yellow-shouldered Blackbird Agelaius xanthomus (Wiley et al. Reference Wiley, Post and Cruz1991, López Ortiz et al. Reference López-Ortiz, Ventosa-Febles, Reitsma, Hengstenberg and Peluca2002) and the ‘Vulnerable’ Saffron-cowled Blackbird Xanthopsar flavus (Fraga et al. Reference Fraga, Pugnali and Casañas1998). The main impact of Shiny Cowbirds when they parasitize hosts that are similar or larger in body mass is the puncture of host eggs, which increases the probability of nest abandonment (Reboreda et al. Reference Reboreda, Mermoz, Massoni, Astié and Rabuffetti2003). Accordingly, our results indicate that the main impact of Shiny Cowbird parasitism on Yellow Cardinals’ reproductive success was the puncture of eggs inflicted by parasite females when they visited their nests. The destruction of cardinal eggs resulted in the reduction of the brood and very often in nest abandonment, thus causing lower survival rates of parasitized nests. We did not detect an effect of the presence of the parasite egg on the probability of hatching of cardinal eggs and we were unable to test if there was a negative effect of the presence of Shiny Cowbird chicks on the survival of cardinal chicks, as all parasitized nests were depredated before fledging. Shiny Cowbird parasitism has been also reported in the closely related Common Diuca Finch Diuca diuca and similarly to our results, the main impact of brood parasitism in this host was the puncture of eggs with consequent nest abandonment (Marin Reference Marin2011).
De Mársico et al. (Reference De Mársico, Mahler, Chomnalez, Di Giacomo and Reboreda2010) showed that Shiny Cowbirds exhibit preferences for certain species within host communities and only parasitize a small fraction of the available hosts at high frequencies (> 50%) while they do not parasitize or parasitize at very low frequencies (< 25%) a large proportion of available hosts. At our study site the frequency of parasitism of Yellow Cardinals was 31%. Because we do not have data on frequencies of parasitism in other hosts at our study site, we cannot ascertain whether this intermediate frequency of parasitism was the result of a low density of cowbirds or a preference for parasitizing other available hosts.
Yellow Cardinals appear not to have evolved defences against brood parasitism (Rothstein Reference Rothstein1990, Krüger 2007). We did not detect any evidence of either ejection of parasite eggs or desertion of the nest after parasitism, although Shiny Cowbird eggs differ considerably in background colour and spotting pattern from cardinal eggs. All cases of nest abandonment in parasitized nests occurred after most cardinal eggs were punctured and therefore the desertion of the nest cannot be considered an antiparasitic defence.
As regards the decrease in nest survival with time of breeding, this trend has also been reported for grassland bird species of North America (Grant et al. Reference Grant, Shaffer, Madden and Pietz2005) and for the vulnerable Strange-tailed Tyrant Alectrurus risora in north-east Argentina (Di Giacomo et al. Reference Di Giacomo, Di Giacomo and Reboreda2011). Grant et al. (Reference Grant, Shaffer, Madden and Pietz2005) proposed that this decrease is the result of an increase in the local abundance of predators. However, because we do not have data on seasonal variation in predators we cannot test this hypothesis.
Our study also shows that Yellow Cardinals are parasitized by botflies. Most studies on botfly parasitism show that it considerably reduces the survival of the chicks (Dudaniec and Kleindorfer Reference Dudaniec and Kleindorfer2006). We found that prevalence of botfly parasitism was 22%. In a study that was also conducted in the Espinal region, in neighbouring Santa Fe province, Antoniazzi et al. (Reference Antoniazzi, Manzoli, Rohrmann, Saravia, Silvestri and Beldomenico2010) found that only 7/33 passerine species have a prevalence of botfly parasitism higher than 20%, which indicates that the Yellow Cardinal would be a preferred host of botflies. Although the number of nests parasitized with botflies was low, our results suggest that botfly parasitism reduces the survival of cardinal chicks and therefore negatively affects the reproductive success of this species. Botfly parasitism results in the abandonment of the nest after all chicks die (Rabuffetti and Reboreda Reference Rabuffetti and Reboreda2007, Segura and Reboreda Reference Segura and Reboreda2012). Because this occurs at an advanced stage of the nesting cycle after the parents have invested considerably in the present brood, it seems less likely that in these cases they attempt to renest.
We found that in most cases the clutch size of Yellow Cardinals was three eggs and did not vary with time of breeding. We also found that approximately 35% of the pairs re-nested after nest failure with up to four nesting attempts during the breeding season. This pattern appears to be relatively frequent in south temperate Neotropical birds, which have high rates of nest predation and attempt many nests per year (Martin Reference Martin1996, Di Giacomo et al. Reference Di Giacomo, Di Giacomo and Reboreda2011, Segura and Reboreda Reference Segura and Reboreda2012). The capability to re-nest several times after nest failure should be considered in future studies analysing the viability of Yellow Cardinal populations, as it increases the prospect that a pair recruits juveniles during the breeding season.
Conservation actions
As Azpiroz et al. (Reference Azpiroz, Isacch, Dias, Di Giácomo, Suertegaray Fontana and Morales Palarea2012) recommended for grassland birds from south-eastern South America, we also believe that the protection and management of habitat should be implemented immediately to improve the conservation status of the Yellow Cardinal. Moreover, our results indicate that Shiny Cowbirds represent a threat for Yellow Cardinals, as female cowbirds puncture cardinal eggs, thus reducing brood size and increasing the likelihood of nest desertion. Because Shiny Cowbird distribution includes the Yellow Cardinals’ entire range and parasite populations have increased in size, parasitism may represent a threat for all remaining cardinal populations. As the cost of parasitism is produced by female puncture behaviour, the main option to reduce this cost is controlling the cowbird population (i.e. egg or chick removal would not be effective). The removal of Shiny Cowbirds has been a successful strategy for the recovery of a Pale-headed Brush-finch population, which showed a marked increase in numbers after seven years of cowbird control (Krabbe et al. Reference Krabbe, Juiña and Sornoza2011), although similar programmes have yielded contrasting results with Brown-headed Cowbirds (Hall and Rothstein Reference Hall and Rothstein1999, Smith et al. Reference Smith, Taitt and Zanette2002). Regarding botfly parasitism, the southern records of botflies in Argentina are at 35°S (Fraga Reference Fraga1984, Rabuffetti and Reboreda Reference Rabuffetti and Reboreda2007, Segura and Reboreda Reference Segura and Reboreda2011). Because the main remaining populations of Yellow Cardinals in La Pampa and Rio Negro provinces are at higher latitudes, it is likely that they are not affected by botflies. Further studies are necessary to better estimate the impact of Shiny Cowbird and botfly parasitism on the viability of the remaining populations of Yellow Cardinals.
Acknowledgements
We thank Conservation Land Trust and Carlos Figuerero for allowing us to conduct this study at 'Reserva Rincón del Socorro' and 'Estancia La Paz', respectively. We are very grateful to I. Pérez, S. Cirignoli, S. Heinonen, Y. Di Blanco, K. Sporring and all the staff of 'Reserva Rincón del Socorro' for their assistance and support. We would also like to thank volunteers Martin Hoffmann and Sergio Briones who aided with field work. I. Berkunsky, M.C. De Mársico and L. Segura provided valuable help for the analyses with MARK. Raissa and Sergio Huykman helped with the elaboration of the map. IDEA WILD donated part of the equipment used in this study. MD was supported by a studentship of Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina (CONICET). BM and JCR are Research Fellows of CONICET. Our study was partially financed by “Becas Conservar la Argentina” from Aves Argentinas/AOP, CONICET PIP 0163 and UBACyT 0107.






