Modern research in CVD indicates that inflammation is the lead underlying the physiopathological mechanism of atherosclerosis(Reference Kriszbacher, Koppan and Bodis1). The ageing process, on the other hand, promotes atherosclerosis, mainly by an association with chronic low-grade inflammation and increased oxidative stress(Reference Herrera, Mingorance and Rodriguez-Rodriguez2, Reference Bruunsgaard, Pedersen and Pedersen3). The combination of the above factors allows us to infer that inflammation and ageing are intimately linked in the development of atherosclerosis. Thus, atherosclerosis in aged persons represents a series of highly specific cellular and molecular responses which may be described as an age-related inflammatory disease(Reference Ross4).
In this baseline low-grade inflammation state associated with age, the postprandial state represents a stressful situation of homeostasis by the increase in lipid pro-inflammatory particles, an increase in oxidative stress and a transient increase in pro-inflammatory molecules released by human leucocytes and endothelial cells(Reference Parks5–Reference Chung, Sung and Jung7).
Peripheral blood mononuclear cells (PBMC) are a subset of leucocytes which include lymphocytes and monocytes, and play a critical role in the immune system. It has been shown that low-grade inflammation is associated with the activation of PBMC and with changes in the expression of a variety of cytokines related to inflammation and the immune response, such as IL-6 and TNF-α (Reference de Mello, Kolehmainen and Schwab8, Reference Ghanim, Aljada and Hofmeyer9). PBMC gene expression has been shown to be useful in distinguishing a disease from a healthy state because in the presence of disease, the PBMC alter the normal protein releasing profile to a much more pro-inflammatory profile(Reference Maas, Chan and Parker10, Reference Burczynski and Dorner11). In addition, these cells are being used increasingly for gene expression studies because they can be easily and repeatedly collected in sufficient quantities in contrast to the more invasive sampling of adipose, muscle and liver tissues(Reference de Mello, Kolehmainen and Schwab8).
Diet, and particularly the fat content of the diet, can modulate the inflammatory and immune responses(Reference Paschos, Rallidis and Liakos12, Reference Baer, Judd and Clevidence13). In contrast, there is an age-related increase in the gene expression of some inflammatory molecules (mainly cytokines)(Reference Chung, Sung and Jung7). However, the maximum evidence of the pro-inflammatory status associated with age is the up-regulation of the expression of NF-κB, a key molecule in the regulation of chronic inflammation(Reference Ross4, Reference Lawrence14).
It has been shown that diet can modulate cytokines and NF-κB expression in the postprandial state in young people. In this population, butter-enriched meals elicit greater postprandial expression of pro-inflammatory cytokines from PBMC compared with the consumption of meals rich in olive oil, the main fat constituent in the Mediterranean diet (Med Diet), or walnuts(Reference Jiménez-Gómez, López-Miranda and Blanco-Colio15). In addition, the Med Diet reduces NF-κB activation in PBMC compared with butter- and walnut-enriched diets or Western diets(Reference Perez-Martinez, Lopez-Miranda and Blanco-Colio16, Reference Bellido, López-Miranda and Blanco-Colio17). Many other studies have shown that the Med Diet reduces the pro-inflammatory response(Reference Esposito, Marfella and Ciotola18–Reference López-Miranda, Pérez-Jiménez and Ros22).
Another factor in atherosclerosis is plaque stability. Atherosclerotic plaques are formed by the accumulation of lipid-rich macrophages, vascular smooth muscle cells, lipids and the extracellular matrix(Reference Kong, Yu and Tang23–Reference Santos-Gallego, Bayón and Badimón25). Although advanced atherosclerotic lesions can grow sufficiently large to block blood flow, the most important clinical complication is an acute occlusion due to the formation of a thrombus or blood clot, usually associated with plaque rupture, and resulting in coronary events or stroke(Reference Lusis26). The major determinants of plaque instability and rupture are progressive lipid accumulation, ongoing inflammation and cap weakening, through which matrix metalloproteinases have been shown to be associated(Reference Kong, Yu and Tang23).
To date, no intervention studies have thoroughly explored the postprandial effects of diet on inflammation in elderly people and the genes involved in atherosclerotic plaque stability, which is relevant considering that the postprandial state is the condition in which humans spend most of the day. Thus, the aim of the present study was to evaluate the long-term effects of dietary fat on the postprandial expression of pro-inflammatory genes and plaque stability-related genes in PBMC in healthy, elderly people.
Methods
Subjects and diets
The study was performed on twenty free-living elderly subjects (ten men and ten women). Recruitment of the patients at dietary intervention took place between 1 January 2006 and 1 January 2007. Informed consent was obtained from all participants and all underwent a comprehensive medical history, physical examination and clinical chemistry analysis before enrolment. None of the subjects showed evidence of chronic illness, such as hepatic, renal, thyroid or cardiac dysfunction, and they were requested to maintain their regular physical activity and lifestyle and asked to record in a diary any event that could affect the outcome of the study, such as stress, change in smoking habits and alcohol consumption, or intake of foods not included in the experimental design. Of those enrolled, six participants had high blood pressure, two had hyperlipidaemia and three had diabetes mellitus. None of the participants showed evidence of high alcohol consumption or family history of early-onset CVD. None of the participants were active smokers. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by The Human Investigation Review Committee at Reina Sofía University Hospital (Córdoba, Spain).
Participants were randomly assigned to receive, in a crossover design, three diets each for a period of 4 weeks: (1) Med Diet enriched in MUFA with virgin olive oil, containing 15 % of energy as protein, 47 % as carbohydrate and 38 % as fat (24 % MUFA (provided by virgin olive oil)), < 10 % SFA, 4 % PUFA of which 0·4 % was α-linolenic acid; (2) SFA-rich diet, with 15 % of energy as protein, 47 % as carbohydrate and 38 % as fat (12 % MUFA, 22 % SFA and 4 % PUFA with 0·4 % α-linolenic acid); (3) low-fat, high-carbohydrate diet enriched in n-3 PUFA (CHO-PUFA diet), with 15 % of energy as protein, 55 % as carbohydrate and 30 % as fat (10 % SFA, 12 % MUFA and 8 % PUFA with 2 % α-linolenic acid). The cholesterol intake was constant ( < 300 mg/d) throughout the three periods. The n-3 PUFA enrichment of the high-CHO and low-fat diet was achieved via the use of natural food components rich in α-linolenic acid of plant origin (based on walnuts; Juglans regia L.). Carbohydrate intake from the CHO-PUFA diet was based on the consumption of biscuits, jam and bread. From the Med Diet, 80 % of the MUFA was provided by virgin olive oil, which was used for cooking, salad dressing and as a spread. Butter was used as the main source of SFA during the SFA dietary period.
At the end of each dietary intervention period and after a 12-h fast, the subjects were given a fat meal consisting of a breakfast with a composition similar to that consumed in each of the diets, and with 50–60 % of the subject's daily normal intake of energy. The methodology of the fat-meal test has been validated and extensively used by our unit(Reference Jiménez-Gómez, López-Miranda and Blanco-Colio15, Reference Jiménez-Gómez, Marín and Pérez-Martínez27, Reference Lopez-Miranda, Williams and Lairon28), and is described next. The composition of the experimental diets was calculated by using the US Department of Agriculture(29) food tables and Spanish food-composition tables for local foodstuffs(Reference Varela30).
Before the start of the intervention period, the volunteers completed a 3-d weighed food diary and an extensive FFQ(Reference Martin-Moreno, Boyle and Gorgojo31), which allowed the identification of foods to be modified. Fat foods were administered by dietitians in the intervention study. At the start of the intervention period, each patient was provided with a handbook for the diet to which they had been randomly assigned, which included fourteen menus elaborated with regular solid foods. Advice was given on the foods to choose and those to avoid when eating outside the home. At baseline, volunteers were provided with a supply of study foods to last for 2 weeks and collected additional study foods every fortnight or when required. At these times, a 24-h recall of the previous day's food intake and a short food-use questionnaire based on the study foods were completed to monitor and motivate volunteers to adhere to the dietary advice. A score was used to assess the number of food exchanges achieved in the 24-h recall and additional advice was given if either the 24-h recall or the FFQ showed inadequate intake of food-exchange options. Volunteers were asked to complete 3-d weighed food diaries at baseline, week 2 and week 4. Weighed food intake over two weekdays and one weekend day was obtained by using scales provided by the investigators. A dietary analysis software program (Dietsource version 2.0; Novartis, Barcelona, Spain) was used in the nutritional evaluation of the menus. Biochemical laboratory personnel were unaware of the dietary period that each participant was following for each determination.
Sample collection
Venous blood samples were obtained at the end of each dietary intervention period on fasting state, after a 12-h fast, before breakfast ingestion and at 1, 2 and 4 h after the ingestion of the breakfast. Samples from the fasting and postprandial states were collected in tubes containing 1 g EDTA/l and were stored in containers with ice water and kept in the dark. Special care was taken to avoid exposure to air and light; and ambient temperature was maintained. Plasma was separated from whole blood by low-speed centrifugation at 1500 g for 15 min at 4°C within 1 h of extraction.
Lipid analysis
Venous blood samples were collected in tubes containing 1 mg/ml. Plasma was obtained by low-speed centrifugation (1500 g) for 15 min at 4°C within 1 h of venepuncture. In order to reduce inter-assay variation, plasma samples were stored at − 80°C and analysed at the end of the study. Lipid parameters were assessed with a DDPPII Hitachi modular analyser (Roche, Basel, Switzerland), using specific reagents (Boehringer-Mannheim, Mannheim, Germany). Total plasma cholesterol and TAG concentrations and lipoprotein fractions were measured by colorimetric enzymatic techniques(Reference Bucolo and David32, Reference Allain, Poon and Chang33). HDL-cholesterol levels were measured using colorimetric assay after precipitating the lipoproteins containing apoB with polyethylene glycol(Reference Brigg, Anderson and Johnson34). LDL-cholesterol concentrations were calculated by using the Friedewald formula based on the cholesterol, TAG and HDL-cholesterol values(Reference Friedewald, Levy and Fredrickson35).
Plasma molecules immunoassay
Plasma concentration of cytokines was determined in duplicate with commercially available ELISA kits: TNF-α (R&D Systems, Inc., Minneapolis, MN, USA), IL-6 (Bender Med System, Vienna, Austria) and monocyte chemoattractant protein 1 (MCP-1, microbead assays, Bio-Plex, Bio-Rad Laboratories, Inc., Hercules, CA, USA), according to the manufacturers' guidelines.
Isolation of peripheral blood mononuclear cells
PBMC were isolated within 2 h after blood was drawn from 30 ml EDTA anticoagulated blood samples. Buffy coats were diluted in a 1:2 ratio in PBS, and the cells were separated in 5 ml Ficoll gradient (lymphocyte isolation solution, Rafer) by centrifugation at 2000 g for 30 min. PBMC were collected and washed twice with cold PBS. Harvested PBMC were preserved in liquid N2 and stored at − 80°C before RNA extraction.
NF-κB activation
NF-κB (p65) DNA binding activity was determined using the NF-κB (p65) Transcription Factor Assay kit (Cayman Chemical, Ann Arbor, MI, USA). PBMC nuclear protein extracts were obtained following the procedure previously described by Hernández-Presa et al. (Reference Hernández-Presa, Ortego and Tuñón36).
RNA extraction and quantitative RT-PCR analysis
Total RNA was extracted from mononuclear cells with TRI Reagent (Sigma-Aldrich, Inc., St Louis, MO, USA) and purified with RNeasy MiniElute Cleanup Kit (Qiagen, Hilden, Germany). Recovered RNA was quantified using a Nanodrop ND-1000 v3.5.2 spectrophotometer (Nanodrop Technology®, Cambridge, UK). RNA integrity was assessed using 1·6 % agarose gel, 1 × TBE (Tris/borate/EDTA). PCR analyses were performed using Mx3005 Thermocycler (Agilent Technologies, La Jolla, CA, USA) and iQ SYBR® Green Supermix (Bio-Rad Laboratories, Inc.) commercial kits in a final volume of 20 μl with 10 pmol of each primer. Each reaction was performed on 1 μl of 1:5 (v/v) dilution of the first complementary DNA strand, synthesised using 1 μg of total RNA by the commercial kit iScript® cDNA Synthesis Kit (Bio-Rad Laboratories, Inc.) according to the manufacturer's instructions. The reaction was incubated at 96°C for 3 min, followed by forty cycles of 30 s at 96°C, 30 s at 62°C, 20 s at 72°C and 10 s at 80°C where fluorescence was measured to avoid primer–dimer and background signals. Primers used were: p65-Up (5′-CCCTGTCCTGATGGTCAGCTCCCT-3′), p65-Low (5′-CTCAAACGCTGGTGTTAGGCACAGGG-3′), IκBα-Up (5′-CACTCCATCCTGAAGGCTACCAAC-3′), IκBα-Low (5′-CACACTTCAACAGGAGTGACACCAG-3′), MCP1-Up (5′-CATGAAAGTCTCTGCCGCCCT-3′), MCP1-Low (5′-CACTTGCTGCTGGTGATTCTTCTAT-3′), TNFα-Up (5′-AAGAGTTCCCCAGGGACCTCT-3′), TNFα-Low (5′-CCTGGGAGTAGATGAGGTACA-3′), MIF1-Up (5′-CTCTCCGAGCTCACCCAGCAG-3′), MIF1-Low (5′-CGCGTTCATGTCGTAATAGTT-3′), MMP-9-Up (5′-CCCATTTCGACGATGACGAGTTGTG-3′), MMP-9-Low (5′-GGAGTAGGATTGGCCTTGGAAGATG-3′), IL6-Up (5′-CACCTCTTCAGAACGAATTGACAAAC-3′), IL6-Low (5′-CTCATTGAATCCAGATTGGAAGC-3′), RPL13-Up (5′-CCTGGAGGAGAAGAGGAAAGAGA-3′) and RPL13-Low (5′-TTGAGGACCTCTGTGTATTTGTCAA-3′). Specificity of PCR amplifications was verified by a melting curve program (60–95°C, with a heating rate of 0·5°C/s and a continuous fluorescence measurement) and analysed by electrophoresis on a 1·6 % agarose gel, TBE 1 × . Expression values were obtained as relative expression of the target gene v. the constitutively expressed RPL13a (ribosomal protein L13a) gene (relative expression = ![]() ).
).
Statistical analysis
SPSS statistical software, version 15.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical comparisons. The Kolmogorov–Smirnov test was used to check the normality of the distribution of variance values. When the data did not show the normality of the distribution, we log-transformed them to obtain a normal distribution. In order to evaluate data variation, an ANOVA for repeated measures was performed, followed by Bonferroni's correction for multiple comparisons. The contrast statistic used was the sphericity assumption. The contrast statistic used when the sphericity assumption was not satisfied was Greenhouse–Geisser. Post hoc statistical analysis was completed to identify significant differences between dietary treatments. A probability value of less than 0·05 was considered significant. A study of the relationship among the parameters was carried out using Pearson's linear correlation coefficient. A probability value of less than 0·05 was considered significant. All data presented in the text and tables are expressed as means and standard errors.
Results
Baseline characteristics of the participants
The baseline characteristics of the twenty healthy participants who completed the three dietary intervention periods have been described by Marin et al. (Reference Marin, Ramirez and Delgado-Lista37). Briefly, subjects (ten men and ten women) had an average age of 67·1 (sem 4·52) years, and an average BMI of 31·9 (sem 5·50) kg/m2. The subjects were non-smokers and none of the subjects had any evidence of CVD.
Dietary intake and inflammation regulatory genes
We studied the effect of the long-term ingestion of the three different diets on NF-κB activation, but no significant differences between the diets were found. In addition, we studied the expression of the p65 gene, a subunit of the NF-κB transcription factor, and the IκBα gene, a member of the IκB family (inhibitors of κB), which is involved in NF-κB inactivation by sequestration in the cytoplasm (Fig. 1).
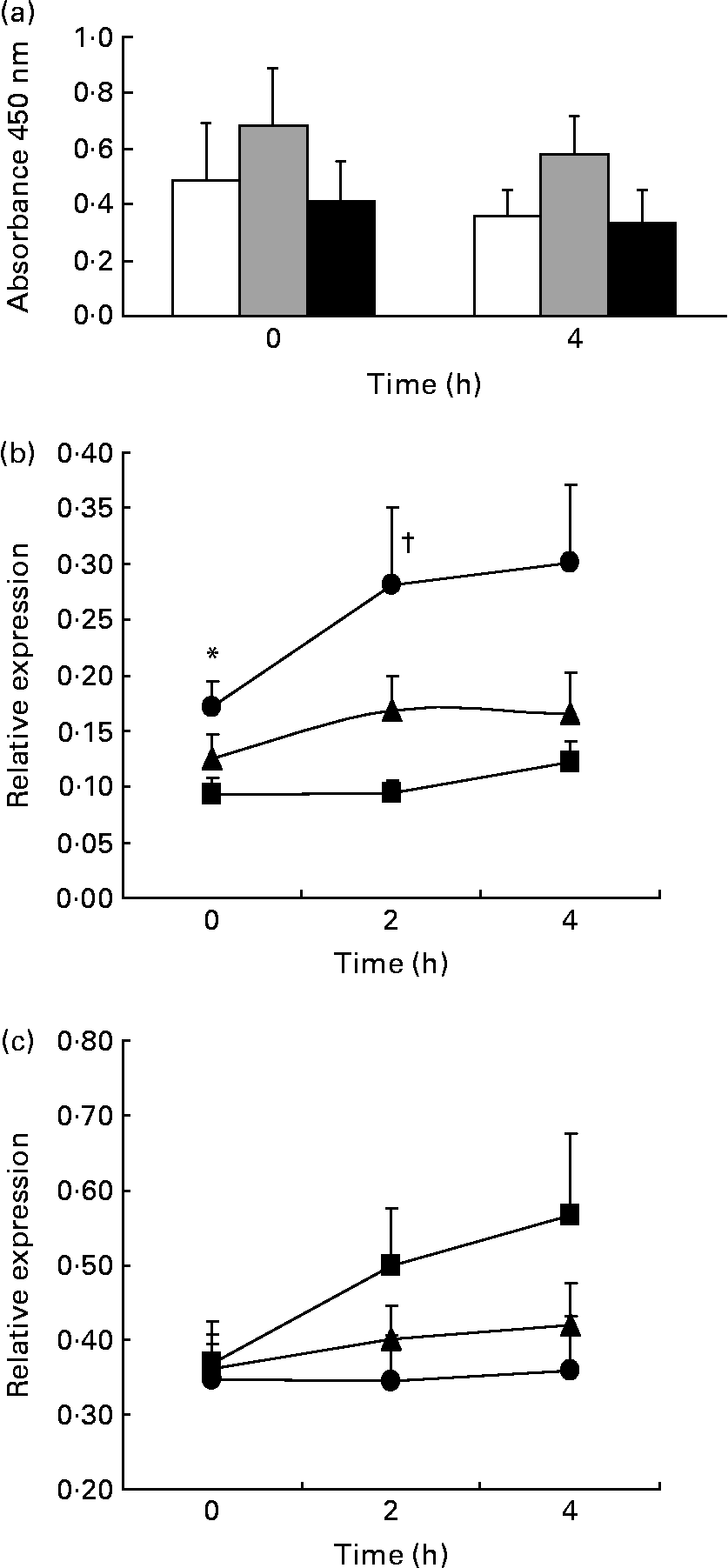
Fig. 1 NF-κB activation and expression of regulatory genes. ANOVA for repeated measurements: P1, effect of diet; P2, time effect; P3, diet × time interaction. Values are means, with their standard errors represented by vertical bars of (a) NF-κB activation (n 13; P1 = 0·274, P2 = 0·206, P3 = 0·786; Mediterranean diet (Med Diet, □), SFA-rich diet (![]() ), low-fat, high-carbohydrate diet enriched with n-3 PUFA (CHO-PUFA diet, ■)), (b) p65 (NF-κB; P1 = 0·002, P2 = 0·045, P3 = 0·382; Med Diet (■), SFA-rich diet (●), CHO-PUFA diet (▲)) and (c) IκBα (P1 = 0·043, P2 = 0·137, P3 = 0·280; Med Diet (■), SFA-rich diet (●), CHO-PUFA diet (▲)) relative expression (n 20) in peripheral blood mononuclear cells. * Mean values were significantly different for SFA-rich diet from those of Med Diet (P < 0·05). † Mean values were significantly different for SFA-rich diet from those of Med Diet and CHO-PUFA diet (P < 0·05).
), low-fat, high-carbohydrate diet enriched with n-3 PUFA (CHO-PUFA diet, ■)), (b) p65 (NF-κB; P1 = 0·002, P2 = 0·045, P3 = 0·382; Med Diet (■), SFA-rich diet (●), CHO-PUFA diet (▲)) and (c) IκBα (P1 = 0·043, P2 = 0·137, P3 = 0·280; Med Diet (■), SFA-rich diet (●), CHO-PUFA diet (▲)) relative expression (n 20) in peripheral blood mononuclear cells. * Mean values were significantly different for SFA-rich diet from those of Med Diet (P < 0·05). † Mean values were significantly different for SFA-rich diet from those of Med Diet and CHO-PUFA diet (P < 0·05).
In the fasting state, we found that consumption of the Med Diet induced lower NF-κB p65 gene expression compared with consumption of an SFA-rich diet (P = 0·019). In addition, we observed that the ingestion of an SFA-rich diet induced higher postprandial expression of the NF-κB p65 gene than the Med Diet (P = 0·006) or the CHO-PUFA diet (P = 0·031) throughout the whole postprandial period. Furthermore, 2 h after the intake of a Med Diet breakfast, we found a statistically significant lower postprandial expression of the p65 gene than after the SFA-rich (P = 0·033) and CHO-PUFA breakfast (P = 0·027). Consumption of the Med Diet induced a higher postprandial expression of IκBα throughout the whole postprandial period (P = 0·043) as compared to the other diets.
Dietary intake and pro-inflammatory cytokines
To determine whether the intake of the three diets could regulate the postprandial expression of different pro-inflammatory cytokines in PBMC, we studied the gene expression for MCP-1, TNF-α and IL-6 (Fig. 2).

Fig. 2 Postprandial cytokine gene expression. Values are means, with their standard errors represented by vertical bars of relative expression in peripheral blood mononuclear cells. Monocyte chemoattractant protein 1 (MCP-1) gene expression values were log-transformed to obtain a normal distribution. ANOVA for repeated measurements (n 20): P1, effect of diet; P2, time effect; P3, diet × time interaction. (a) MCP-1 (P1 = 0·017, P2 = 0·150, P3 = 0·199), (b) TNF-α (P1 = 0·028, P2 = 0·178, P3 = 0·840) and (c) IL-6 (P1 = 0·246, P2 = 0·307, P3 = 0·898). Mediterranean diet (Med Diet, ■); SFA-rich diet (●); low-fat, high-carbohydrate diet enriched with n-3 PUFA (CHO-PUFA diet, ▲). * Mean values were significantly different for SFA-rich diet v. Med Diet (P < 0·05).
No statistically significant differences in the expression of these genes were detected during the fasting state between diets. However, we observed that the ingestion of an SFA-rich diet induced a higher expression of the MCP-1 gene than the Med Diet (P = 0·022) when assessing the postprandial measurements together (0, 2 and 4 h). In the post hoc tests, these differences were significant at 2 h (P = 0·018). When we studied the expression of the TNF-α gene, we showed that the ingestion of the CHO-PUFA diet induced a higher TNF-α postprandial gene expression than the Med Diet (P = 0·047). We also studied the expression of the IL-6 gene, but no significant differences were found between the three diets.
In addition, we also analysed the plasma levels of MCP-1, TNF-α and IL-6 (Fig. 3). Consumption of an SFA-rich diet induced a postprandial increase in the MCP-1 plasma concentration (P = 0·015). The TNF-α plasma level decreased (P = 0·008) and the IL-6 increased (P < 0·001) in the postprandial state, irrespective of the diet consumed.

Fig. 3 Monocyte chemoattractant protein 1, TNF-α and IL-6 plasma levels. ANOVA for repeated measurements (n 20): P1, effect of diet; P2, time effect; P3, diet × time interaction. (a) Monocyte chemoattractant protein 1 (P1 = 0·293; P2 = 0·026; P3 = 0·266), (b) TNF-α (P1 = 0·723; P2 = 0·008; P3 = 0·817) and (c) IL-6 (P1 = 0·933; P2 < 0·001; P3 = 0·176) plasma levels. Values are means, with their standard errors represented by vertical bars of plasma levels. Mediterranean diet (Med Diet, □); SFA-rich diet (![]() ); low-fat, high-carbohydrate diet enriched with n-3 PUFA (CHO-PUFA diet, ■). * Mean values were significantly different (P < 0·05).
); low-fat, high-carbohydrate diet enriched with n-3 PUFA (CHO-PUFA diet, ■). * Mean values were significantly different (P < 0·05).
Dietary intake and atherosclerotic plaque stability
To evaluate whether the ingestion of three different diets could affect atherosclerotic plaques, we studied the expression of matrix metalloproteinase 9 (MMP-9), a metalloproteinase shown to contribute to plaque instability, and macrophage migration inhibitory factor-1 (MIF-1), one of the stimulators of MMP-9 (Table 1). Although no statistically significant differences between the diets were found on MIF-1 or MMP-9 gene expression in the fasting state, the ingestion of an SFA-rich diet induced a higher postprandial MMP-9 gene expression than the Med Diet (P = 0·041). We did not find any differences in the gene expression of MIF-1 depending on the diets.
Table 1 Gene expression of atherosclerotic plaque stability-related genes*
(Mean values with their standard errors)
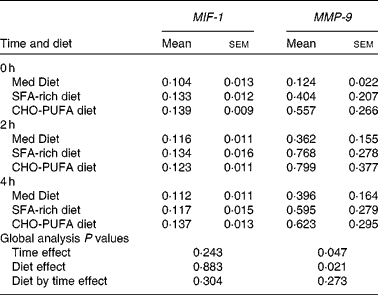
MIF-1, migration inhibitory factor-1; MMP-9, matrix metalloproteinase 9; Med Diet, Mediterranean Diet; CHO-PUFA diet, low-fat, high-carbohydrate diet enriched in n-3 PUFA.
* Mean values with their standard errors of relative expression in peripheral blood mononuclear cells. MMP-9 gene expression values were log-transformed to obtain a normal distribution. ANOVA for repeated measures (n 20).
Correlation between pro-inflammatory cytokine level and lipid parameters
We studied the relationship between the expression of inflammatory genes and lipid parameters. Postprandial lipaemia for this population under study has been described by Marin et al. (Reference Marin, Ramirez and Delgado-Lista37). The plasma total cholesterol concentration positively correlated with the NF-κB p65 sub-unit gene expression (R 0·260, P = 0·044) in the fasting state. In addition, both the total cholesterol and LDL-cholesterol plasma concentration positively correlated with the MIF-1 gene expression (R 0·257, P = 0·047; and R 0·257, P = 0·049, respectively) in the postprandial state (4 h after breakfast). Interestingly, the plasma level of MCP-1 and the HDL-cholesterol plasma concentration were negatively correlated in the fasting and postprandial states (4 h after breakfast (R − 0·257, P = 0·047; and R − 0·335, P = 0·009, respectively)).
Discussion
In the present study, we observed a lower expression of several inflammatory genes after the consumption of a Med Diet compared with SFA-rich (p65 and MCP-1) and CHO-PUFA diets (p65 and TNF-α), and an increase in the expression of the anti-inflammatory gene, IκBα, in elderly people during the postprandial state. Additionally, the gene expression of MMP-9, a marker of plaque instability, was lower after consumption of a Med Diet compared with an SFA-rich diet during the postprandial state.
Recent pathological evidence indicates that major chronic age-related diseases, such as atherosclerosis, arthritis, dementia, osteoporosis and CVD, are linked to a state of increased inflammation(Reference Chung, Sung and Jung7, Reference Ross38). The NF-κB transcription factor and its target genes, which include several pro-inflammatory cytokines, are key factors in the regulation of inflammation. In summary, the NF-κB transcription factor acts as a ‘trigger’, the activation of which leads to a cascading expression of genes and proteins, which leads to inflammatory activation. NF-κB is involved, among other molecules, in the pathogenesis of atherosclerosis(Reference Kutuk and Basaga39). The activation of this factor may be, among others, regulated by dietary fat(Reference Perez-Martinez, Lopez-Miranda and Blanco-Colio16). Our group has already demonstrated that the Med Diet decreases NF-κB activation in PBMC when compared with butter- and walnut-enriched diets or a typical Western diet(Reference Perez-Martinez, Lopez-Miranda and Blanco-Colio16, Reference Bellido, López-Miranda and Blanco-Colio17) in healthy young people. This factor is constitutively formed by two sub-units (p65 and p50), the activation of which leads to the split from IκB and migration to the nucleus. IκB stabilises the NF-κB molecule in the cytoplasm, maintaining it in an ‘unactivated’ state. Only the release of this third sub-unit allows p65 and p50 to migrate to the nucleus to initiate the pro-inflammatory effects of NF-κB(Reference Lee, Allport and Sykes40). In the present study, we observed an increase in the expression of the inhibitory IκBα sub-unit and a decrease in the activating p65 sub-unit after the Med Diet, especially in comparison to the SFA-rich, but also in comparison to the low-fat diet. These combined effects show the underlying mechanisms that cause the lower NF-κB activation reported after the Med Diet, and demonstrate that this lower activation is induced by a double mechanism as follows: IκBα gene expression is increased, while the pro-activator p65 sub-unit is reduced.
We administered a Med Diet enriched in MUFA with virgin olive oil. In addition to the benefits conferred by its lipid content, virgin olive oil has other biological effects as it contains hundreds of non-fat components with great biological potential, including vitamin E, carotenes, squalene, flavonoids, phenolic compounds and others with antioxidant properties(Reference Perez-Jimenez, Ruano and Perez-Martinez41–Reference Visioli and Bernardini43), which could be responsible for a lower postprandial inflammatory response by reducing the pro-oxidative state that activates the genes implicated in the inflammatory response during the postprandial period(Reference Kreeft, Moen and Porter44).
Additionally, minor components of olive oil, such as phenolic compounds, which have also been shown to exert anti-inflammatory properties(Reference Perez-Jimenez, Ruano and Perez-Martinez41–Reference Visioli and Bernardini43), could be responsible for at least some of the observed effects on NF-κB shown in in vitro studies(Reference Dell'Agli, Fagnani and Galli45, Reference Carluccio, Siculella and Ancora46).
However, one of the limitations of this study was deducing whether the observed effects after Med Diet consumption are due to MUFA content, minor components of olive oil, or a combination of both.
In contrast to the report from the PREDIMED (Prevención con Dieta Mediterránea) study, which showed the effects of the Med Diet on plasma inflammatory markers(Reference Llorente-Cortes, Estruch and Mena47) and expression of inflammatory genes on PBMC(Reference Mena, Sacanella and Vazquez-Agell48), our study did not show significant differences between the diets during the fasting state on the inflammatory markers analysed.
Nevertheless, our study showed that consumption of the Med Diet induced a lower expression of the MCP-1 gene than an SFA-rich diet when assessing the postprandial measurements together. MCP-1 (also known as small inducible cytokine A2) and monocyte chemotactic and activating factor play a fundamental role in the recruitment of monocytes to sites of injury and infection, and have been shown to increase in clinical models of chronic inflammation, such as rheumatoid arthritis or lupus. Furthermore, MCP-1 plays a critical role in atherosclerotic plaque formation, by initiating fatty streak formation(Reference Niu and Kolattukudy49). In addition, in the present study, the Med Diet was associated with a lower expression of the TNF-α gene, which acts as an activator of a cascade of cytokine production(Reference Grimble50), compared with the consumption of a CHO-PUFA diet.
Along with the progression of atherosclerosis, rupture of the atherosclerotic plaque is fundamental to the appearance of cardiovascular events. Furthermore, this rupture of the plaque and the subsequent inflammatory and pro-aggregant responses is the key mechanism responsible for these clinical events(Reference Badimon, Ibanez and Cimmino51, Reference Corti, Farkouh and Badimon52). In our study, the postprandial expression of MMP-9, a metalloprotease with an important role in the unstabilisation of the plaque, was lower after consumption of the Med Diet. This effect of the consumption of the Med Diet could be due to the olive oil phenolic compound, as in vitro studies have shown that these compounds prevent the stimulation of MMP-9 expression and secretion in TNF-α-treated THP-1 (human acute monocytic leukaemia cell line) cells(Reference Dell'Agli, Fagnani and Galli45) by impairing NF-κB signalling. Thus, consumption of a Med Diet may not only influence the rate by which atherosclerosis develops, but also promotes the stability of the plaque(Reference Mena, Sacanella and Vazquez-Agell48) by reducing MMP-9 gene expression, possibly by the inhibition of metalloproteinase actions performed by olive oil phenolic compounds as suggested by Hashim et al. (Reference Hashim, Eng and Gill53), which has been shown in green tea polyphenols(Reference Adhami, Ahmad and Mukhtar54, Reference Annabi, Lachambre and Bousquet-Gagnon55).
When analysing our results, we should keep in mind that we performed the study during the postprandial state (not the fasting state), which is the most common state in the populations of Western countries. The postprandial state following a fatty meal induces a ‘physiological’ inflammatory response(Reference Paschos, Rallidis and Liakos12, Reference Baer, Judd and Clevidence13), which may have allowed us to discover the differences in gene expression of the PBMC, depending on the type of diet. Thus, part of the differences in the gene expression of the PBMC may be due to the pro-inflammatory state, which is why the genes could not be detected in the fasting state. To confirm the pro-inflammatory state in which the PBMC were extracted, other pro-inflammatory markers (MCP-1 and IL-6 plasma levels) were augmented, regardless of the diet. Interestingly, gene expression of these proteins was not increased; proteins increased in the blood, but the gene expression in PBMC did not rise, which may indicate that the increased level in the postprandial state may exceed the regulation of PBMC. Other additional potential causes for this apparent lack of correlation between IL-6 plasma levels and mononuclear gene expression may be that the synthesis and secretion processes of these proteins do not occur simultaneously(Reference Futterman and Lemberg56, Reference Kishimoto57) and/or that there are additional regulatory mechanisms in the secretory pathways(Reference Stow, Ching-Low and Offenhäuser58).
It has been suggested that ageing may contribute to changes in immune system activity, resulting in altered cell surface marker expression and cytokine levels in the elderly population compared with young people(Reference Horvathova, Jahnova and Szabova59). Our findings show that this age-related increase in inflammation may be, at least partially during the postprandial state, modulated by diet.
In conclusion, our data suggest that consumption of the Med Diet reduces the expression of inflammatory genes in PBMC compared with the SFA-rich and CHO-PUFA diets. Additionally, the Med Diet reduced the expression of metalloproteinases in relation to atherosclerotic plaque instability compared with an SFA-rich diet during the postprandial state. These findings may be partly responsible for the lower CVD risk in populations with a high adherence to the Med Diet.
Acknowledgements
The CIBER Fisiopatología de la Obesidad y Nutrición is an initiative of the Instituto de Salud Carlos III, Madrid, Spain. This study was supported in part by research grants from the Spanish Ministry of Science and Innovation (AGL 2004-07907, AGL2006-01979 and AGL2009-12270 to J. L.-M., SAF07-62005 to F. P.-J. and FIS PI10/01041 to P. P.-M., PI10/02412 to F. P.-J.); Consejería de Economía, Innovación y Ciencia, Proyectos de Investigación de Excelencia, Junta de Andalucía (P06-CTS-01425 to J. L.-M., CTS-03039 to M. M. M., CTS5015 and AGR922 to F. P.-J.); Consejería de Salud, Junta de Andalucía (06/128, 07/43, and PI0193/09 to J. L.-M., 06/129 to F. P.-J., 0118/08 to F. F.-J., PI-0252/09 to J. D.-L. and PI-0058/10 to P. P.-M.); Fondo Europeo de Desarrollo Regional. We thank Ma Jose Gomez-Luna for technical support. The contributions of the authors to the study were as follows: A. C., J. D.-L., J. L.-M. and F. P.-J. designed the research; A. C., J. D.-L. and P. P.-M. contributed to the provision of study materials and subjects; A. C., C. C.-T., E. Y.-S., F. G.-M., F. R.-C. and P. L.-A. performed the research; F. R.-C. contributed new reagents/analytical tools; A. C., F. G.-M. and P. L.-A. were responsible for the collection and assembly of data; A. C., J. D.-L., A. G.-R., C. C.-T., E. Y.-S. and P. P.-M. analysed the data; J. D.-L., P. P.-M., J. L.-M. and F. P.-J. provided statistical expertise; A. C. wrote the paper; F. F.-J., F. J. T., M. M. M., F. P.-J. and J. L.-M. were responsible for critical review of the manuscript; P. P.-M., J. D.-L., M. M. M., F. P.-J. and J. L.-M. obtained funding for the present study. None of the authors has any conflict of interests that could affect the performance of the work or the interpretation of the data.






