1. Introduction
Deterioration has historically been considered a cardinal feature of schizophrenia [Reference Kraepelin and Kraepelin1]. Nonetheless, a significant number of patients have the potential to achieve clinical remission and functional recovery after the onset of the illness [Reference AlAqeel and Margolese2, Reference Lally, Ajnakina, Stubbs, Cullinane, Murphy and Gaughran3]. Historical research on early detection and intervention in schizophrenia suggested that lengthy active psychotic symptoms might prompt a worse outcome [Reference Wyatt4]. Active positive symptoms represent a dangerous mental state that might be “biologically toxic,” leading to the notion of the deleterious effect on the brain in patients with acute active psychosis [Reference McGlashan5]. Based on the weight of accumulating evidence against a uniformly deteriorating or degenerative course across time, the concept of a “critical period” proposes that most of the clinical and psychosocial deterioration occurs within the first 2 to 5 years after psychosis onset [Reference McGlashan and Johannessen6, Reference Lieberman, Perkins, Belger, Chakos, Jarskog and Boteva7]. This period is notable for a high risk of antipsychotic treatment dropout, relapse and suicide [Reference Pelayo-Terán, Gajardo Galán, Martínez-García, Tabarés-Seisdedos and Crespo-Facorro8, Reference Ayesa-Arriola, Alcaraz, Hernández, Pérez-Iglesias, López Morííñigo and Duta9].
A lengthy DUP may negatively influence illness prognosis with regard to symptomatic response, remission and functional outcomes [Reference Perkins, Gu, Boteva and Lieberman10–Reference Penttilä, Jääskeläinen, Hirvonen, Isohanni and Miettunen12]. Some cognitive [Reference Murru and Carpiniello13, Reference Ito, Nemoto, Tsujino, Ohmuro, Matsumoto and Matsuoka14] and imaging studies [Reference Anderson, Voineskos, Mulsant, George and McKenzie15, Reference Malla, Bodnar, Joober and Lepage16], but not all [Reference Crespo-Facorro, Roiz-Santiáíñez, Pelayo-Terán, Rodríguez-Sánchez, Pérez-Iglesias and González-Blanch17, Reference Rund, Barder, Evensen, Haahr, ten Velden Hegelstad and Joa18], have provided evidence to support this hypothesis, and it has been suggested that there is minimal evidence of an association between untreated psychosis and brain structure in psychosis [Reference Anderson, Rodrigues, Mann, Voineskos, Mulsant and George19, Reference Rund20].
Surprisingly, little attention has been paid to the likely harmful effects of the duration of active psychotic symptoms after treatment is initiated (DAT) on clinical and functional outcomes in schizophrenia. This relationship can be suspected by the association clinical and functional outcomes with time to remission or relapses in patients with a first episode of psychosis. Time to remission and non-early remission has been previously associated to both clinical and functional outcomes at long term [Reference Jordan, Veru, Lepage, Joober, Malla and Iyer21–Reference Friis, Melle, Johannessen, Røssberg, Barder and Evensen23]. Number of relapses have been previously associated to a poorer outcome in patients with schizophrenia or a first episode of psychosis [Reference Wiersma, Nienhuis, Slooff and Giel24, Reference Emsley, Chiliza, Asmal and Harvey25]. With regard to the period of active psychosis, in a previous study, patients with first episode of psychosis and a longer DAT showed a negative intellectual course [Reference Barder, Sundet, Rund, Evensen, Haahr and ten Velden Hegelstad26]. Additionally, it has been reported that the entire duration of active psychosis (DAP: DUP plus the DAT) is a better predictor of severe negative symptoms at 24 months than DUP in patients with a first episode of psychosis [Reference Lyne, Joober, Schmitz, Lepage and Malla27].
We aimed to investigate the effect of the DAP before or after the start of treatment (DUP or DAT, respectively) on clinical and functional outcomes in the long term (3 years) in early psychosis. We hypothesized that both variables, DUP and DAT may have an additive negative effect on the long term functional outcomes.
2. Experimental procedures
2.1 Study setting
This cohort was obtained from an ongoing epidemiological and three-year longitudinal intervention program of first-episode psychosis (PAFIP) conducted at the outpatient clinic and the inpatient unit at the University Hospital Marques de Valdecilla (Cantabria, Spain) [Reference Pelayo-Terán, Pérez-Iglesias, Ramírez-Bonilla, González-Blanch, Martínez-García and Pardo-García28]. Conforming to the international standards for research ethics, this program was approved by the local Institutional Review Board and conforms to the provisions of the Declaration of Helsinki. Patients meeting the inclusion criteria provided their written informed consent to be included in the PAFIP.
2.2 Subjects
All referrals to PAFIP between February 2001 and May 2011 were screened for eligibility with respect to the following criteria: 1) age 15–60 years; 2) living in the catchment area; 3) experiencing their first episode of psychosis; 4) no prior treatment with antipsychotic medication or, if previously treated, a total lifetime of adequate antipsychotic treatment of less than 6 weeks; and 5) DSM-IV criteria for brief psychotic disorder, schizophreniform disorder, schizophrenia, or schizoaffective disorder. Patients were excluded for any of the following reasons: 1) DSM-IV criteria for drug dependence or mental retardation and 2) a history of neurological disease or head injury. The diagnoses were confirmed using the Structured Clinical Interview for DSM-IV (SCID-I) [Reference First, Spitzer, Gibbon and Williams29] conducted by an experienced psychiatrist 6 months after the baseline visit. Our operational definition for a “first episode of psychosis” included individuals with non-affective psychosis who had not previously received antipsychotic treatment, regardless of the duration of untreated psychosis.
2.3 Study design
This prospective clinical study evaluated the effects of DUP, DAT and DAP in the clinical and functional outcomes in individuals with first-episode non-affective psychosis (DSM-IV criteria). All patients with DUP and DAT measurements followed up in PAFIP and with clinical and functional assessments at the end point (3 years) were included in the final analysis.
2.4 Medication
This is an analysis of three different, randomized, flexible-dose and open-label clinical trials, PAFIP I, II and III [Reference Crespo-Facorro, Pérez-Iglesias, Ramirez-Bonilla, Martínez-García, Llorca and Luis Vázquez-Barquero30, Reference Crespo-Facorro, Pérez-Iglesias, Mata, Mata and Valdizan31]. In each trial, the patients were randomly assigned to receive olanzapine (5–20 mg/day), risperidone (2–6 mg/day), haloperidol (3–9 mg/day), aripiprazole (5–30 mg/day), ziprasidone (40–160 mg/day) or quetiapine (100–600 mg/day). A rapid titration schedule (5 days) until an optimal dose was reached was considered the rule unless severe side effects occurred. Based on the clinical efficacy and side effects during the follow-up period, the dose and type of antipsychotic medication could be changed by the treating physician. The mean equivalent chlorpromazine doses of antipsychotic medications [Reference Gardner, Murphy, O’Donnell, Centorrino and Baldessarini32] were 212.18 mg (SD: 92.22) at baseline and 294.12 mg (SD: 260.39) at the 3-year follow-up. The study protocol allowed for the use of anticholinergic agents, benzodiazepines and antidepressants for clinical reasons. Anticholinergic medication was never used prophylactically.
2.5 Assessments
2.5.1 Premorbid and sociodemographic variables
Premorbid and sociodemographic information was recorded from patients, relatives and medical records. The age at the time of onset of psychosis was defined as the age when the emergence of the first continuous (present most of the time) psychotic symptom occurred. The duration of untreated illness (DUI) was defined as the time from the first unspecific symptoms related to psychosis (for such a symptom to be considered, there should be no return to previous stable level of functioning) to initiation of adequate antipsychotic drug treatment; the duration of untreated psychosis (DUP) was defined as the time (months) from the first continuous (present most of the time) psychotic symptom to the initiation of adequate antipsychotic treatment (date when the first antipsychotic treatment in PAFIP was assigned and initiated). DUP was measured systematically to guarantee a valid and reliable measurement. Dating the onset of positive psychotic symptoms relied on information collected in a semi structured interview, based on the Symptom Onset in Schizophrenia (SOS) inventory [Reference Perkins, Leserman, Jarskog, Graham, Kazmer and Lieberman33] and SCID and was operationalized by estimating the date on the total SAPS score that would have met the threshold of ≥3. Cross-referencing with milestones and memorable events was used to enhance the accuracy of dating. All of the interviews were conducted during the patient’s first episode of psychosis. Family members and other carers also provided collateral reports for dating the onset of positive symptoms. Information gathered by a senior psychiatrist, nurses and social workers was considered to establish the DUP. After completion of all interviews, consensus-based best estimates were determined for variables in which there may have been discrepancies between clinician, patient, and family reports.
Other variables were gender; educational level (1. Primary education; 2.10 years of education or higher); living arrangements at the onset of psychosis (1. Living with relatives; 2. Living alone and other status); occupational status for 2 years prior to the initial interview (1.Employment/student; 2.Unemployed) and premorbid adjustment scale (PAS) [Reference van Mastrigt and Addington34].
2.5.2 Clinical variables
Clinical symptoms of psychosis were assessed using the Scale for the Assessment of Negative Symptoms (SANS) [Reference Andreasen35], the Scale for the Assessment of Positive Symptoms (SAPS) [Reference Andreasen36] and their positive, disorganized and negative dimensions [Reference Grube, Bilder and Goldman37] and the 24-item Brief Psychiatric Rating Scale (BPRS) [Reference Overall and Gorham38].
Complete clinical evaluations were conducted at baseline, 6 weeks, 12 months and 36 months. The patients were followed in our outpatient clinic and were permitted rapid and easy access to a clinical appointment at any time, for any possible signs/symptoms of clinical exacerbation that might appear. A thorough clinical assessment was performed to evaluate the severity, duration and course of clinical symptomatology.
2.5.3 Duration of psychosis after starting treatment (DAT)
The SAPS scale was used to determine the duration of psychosis after treatment (DAT) at frequent intervals for 36 months after the initial presentation. Subscale scores were calculated prospectively for each week for hallucinations, delusions, bizarre behavior and positive formal thought disorder. DAT was estimated as the total number of weeks with a score of 3 or higher on any SAPS subscale during the 3-year follow-up. The DAT was recorded based on the severity of symptomatology during exacerbations and relapses throughout the follow-up period, completed with the medical records and discussed at consensus meetings involving two senior psychiatrists and a clinical nurse. All patients enrolled in PAFIP are regularly interviewed at PAFIP outpatient clinic at least during a 3-year follow-up period; during this period, the frequency of clinical interviews varies based on patients clinical status and to the discretion of PAFIP clinical team (from weekly to quarterly visits). In addition to these, protocol clinical interviews were regularly set up at baseline, 6 weeks, 3 months, 1 year, 2 years and 3 years. Relapse was defined among patients who achieved clinical improvement and stability (CGI rating ≤4 and a decrease of at least 30% on BPRS total score and all BPRS key symptom items, by being rated ≤3 for more than 4 consecutive weeks at some point during the first six months following program td) and was defined as any of the following criteria occurring after clinical improvement: 1.- a rating of 5 or above on any key BPRS symptom items for at least 1 week; 2.- CGI rating of ≥6 and a change score of CGI of “much worse” or “very much worse” for at least 1 week; 3.- hospitalization for psychotic psychopathology; 4.- completed suicide. Exacerbation was defined as any 2-point increase of any of the key BPRS symptoms, excluding changes in which the rating remained at the nonpsychotic level (i.e, <3). The key BPRS symptoms were unusual thought content, hallucinations, suspiciousness, conceptual disorganization and bizarre behavior. Patients were considered to have relapsed if the relapse state lasted at least one week [Reference Caseiro, Pérez-Iglesias, Mata, Martínez-Garcia, Pelayo-Terán and Tabares-Seisdedos39, Reference Mayoral-van Son, Martinez-Garcia, Moreno, Parrilla-Escobar and Valdizan40].
The DAT was active psychotic symptoms during relapse/exacerbation, defined as the number of weeks with a score of 3 or higher on any of the four SAPS items during the 3 years follow-up. After a patient was considered to have had a relapse/exacerbation, weekly assessments (SAPS scores) were performed to determine prospectively the duration of active psychotic symptoms. However, an objective quantification of commence or severity of psychotic experienced by the patient is not easy to accurately attain. It can only be retrospectively assessed indirectly based on a patient's and familys overt communication of hallucinations, thought disorders or behavioral alterations and clinical history. Based on information gathered, the starting date of relapse was confirmed in a consensus meeting by the clinical team. The DAT was estimated as the total number of weeks with a score of 3 or higher on any SAPS items during the 3 years follow-up. This definition was based in previous criteria for DAT [Reference Lyne, Joober, Schmitz, Lepage and Malla27] and on the standardized positive remission criteria without the time criteria [Reference Andreasen, Carpenter, Kane, Lasser, Marder and Weinberger41]. The active psychosis measurement after DUP was in the unit of weeks; however, as DUP had been measured in the unit of months, DAT was multiplied by a factor of 0,23 (divided by 52 weeks and multiplied by 12 months) to convert into months. This duration was added to the Duration of Untreated Psychosis (DUP) prior to presentation in order to create a new variable, Duration of Active Psychosis (DAP). To compare groups with higher and lower DUP, DAT and DAP, the data sets were divided into tertiles.
2.5.4 Social functioning and functional recovery
The Disability Assessment Scale (DAS) [Reference World Health Organization42] was used to assess functional outcome at the 3-year follow-up by a psychiatrist and a social worker. At the end of the interview, an overall judgment of total functioning was established using the Global Evaluation (GE) (ranging from 0, normal adjustment, to 5, severe maladjustment), with a consensus rating reached between the psychiatrist and the social worker. Good social functioning was defined as a score of 0 or 1 in the 3-year, whereas a score of 2 or more was considered poor social functioning.
Functional recovery was determined at the 3-year follow-up by collecting information independently from the patients and their relatives by a psychiatrist and a social worker. Information was gathered to determine whether the patient was in full- or part-time work or at school at the 3-year follow-up. The raters reached a consensus after evaluating a structured assessment of the academic or work performance. According to our previous study [Reference González-Blanch, Perez-Iglesias, Pardo-García, Rodríguez-Sánchez, Martínez-García and Vázquez-Barquero43], we considered the patient to have achieved functional recovery when he/she was currently in part-time or full-time work or study with minimal disability (scores of 0 or 1 in the DAS).
2.6 Statistical analysis
Univariate analyses were conducted to explore the of functional outcome and recovery. The differences between patients with good or poor DAS and those between subjects who achieved recovery or did not were assessed using Student’s t test or the Mann-Whitney U in the case of quantitative variables and chi-squared tests in the case of dichotomous or qualitative variables.
Two regression logistic models were performed to predict both functional outcome assessed by DAS and functional recovery including all of the significantly associated variables in the univariate analysis as predictors in a backward “Likelihood Ratio” test. DAP showed high collinearity with both DUP and DAT; therefore, two different models were performed, one including DAP and the other including DAT and DUP.
Finally, the receiver operating characteristic (ROC) curves were conducted to evaluate the area under the curve (AUC) for DAP, DAT and DUP and to assess the best cut-off values and their sensitivity, specificity and maximized Youden’s index. An ROC curve provides a representation of diagnostic performance across the complete test’s possible cut-offs. AUC, ranging from 0.5 to 1, is a measure of the discriminative ability of the test. It has been shown that values of AUC = 0.5 indicate no discrimination, AUC ≥0.7 indicates acceptable discrimination, AUC ≥ 0.8 indicates excellent discrimination, and AUC ≥0.9 indicates outstanding discrimination [Reference Hajian-Tilaki44]. As a sensitivity analysis, the optimal DUP, DAT and DAP thresholds were obtained by maximizing Youden’s index. This is a simple approach to minimizing error, equivalent to maximizing the sum of sensitivity and specificity (Youden’s index = Sensitivity + Specificity−1). It ranges from 0 to 1 and can be interpreted as maximizing the true positive rate while minimizing the false positive rate [Reference Youden45].
The analyses were performed using the Statistical Package for Social Science, version 19.0 (SPSS Inc., Chicago, IL, USA). All of the statistical tests were two-tailed, and statistical significance was determined at the 0.05 level.
3. Results
Of 541 individuals referred to PAFIP from February 2001 to May 2011, 415 persons met the inclusion criteria and gave their written consent to participate in the study. A total of 307 subjects had sufficient information to assess functional recovery at three years. The patients who dropped out during the study or did not have sufficient available data to evaluate functional recovery at 3 years did not significantly differ from the final analyzed sample with respect to their initial sociodemographic and clinical characteristics, excepting in the academic level and the percentage of cannabis use (see Supplementary Table 5). DUP was comparable in the two groups. Interestingly, although the average DAT and DAP appear to be longer in the dropout subjects, they were more frequently classified in the tertiles of shorter DAT and DAP. The subjects included in the three clinical trials did not significantly differed in the good functional outcome or recovery rates.
The mean and median DUP were 13.62 months and 3 months, respectively; the cut-off points for 33.33th and 66.67th percentiles were 1 month and 8 months, respectively. The mean and median DAT were 4.88 and 2.66, respectively; the cut-off points for 33.33th and 66.67th percentiles were 1.53 months and 4.22 months, respectively. The mean and median DAP were 18.42 months and 8.80 months, respectively; the cut-off points for 33.33th and 66.67th percentiles were 4.55 months and 15.17 months, respectively.
3.1 Demographic and clinical profile
The baseline characteristics of the sample and comparisons between groups with good and poor functional recovery status and between groups with good and poor social functioning are depicted in Tables 1 and 2. Comparisons between the groups (according to 33.33th and 66.67th percentiles) of DUP, DAT and DUP are available in the supplementary Tables 1, 2 and 3. A total of 180 patients had good social functioning (58.63% of the included subjects and 43.37% of the total sample), and 124 achieved functional recovery (40.39% of the included subjects and 29.88% of the total sample) at the 3-year follow-up.
Table 1 Clinical variables by functional recovery at 3-years follow-up.

1:n = 299; 2:n = 306; 3:n = 304; 4: n = 303; 5:n = 282; 6:n = 278; 7:n = 218; 8:n = 259: 9: n = 273; 10:n = 245; 11:n = 302.
Table 2 Clinical variables by social functioning at 3-years follow-up.
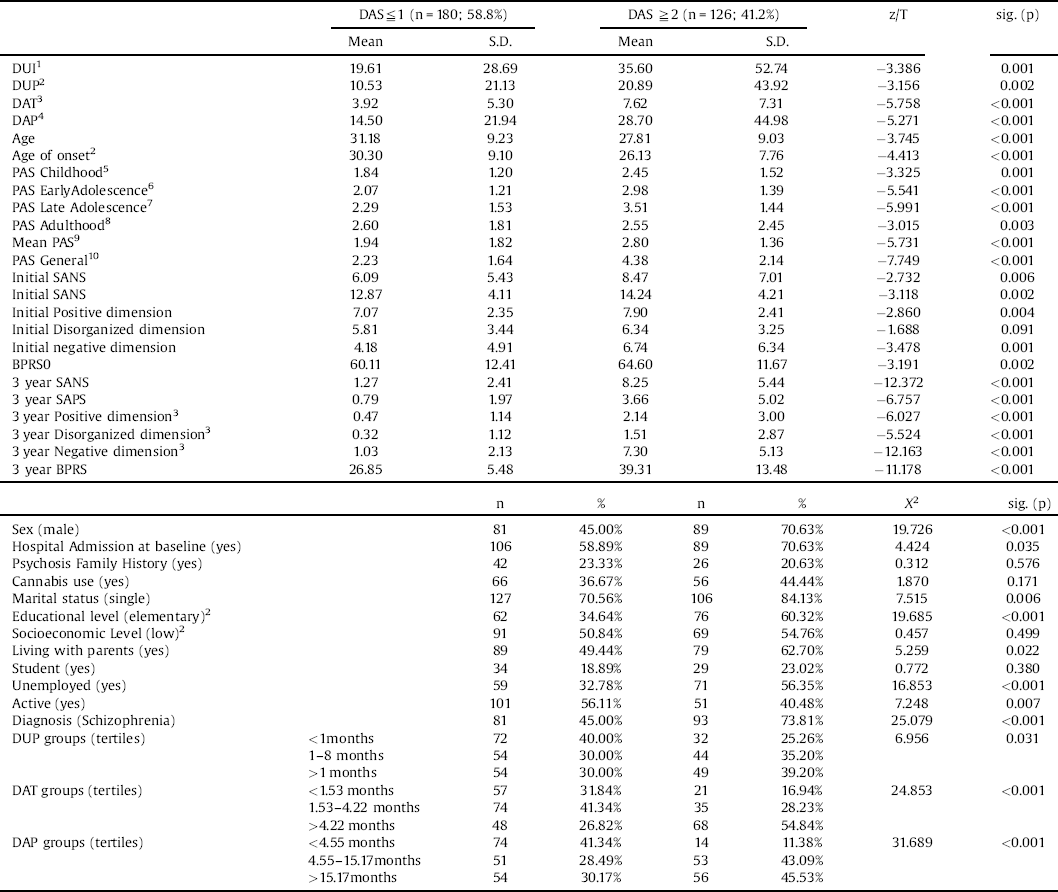
1:n = 298; 2:n = 305; 3:n = 303; 4:302; 5:N = 281; 6:n = 277; 7:n = 218; 8: n = 258; 9:n = 282; 10:n = 244.
Table 3 Logistic regressions including DAT and DUP.

1Model summary: R2:77.939; p < 0.001; Nagelkerke R2: 0.330; Method: Backward – LR; Initial variables: DUP, DAT, Age of onset, Mean PAS; Initial BPRS, Initial Positive, Disorganized and Negative Dimensions, Gender, Hospitalization, Educational Level, Marital Status, Unemployment.
2Model summary: R2: 41.663; p < 0.001; Nagelkerke R2: 0.188; Method: Backward – LR; Initial variables: DAT, DUP, Age of onset, Mean PAS; Initial BPRS, Initial Positive and Negative Dimensions, Gender, Hospitalization, Educational Level, Marital Status, Unemployment.
3.2 Predicting social functioning at 3 years
Gender, educational level, employment status at baseline, diagnosis, age of onset, mean premorbid adjustment, positive and negative dimensions, baseline BPRS, hospital admission, DUP and DAT were included in the logistic regression model to predict social function status at the third year (Table 3). The final model (χ2: 77.939; Nagelkerke R2: 0.330; p < 0.001) included the following as predictors: age of onset, mean PAS score, initial BPRS score, gender, diagnosis and DAT. DAT was the main predictor in the logistic regression analysis (Wald: 13.974; p < 0.001).
The result of the second model that included DAP instead of DAT and DUP (χ2: 71.726; Nagelkerke R2: 0.307; p < 0.001) had the following as final predictor variables (Supplementary Table 4): mean PAS score, initial BPRS score, age of onset, gender, educational level, diagnosis and DAP (Supplementary Table 4). Mean PAS was the main predictor in the logistic regression analysis (Wald: 8.668; p = 0.003).
The comparative ROC curves for DUP, DAT and DAP are shown in Fig. 1. The AUC was statistically significant for DUP (AUC = 0.604; p = 0.002), DAT (AUC = 0.693; p < 0.001) and DAP (AUC = 0.678; p < 0.001). The optimal cut-off points were 0.85 months for DUP (sensitivity: 0.94; specificity: 0.27; Youden index: 0.203), 3.17 months for DAT (sensitivity: 0.68; specificity: 0.64; Youden index: 0.314) and 6.33 months for DAP (sensitivity: 0.84; specificity: 0.54; Youden index: 0.373). Relationships between sensitivity, specificity, Youden’s Index and DAT thresholds are shown in Fig. 2.

Fig 1. Curves for DUP, DAT and DAP.

Fig 2. Relationship between sensitivity, specificity and Youden’s index (thresholds of DAT for the prediction of bad social functioning).
3.3 Predicting functional recovery at 3 years
Gender, educational level, employment status at baseline, diagnosis, mean PAS, positive dimension and BPRS scores at baseline, DUP and DAT were included in the logistic regression model to predict functional recovery status at the third year. The final model (χ2: 41.663; Nagelkerke R2: 0.188; p < 0.001) included the following as predictors: mean PAS, educational level, working status at onset and DAT (Table 3).
The result of the second model that included DAP instead of DAT and DUP (χ2: 40.529; Nagelkerke R2: 0.183; p < 0.001) had the following as final predictor variables: mean PAS, initial positive dimension score, educational level and DAP (Supplementary Table 4).
The comparative ROC curves for DUP, DAT and DAP for predicting nonfunctional recovery at three years are shown in Fig. 1. The AUC was statistically significant for DUP (AUC = 0.585; p = 0.012), DAT (AUC = 0.612; p = 0.001) and DAP (AUC = 0.629; p < 0.001). The optimal cut-off points were 1.75 months for DUP (sensitivity: 0.70; specificity: 0.47; Youden index: 0.166), 2.99 months for DAT (sensitivity: 0.59; specificity: 0.61; Youden index: 0.196) and 6.92 months for DAP (sensitivity: 0.69; specificity: 0.56; Youden index: 0.255).
4. Discussion
The duration of active positive symptoms in the early phases of the illness has a significant impact on patients’ functionality in the long term. Interestingly, in our study, the duration of active psychosis before antipsychotic treatment was initiated (DUP) did not show an independent significant association with functional outcome.
Active positive symptoms represent a dangerous mental state that might be “toxic” for the outcomes of patients with a first episode of psychosis. However, many previous studies have focused only on the effect of DUP on outcome; subsequently, longer DUP has been described as one of the most replicated predictors of worse clinical, functional and cognitive outcomes [Reference Penttilä, Jääskeläinen, Hirvonen, Isohanni and Miettunen12], whereas the active psychotic symptoms after treatment initiation has been poorly understood as a risk factor of worse outcome.
Interestingly, we initially found a relationship between a longer DUP and poorer social functioning or non-recovery (Tables 1 and 2), according to previous research [Reference Santesteban-Echarri, Paino, Rice, González-Blanch, McGorry and Gleeson46], that did not remain significant in the multivariate analysis. This suggest that the association of DUP with functional outcomes is not independent but mediated by other predictors such as premorbid adjustment and other social factors. It has been previously suggested that DUP effects on functional outcomes during the first years of treatment may be mediated by social support and other social factors [Reference Norman47] and this may explain the inconclusive results in other neurotoxic effects such as cognition [Reference Bora, Yalincetin, Akdede and Alptekin48] or neuroimaging [Reference Anderson, Rodrigues, Mann, Voineskos, Mulsant and George19].
Only a limited number of studies have explored the impact of DAT or DAP on the evolution of the illness. Lyne et al. [Reference Lyne, Joober, Schmitz, Lepage and Malla27] reported that prolonged periods of DAP, but not DUP, were associated with negative symptoms at 18- and 24-month follow-up [Reference Lyne, Joober, Schmitz, Lepage and Malla27]. Barder et al. [Reference Barder, Sundet, Rund, Evensen, Haahr and ten Velden Hegelstad26] evaluated DUP and DAT (defined as the period in weeks per year with a score of at least 4 in any of the items of the PANSS positive subscale) in patients with an FEP and divided them into three equally sized groups [Reference Barder, Sundet, Rund, Evensen, Haahr and ten Velden Hegelstad26]. Only the subgroup with a longer DAT showed a significant intellectual decline during the 10-year follow-up. In accordance with these findings, we observed that longer DAT and DAP periods, but not DUP, were significantly associated with poorer social functioning at 3 years and with a lower likelihood of achieving functional recovery, that may be related to a poorer outcome on cognitive or negative symptomatology [Reference Santesteban-Echarri, Paino, Rice, González-Blanch, McGorry and Gleeson46].
Whereas DAT was the main predictor for social functioning in the long term in our study, functional recovery was primarily predicted by premorbid adjustment and educational level. Given that the definition of recovery includes returning to work or academic activities, a number of variables that are not directly related to the illness process may limit the recovery rates, such as personal, social and economic factors [Reference Jääskeläinen, Juola, Hirvonen, McGrath, Saha and Isohanni49].
When the ROC curves were analyzed, we only obtained a near acceptable value of 0.693 in the evaluation of thresholds of DAT for predicting social functioning. The optimal threshold of DAT, with a sensitivity of 0.68 and a specificity of 0.64 may help to discriminate the subjects with a poor social functional outcome and suggests that individuals with a total DAT less than 3.17 months may obtain greater benefit from symptomatic remission and relapse prevention. This cut-of suggests that even small periods of active psychotic symptoms may be associated to poorer social outcomes. The results regarding functional recovery do not allow for the establishment of an acceptable threshold for any of the periods. Premorbid adjustment and other predictor variables may moderate the relationship between active psychosis periods and recovery.
Our results suggest that the DAP period, as a modifiable variable, is the main focus of intervention to improve long-term functional results. Reducing the DAP period includes reducing the DUP, as previously stated, as well as the DAT period. Educational campaigns, improvements in health care networks and availability of assertive outreach teams seem to help reduce DUP periods [Reference Larsen, Joa, Langeveld and Johannessen50]. As a period of active positive symptoms, DAT involves initial remission and also the number of relapses and the duration of symptomatic relapse. In this regard, DAT may be reduced by improving remission rates and time to remission and preventing relapses. Engaging patients in effective specialized treatments may help to increase adherence to treatment [Reference Mayoral-van Son, Martinez-Garcia, Moreno, Parrilla-Escobar and Valdizan40] and reduce time to remission [Reference Jordan, Veru, Lepage, Joober, Malla and Iyer21] and specific interventions should be used to reduce substance that may worsen clinical outcome [Reference Oluwoye, Monroe-DeVita, Burduli, Chwastiak, McPherson and McClellan51]. Since up to a quarter of patients with a first episode of psychosis may be treatment resistant [Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha and Kolliakou52], early detection and treatment of refractory psychosis should be a main focus of intervention to improve remission. Early use of clozapine in individuals with refractory psychosis may reduce DAT by reducing time to remission in selected patients [Reference Agid, Remington, Kapur, Arenovich and Zipursky53]. Finally, improving adherence to medication as the best modifiable predictor of relapse in schizophrenia and first-episode psychosis patients may help to maintain symptomatic remission and reduce relapse rates [Reference Alvarez-Jimenez, Priede, Hetrick, Bendall, Killackey and Parker54].
Some limitations should be considered when interpreting our results. First, these results do not clarify the direction of causality between DAT and functional outcome. An alternative explanation is that a third variable associated with functional outcome is also associated with DAT. Second, the evaluation of the DAT period has been based on all possible information sources, including clinical records and prospective assessment. However, missing data and recall bias may be further limitations. Third, although the DAT definition is based on the persistence of positive symptoms, it is likely that other symptoms, such as negative symptoms, may have additional predictive value and toxicity effects. Finally, although our definitions of social functioning and functional recovery included standardized assessments and real-world outcomes, there is no consensus in these definitions, which may limit generalization of the results.
5. Conclusions
Diminishing the duration of active psychotic symptoms after the initiation of treatment is crucial to achieve long-term functional recovery. Specialized interventions aimed at improving remission rates and reducing relapses should result in shorter DAT periods and, subsequently, better functional outcomes. Mental health professionals may be fully aware of the modifiable factors that influence the functional recovery and design interventions to maximize clinical response and minimize the risk of relapse after clinical stabilization.
Funding sources
The study was carried out at the Hospital Marqués de Valdecilla, University of Cantabria, Santander, Spain, under the following grant supports: Plan Nacional de Drogas Research (2005-Orden sco/3246/2004); SENY Fundació (CI 2005–0308007); and Fundación Marqués de Valdecilla (API07/011); Gerencia Regional de Salud de Castilla y León (INT/M/04/17)
Unrestricted educational and research grants from AstraZeneca, Pfizer, Bristol-Myers Squibb, and Johnson & Johnson provided support for PAFIP activities. No pharmaceutical industry or institutional sponsors participated in the study design, data collection, analysis and interpretation of the results.
Declaration of interest
Dr. José María Pelayo-Terán has received lecture honoraria and travel support form Janssen Johnson & Johnson, Lundbeck, Otsuka Pharmaceuticals, GlaxoSmithkline and EiLilly.
Prof. Benedicto Crespo-Facorro has received honoraria for consulting/advisory boards from Otsuka Pharmaceuticals and lecture honoraria from Janssen Johnson & Johnson, Lundbeck, Roche and Otsuka Pharmaceuticals.
Prof. Rafael Tabarés-Seisdedos has received grants from or acted as a consultant for the following companies: AstraZeneca, Janssen, Eli- Lilly, Lundbeck, Novartis, Pfizer, Sanofi-Aventis, and Wyeth that were deposited into research accounts at the University of Valencia.
Dr. Rosa Ayesa-Arriola has received lecture honoraria and travel support form Lundbeck and Otsuka Pharmaceuticals.
Dr. Virginia Gajardo Galán, Dr. Marcos Gómez-Revuelta and Victor Ortiz-Garcia de la Foz report no additional financial support or other relationship relevant to the subject of this article.
Acknowledgements
This study was conducted as part of the clinical trial Comparative Study of Aripiprazole, Quetiapine and Ziprasidone in the Treatment of First-Episode Non-affective Psychosis (AZQ2005), ClinicalTrials.gov Identifier: NCT02305823.
The authors wish to thank the “Programa Asistencial de las Fases Iniciales de Psicosis” (PAFIP) research team and all patients and family members who participated in the study.
Appendix A Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eurpsy.2018.03.003.








Comments
No Comments have been published for this article.