An elevated serum cholesterol level is considered to be one of the major risk factors associated with atherosclerosis and CHD(Reference Brown and Goldstein1, Reference Khedkar, Mantri and Garge2) – the major cause of morbidity and mortality around the world(3, 4). The WHO predicts that by the year 2020, up to 40 % of all deaths will be related to CVD. Although drug-based therapy (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors or drugs) is currently being used to treat this condition(Reference Levine, Keaney and Vita5), it is often suboptimal, expensive and suffers from unwanted side effects(6–Reference Schuster8). Another natural cost-effective and safe alternative approach recently being explored to manage cholesterol-related problems is based on probiotic intervention. Probiotics particularly belonging to the genera Lactobacillus and Bifidobacterium as biotherapeutics have been investigated for reducing the level of cholesterol in the serum by virtue of bile salt hydrolase (Bsh) activity through direct impact on host bile salt metabolism(Reference De Smet, Van Hoorde and De Saeyer9–Reference Taranto and Font de Valdez12). Bsh-producing lactobacilli have the selective advantage of surviving and colonising the lower small intestine where the enterohepatic cycle takes place and, therefore, Bsh activity could be considered as one of the important colonisation factors(Reference De Smet, Van Hoorde and Woestyne13). Bile salts are secreted as bile into the duodenum in the form of N-acyl compounds conjugated with glycine or taurine(Reference Hofmann and Mysels14), and enhance the emulsification of lipids and aid in the absorption of lipid nutrients(Reference Salter, Chaplin and Dickerson15) apart from undergoing enterohepatic circulation(Reference Hofmann and Roda16). During this process, deconjugation of bile salts by Bsh results in the liberation of the amino acid residue and the formation of deconjugated bile acids. Deconjugated bile acids are excreted more rapidly from the intestinal tract than the conjugated ones. Since free bile acids are excreted from the body, the synthesis of new bile salts from cholesterol can reduce the total cholesterol concentration in the body(Reference Gilliland and Speck17). Furthermore, deconjugated bile salts are known to co-precipitate cholesterol at pH values lower than 5·5(Reference Klaver and van der Meer18) and bind to bacterial cells and dietary fibre, which enhance their faecal excretion(Reference Chikai, Nakao and Uchida19). Free bile salts are less effective detergents than conjugated bile salts and less efficient in forming mixed micelles(Reference Macdonald, Bokkenheuser and Winter20). Therefore, the deconjugation of bile salts can decrease the solubility of cholesterol(Reference De Smet, Van Hoorde and De Saeyer9, Reference Reynier, Montet and Gerolami21). In recent years, the possibility of using bile salt deconjugation by intestinal micro-organisms to lower serum cholesterol levels in vivo has received considerable attention(Reference De Smet, De Boever and Verstraete10, Reference Ha, Cho and Chai22, Reference Park, Jong and Young23). However, in order to bring these beneficial effects, the probiotic lactobacilli have to overcome the acidity of the gastric juices, numerous digestive enzymes, bile acid, intestinal peristaltic movement, immune response and low surface tension(Reference Gilliland, Nelson and Maxwell24). Therefore, to be effective as probiotics, lactic acid bacteria must survive the low pH of the stomach and arrive at the intestines as live microbes(Reference McDonald, Fleming and Hassan25). Although the cholesterol-lowering ability of probiotics and probiotic-based dairy foods has been investigated by different groups across the world(Reference Anderson and Gilliland11, Reference Taranto, Medici and Perdigon26–Reference El-Shafie, Yahia and Ali30), there is very little information available on direct or indirect involvement of Bsh activity associated with such cultures in this process(Reference De Smet, De Boever and Verstraete10, Reference Ha, Cho and Chai22, Reference Park, Jong and Young23, Reference Nguyen, Kang and Lee31). Moreover, whatever information that is available on this subject is mainly limited to probiotic strains of Western origin, which may not be as effective in expressing their bioactive functions in Indian subjects as in their own native population due to different gut ecology and food habits. The cholesterol-lowering functions of indigenous probiotic strains of lactobacilli have not been worked out as yet. Hence, the present study was primarily focused to tap the functional properties of putative probiotic Lactobacillus plantarum strains isolated from an Indian gut in a rat model with the long-term objective of using them as biotherapeutics for the management of high cholesterol in the Indian population.
Experimental methods
Bacterial strains
L. plantarum Lp91 and Lp21, the subject of the present study, were laboratory isolates recovered from an Indian gut whose identity and probiotic attributes were established previously in our laboratory as per FAO/WHO guidelines(32). L. plantarum NCDO82 (also designated as CSCC5276 or VTTE-71 034) used as a reference culture in the present study was procured through the courtesy of Dr N. P. Shah from Victoria University, Australia. Stock cultures were maintained in 15 % glycerol at − 70°C. Before their use, Lactobacillus cultures were subcultured three times in de Man, Rogosa and Sharpe (MRS) broth (Difco Laboratories, Detroit, MI, USA) supplemented with 0·2 % sodium thioglycolate (Sigma Chemical Company, St Louis, MO, USA) and incubated anaerobically (10 % H2, 10 % CO2 and 80 % N2) in a MK3 Anaerobic workstation (Don Whitley Scientific Limited, Shipley, West Yorkshire, UK) at 37°C for 18–22 h.
In vitro tolerance to pH 2·5 and 2 % bile
Initially, the cell suspension of each of the test cultures, namely Lp91, Lp21 and NCDO82, was prepared by pelleting the overnight grown cultures (37°C) followed by washing and resuspension in peptone saline at the rate of 109 colony-forming units (cfu)/ml. MRS medium preadjusted to pH 2·5 and 6·8 was inoculated individually with approximately 108 cfu/ml of Lp91 and Lp21 as well as NCDO82 followed by incubation at 37°C for 3 h. Viability was determined using the plate count method. An aliquot of 1 ml sample was drawn from each tube at 0, 1, 2 and 3 h, pelleted and washed, and tenfold serial dilutions were made using peptone saline diluent and plated on MRS-Thio agar followed by anaerobic incubation of plates at 37°C for 24 h.
Bile salt mix (Hi-Media, Mumbai, India) was added at 2 % to MRS-Thio broth for the determination of bile tolerance of Lactobacillus strains. Overnight grown cultures were pelleted (4300 g for 10 min at 4°C), washed, resuspended in peptone saline and inoculated (approximately 108 cfu/ml) into MRS broth with bile salt (2 %) and without bile salt, and their viability was determined using the plate count method. The cultures were incubated in the medium at 37°C for 0, 1, 2 and 3 h. The samples were collected, pelleted and washed, and cfu of the samples were determined by serially diluting in peptone saline water followed by plating onto the MRS agar medium and incubating at 37°C under anaerobic conditions for 24 h.
Bile salt hydrolase activity
Direct plate assay
Initially, the Bsh activity of the test cultures was assessed by the qualitative direct plate assay. Bacterial cells were streaked on MRS-Thio agar plates supplemented with 0·5 % (w/v) taurodeoxycholic acid sodium salt (filter sterilised) and 0·37 g of CaCl2/litre. The plates were incubated anaerobically at 37°C for 72 h, while MRS agar plates without supplementation of taurodeoxycholic acid sodium salt were used as controls. The presence of precipitated bile acid around colonies (opaque halo) or the formation of opaque granular white colonies with a silvery shine was considered as a positive reaction(Reference Dashkevicz and Feighner33, Reference Ahn, Kim and Lim34).
Resting cell bile salt hydrolase assay
The Bsh assay was performed by measuring the release of amino acids from hydrolysis of conjugated bile salts by Lactobacillus strains used in the study as described by Liong & Shah(Reference Liong and Shah35), Tanaka et al. (Reference Tanaka, Hashiba and Kok36) and Grill et al. (Reference Grill, Cayuela and Antoine37) with some modifications. Briefly, the test cultures were inoculated at 1 % into freshly prepared MRS-Thio broth and incubated at 37°C for 16–18 h. Exponentially grown cells were harvested (12 000 g for 30 min at 4°C), washed twice with peptone saline and resuspended in 0·1 m- sodium acetate buffer (pH 5·0) to give an optical density of 5·0 at 600 nm. The bacterial suspensions were incubated at 37°C with 10 mm (final concentration) sodium glycocholate. The reaction mixture (200 μl) contained 100 μl cell suspension and 100 μl of 20 mm-conjugated bile salt (sodium salt of glycocholic acid). Reaction mixes were incubated at 37°C for 30 min. The enzymatic reaction was terminated by the addition of an equal volume (200 μl) of 15 % TCA (w/v) followed by centrifugation at 18 000 g for 15 min. The amount of amino acids present in the supernatant was measured as described by Liong & Shah(Reference Liong and Shah35). An aliquot of 0·2 ml of supernatant obtained after centrifugation was added to 1 ml of distilled water and 1 ml of ninhydrin reagent (0·5 ml of 1 % ninhydrin in 0·5 m-citrate buffer, pH 5·5, 1·2 ml of 30 % glycerol and 0·2 ml of 0·5 m-citrate buffer, pH 5·5). The preparation was vortexed vigorously and kept in a boiling water-bath for 14 min. After subsequent cooling, absorbance was determined at 570 nm using glycine or taurine as standards. Bsh activities were quantified from the standard curve prepared for glycine or taurine. One unit of Bsh activity (U/ml) was defined as the amount of enzyme that liberated 1 nmol of amino acid from substrate per min per OD600.
Bile salt deconjugation
MRS-Thio broth supplemented with 6 mm-sodium glycocholate was inoculated with overnight grown culture at 1 % and incubated anaerobically at 37°C for 20 h. Bile salt deconjugation ability was measured as per the protocol given by Liong & Shah(Reference Liong and Shah35) based on the release of free cholic acid. After incubation, 10 ml culture of each organism was adjusted to pH 7·0 with NaOH (1 m) and centrifuged at 10 000 g at 4°C for 10 min. The pH of the supernatant thus obtained was adjusted to 1·0 with HCl (10 m). The assay reaction was set up by adding 1 ml of the supernatant into 2 ml of ethyl acetate followed by vortexing for 1 min. Then 2 ml of the ethyl acetate layer was transferred into a glass tube and evaporated under N2 at 60°C. The residue was immediately dissolved in 1 ml of NaOH (0·01 m). After complete mixing, 1 ml of furfuraldehyde (1 %) and 1 ml of H2SO4 (8 m) were added, and the mixture was vortexed for 1 min before heating at 65°C in a water-bath for 10 min. After cooling, 2 ml of glacial acetic acid was added, and the mixture was vortexed for 1 min. Absorbance was read at 660 nm. The amount of cholic acid released was determined from the cholic acid standard curve (Sigma Chemical Company).
Cholesterol assimilation
Water-soluble cholesterol (polyoxyethanyl-cholesteryl sebacate, Sigma) was dissolved in 50 % ethanol (5 mg/ml), filter sterilised and added to MRS-Thio broth supplemented with 0·3 % ox-bile at a final concentration of 80–100 μg/ml. Medium was inoculated with each test culture at the 1 % level and incubated anaerobically at 37°C for 20 h. After the incubation period, cells were centrifuged (10 000 g at 4°C for 10 min), and the remaining cholesterol concentration in the broth was determined using a colorimetric method as described by Liong & Shah(Reference Liong and Shah35). Then 1 ml of the aliquot was added with 1 ml of KOH (33 %, w/v) and 2 ml of absolute ethanol, vortexed for 1 min and left at 37°C for 15 min. After cooling, 2 ml of distilled water and 3 ml of hexane were added followed by vortexing for 1 min. Then 1 ml of the hexane layer was transferred into a glass tube and evaporated under N2. The residue was immediately dissolved in 2 ml of o-phthalaldehyde reagent (Sigma). After complete mixing, 0·5 ml of concentrated H2SO4 was added, and the mixture was again vortexed for 1 min. Finally, the absorbance was read at 550 nm after 10 min. The amount of cholesterol removed from broth was determined by subtracting the amount in each broth sample (μg/ml) from the amount present in the uninoculated control.
Co-precipitation of cholesterol with deconjugated bile
Freshly prepared sterile MRS-Thio broth was supplemented with 6 mm-sodium glycocholate. Filter-sterilised water-soluble cholesterol was added to the broth at a final concentration of 80–100 μg/ml. Co-precipitation of cholesterol with cholic acid formed was determined by the difference between cholesterol level in the control (inoculated MRS broth without bile) after the incubation period and the final cholesterol level in the inoculated MRS broth supplemented with sodium glycocholate.
Microencapsulation of probiotic lactobacilli
The probiotic bacterial cells were microencapsulated in sodium alginate matrix as described by Sheu & Marshall(Reference Sheu and Marshall38). A 4 % sodium alginate solution was prepared, sterilised by autoclaving (120°C for 15 min) and cooled to 38–40°C. Then 20 ml of sodium alginate solution and 4 ml of cell suspension (approximately 1011 cfu/ml) were transferred into a centrifuge tube (40 ml), and the contents were mixed homogenously. The alginate cell mixture was added drop wise to 100 ml of soyabean oil containing 0·2 % Tween 80 (emulsifier) with continuous magnetic stirring. Within 5 min, a uniformly turbid emulsion was obtained into which 0·1 m-CaCl2 (100 ml) was added quickly to break the emulsion and for the hardening of alginate microcapsules. The capsules were then harvested by gentle centrifugation at 1000 rpm for 10 min at 4°C and washed with 0·1 m-CaCl2. The beads were separated by decantation and stored in a refrigerator (7 ± 1°C).
Experimental animals
A total of thirty adult male Sprague–Dawley (SD) rats (mean body weight 150 (sem 10) g) were procured from the National Institute of Pharmaceutical Education and Research, Mohali, India, and were kept at the small animal house maintained at the Institute. The animals were divided into six homogeneous groups comprising of six animals each, housed in individual Al cages and maintained under a constant 12 h light–12 h dark cycle. Room temperature was controlled at 22–25°C with about 56–60 % relative humidity. Before conducting the animal trial, prior approval of the Institute's Animal Ethics Committee was obtained, and the mice were maintained in accordance with the National Institute of Nutrition, India guidelines for the care and use of laboratory animals.
Diet and experimental design
The base composition of the experimental diet is recorded in Table 1. Animal diets were formulated based on AIN-76. Table 2 provides the detailed formula of experimental diets used in the present study. Five experimental diets included a normal diet, a hypercholesterolaemic diet (HD), a HD with L. plantarum Lp91 (HD91), a HD with microencapsulated L. plantarum Lp91 (HDCap91) and a HD with L. plantarum Lp21 (HD21). The HD contained 0·5 % (w/w) supplemental cholesterol.
Table 1 Composition of experimental high-cholesterolaemic diet*

* Normal diet = hypercholesterolaemic diet − cholesterol.
† AIN-76 vitamin mixture.
‡ AIN-76 mineral mixture.
Table 2 Experimental diets of different treatment groups

NDCtrl, normal diet control; HDCtrl, hypercholesterolaemic diet control; HD91, HD containing L. plantarum Lp91; cfu, colony-forming units; HDCap91, HD containing microencapsulated L. plantarum Lp91; HD21, HD containing L. plantarum Lp21.
Preparation of diets
Overnight grown test cultures were pelleted at 5000 rpm for 30 min at 4°C and washed two times with saline. The cell pellet of each culture was mixed with the HD to achieve the final cell concentration of>108 cfu/g of diet. Microencapsulated L. plantarum Lp91 was mixed with the HD to achieve the desired cell concentration, and the cfu/g of diet was determined by suspending 1 g of diet in 9 ml of phosphate buffer saline (137 mm-NaCl, 2·7 mm-KCl, 10 mm-Na2HPO4 and 2 mm-KH2PO4, pH 7·4), and then the subsequent dilutions were made in peptone saline and plated on MRS-Thio agar to determine the exact cfu/g of diet.
Feeding schedule
Rats were fed a rodent chow diet for 7 d to remove the effect of stress experienced by the animals due to separation from the main stock and to become accustomed to the testing regimen. At the end of the adaptation period, all the groups were fed on their assigned experimental diets for the next 21 d. After 21 d of the treatment period, all the animals were fed on the rodent chow for a further 7 d, which was considered as the post-treatment period. During the entire course of the experiment, the rats had free access to water and to the group-specific diet (20 g/100 g body weight per d). Feed intake was recorded daily and body weight was measured weekly.
Blood sampling and analytical procedures
The overnight fasted rats were bled from the tail vein at the 21st day for plasma lipid analysis. The blood samples collected were placed in heparinised sterile microfuge tubes and centrifuged at 2000 g for 15 min at 4°C. The samples were analysed for total cholesterol, TAG and HDL-cholesterol using commercial enzymatic kits (Autopak, M/s Siemens Diagnostics Limited, Gujarat, India). Friedewald's equation(Reference Friedewald, Levy and Frederickson39) was applied to analyse the following other plasma lipid fractions:
(1) LDL-cholesterol = total cholesterol − HDL-cholesterol − (TAG/5). All the concentrations are given in mg/l.
(2) VLDL-cholesterol = The quotient (TAG/5) is used as an estimate of VLDL-cholesterol.
(3) Atherogenic index (AI) = The AI was calculated as (total cholesterol − HDL-cholesterol)/HDL-cholesterol.
Determination of faecal cholic acid
The faecal droppings of the experimental animals were collected over a 2 d period (days 19 and 20) using disposable wooden chopsticks to avoid contamination by feed or rodent hair. The samples were dried in an oven (55°C) for 2 d, and any remaining hair or dust contaminants were blown away using an air blower; the remaining material was finely ground using an analytical mill for further analysis. Faecal cholic acid levels were measured as per the published methods(Reference Reinhold and Wilson40–Reference Irvin, Johnston and Kopala43) with some modifications. Dried faeces (0·1 g) were extracted twice with 3·5 ml ethanol at 80°C for 1 h. After two extractions, the ethanol was evaporated under N2 gas at 50°C, and the residue was dissolved in 2·5 ml ethanol. Aliquots of these alcoholic extracts were evaporated to dryness in a water-bath, and the residue was dissolved in 2·5 ml of glacial acetic acid (60 %). Then 1 ml portions of the resulting solution were pipetted into each of two small test tubes, to which 6 ml of 8 m-H2SO4 was added. Then 1 ml of 1 % furfural solution (Hi-Media) in 60 % glacial acetic acid was added to one of these tubes, and 1 ml of 60 % glacial acetic acid was added to other tubes that served as a blank. The content of these tubes was mixed thoroughly, heated in a water-bath at 67°C for 15 min and cooled to room temperature. The absorbance of the resulting solutions was monitored at 610–690 nm with that of a suitable cholic acid (sodium salt) standard. Cholic acid standards were prepared as follows: 5 ml portions of two stock alcoholic solutions containing 0·25 and 0·5 mg of sodium cholate were evaporated to dryness and treated exactly as were the unknowns. The cholic acid concentration in faecal samples was calculated by deducting the colour equivalent of the blank tube from that of the tube containing furfural:

where ASF is the absorbance of the sample with furfural, ASB is the absorbance of the sample blank, ACF is the absorbance of the cholic acid standard with furfural, and ACB is the absorbance of the cholic acid standard blank.
Microbiological analysis of faecal samples
Faecal samples from the experimental SD rats were collected weekly in separate sterile tubes for microbial analyses. Samples for microbial analyses were processed within 1 h of collection. Each sample was homogenised with a stomacher using sterile PBS and peptone saline diluents. Subsequent tenfold serial dilutions of each sample were plated in triplicate. All the enumeration media were obtained from Hi-Media Pvt Limited (India). Nutrient agar was used for total plate count, whereas eosin methylene blue agar was used for Escherichia coli. Violet red bile agar was used for coliforms, and MRS agar was used for total lactobacilli. Plates of total lactobacilli were incubated anaerobically at 37°C for 48 h in an anaerobic workstation (Don Whitley Scientific Limited, Shipley, West Yorkshire, UK), while plates for the enumeration of total plate count, E. coli and coliforms were incubated at 37°C for 48 h.
Statistical analysis
Data analysis was carried out with SPSS, Inc. software (version 10.0). One-way ANOVA was used to study any significant difference between means with a significance level of P ≤ 0·05. Critical difference values were used to perform multiple comparisons between means. All the data are presented as means with their standard errors (n 6).
Results
The main focus of the present study was to assess the role of two putative Bsh-producing indigenous probiotic L. plantarum strains Lp91 and Lp21 on their survival at high bile salt concentration and their cholesterol-lowering effects both under in vitro and in vivo conditions. However, before evaluating the hypocholesterolaemic effect of these strains in SD rats, the survivability of the strains at acidic pH and high bile salt concentrations was checked under in vitro conditions along with the quantification of Bsh production, bile salt deconjugation, cholesterol assimilation and co-precipitation of cholesterol with deconjugated bile salts. The results obtained from these experiments are presented later.
Survivability of probiotic Lactobacillus strains at low pH and high bile concentration
The effect of acidic pH (2·5) on the survivability of Lactobacillus strains at different incubation periods has been recorded in Table 3. All the three strains exhibited adequate survival at pH 2·5 for 3 h despite marginal variations in the degree of viability. However, L. plantarum Lp91 exhibited the highest survival rate of 86·89 (sem 0·23) % after 1 h followed by L. plantarum Lp21 and NCDO82, which showed 85·07 (sem 0·68) and 84·71 (sem 0·61) % survival rates, respectively, after the same period. After 2 h, the survival rates of L. plantarum Lp91, Lp21 and NCDO82 were recorded as 74·87 (sem 0·66), 73·87 (sem 0·72) and 73·72 (sem 0·99) %, respectively. The highest decrease in survivability appeared after 3 h of incubation in respect of all the three strains of L. plantarum Lp91, Lp21 and NCDO82 examined in the present study. In general, L. plantarum Lp91 was more acid tolerant than L. plantarum Lp21 and NCDO82, which showed almost similar level of tolerance. However, statistical analysis of results obtained in this experiment did not reveal any significant differences (P>0·05) in the percentage survivability of the three Lactobacillus strains.
Table 3 Probiotic attributes of selected Lactobacillus strains
(Mean values with their standard errors, n 6)

Lp91, Lactobacillus plantarum Lp91; Lp21, L. plantarum Lp21; NCDO82, L. plantarum NCDO82.
* One unit of bile salt hydrolase activity has been defined as the liberation of 1nmol amino acid from substrate per min per OD600.
The presence of bile salts in the environment of bacterial cultures under in vitro conditions is much more detrimental than the effect of low pH. The concentration of bile salts used in this experiment was very high as a strong selective factor, while the in vivo bile concentration in the intestine is much lower. Data presented in Table 3 indicate that the most bile-tolerant strain was L. plantarum Lp91, which survived after 0, 1, 2 and 3 h of incubation in 2 % bile up to 96·82 (sem 0·81), 88·34 (sem 0·25), 76·23 (sem 0·39) and 62·5 (sem 0·71) %, respectively. On the other hand, L. plantarum Lp21 and NCDO82 survived bile treatment at the rate of about 61·93 (sem 0·35) and 60·16 (sem 0·98) %, respectively, after 3 h. All Lactobacillus strains exhibited resistance against high concentrations of bile salts, which is a normal characteristic of commensal gut flora. However, statistical analysis did not prove the significance of differences (P>0·05) observed between the survival of lactobacilli strains.
Bile salt hydrolase activity
The Bsh activity of the test cultures was estimated both by qualitative and quantitative assays.
Qualitative taurodeoxycholic acid sodium salt plate assay
The ability of Lactobacillus strains to hydrolyse taurodeoxycholic acid sodium salt was indicated by the precipitation of the bile salt along with the formation of opaque granular white colonies with a silvery shine around them on the medium plates (Fig. 1). The three strains differed in their taurodeoxycholic acid sodium salt hydrolysing activity as can be evidenced from the intensity of the precipitated opalescent zones, which was relatively higher in L. plantarum Lp91 as compared to the L. plantarum Lp21 and NCDO82 strains.

Fig. 1 Bile salt hydrolase activity of Lactobacillus plantarum Lp91, Lp21 and NCDO82 on solid de Man, Rogosa and Sharpe-Thio medium. Plates were incubated anaerobically for 72 h at 37°C. The control medium plate is shown in (a); the assay medium plate containing 0·5 % taurodeoxycholic acid sodium salt is shown in (b). The precipitation or the formation of opaque granular colonies with a silvery shine in the agar is indicative of bile salt hydrolase activity.
Quantitative ninhydrin assay
All the three Lactobacillus strains, when subjected to the resting cell ninhydrin assay (Table 3) to quantify their ability to hydrolyse glycocholic acid, showed that L. plantarum Lp91 and Lp21 possessed significantly (P ≤ 0·05) higher Bsh activity (99·29 (sem 4·46) and 88·63 (sem 2·50) nmol/ml per min, respectively) in comparison to NCDO82 (59·79 (sem 1·79) nmol/ml per min). By expressing strong Bsh activity, both Lp91 and Lp21 were expected to play a positive health-promoting role specifically by lowering serum cholesterol levels.
Bile salt deconjugation
L. plantarum Lp91, Lp21 and NCDO82 demonstrated deconjugation activity at different levels (Table 3). There were significant differences (P ≤ 0·05) among the Lactobacillus strains in the deconjugation of sodium glycocholate from suspension in the growth medium during 20 h of incubation. L. plantarum Lp91 and Lp21 deconjugated the highest level of sodium glycocholate as indicated by the release of 3·87 (sem 0·12) and 3·29 (sem 0·19) mm-cholic acid, respectively, in comparison to 2·99 (sem 0·28) mm-cholic acid released by NCDO82.
Cholesterol assimilation
The test strains were also able to remove cholesterol from MRS-Thio broth supplemented with 0·3 % ox-bile plus cholesterol during growth (Table 3). L. plantarum Lp91 and Lp21 could assimilate cholesterol appreciably as indicated by high cholesterol removal values, i.e. 68·97 (sem 0·63) and 68·54 (sem 0·71) μg/ml, which were significantly different (P ≤ 0·05) from L. plantarum NCDO82 (64·94 (sem1·37) μg/ml).
Co-precipitation of cholesterol with deconjugated bile
Cholesterol was co-precipitated concurrently with deconjugation of sodium glycocholate at varying levels (P>0·05), ranging from 2·39 to 3·77 μg/ml by all the three cultures (Table 3). Deconjugation of sodium glycocholate by L. plantarum Lp91 brought about a relatively higher amount of cholesterol co-precipitation (3·77 (sem 0·64) μg/ml) compared to L. plantarum Lp21 (3·12 (sem 0·75) μg/ml) and L. plantarum NCDO82 (2·39 (sem 0·61) μg/ml).
Cholesterol-lowering ability of bile salt hydrolase-positive Lactobacillus strains in Sprague–Dawley rat model
L. plantarum Lp91 and Lp21 were assessed for their cholesterol-lowering ability under in vivo conditions using the SD rat as the model system. L. plantarum Lp91 cells in encapsulated and non-encapsulated formats were separately mixed with experimental diets to achieve the final concentration of approximately 108 cfu/g. The final concentration of bacterial cells in the individual diets was ≥ 108 cfu/g at 0 h and 107 cfu/g after 24 h.
Body weight and feed efficiency
All the experimental rats used in the study remained healthy, and their body weight gain, feed intake and feed efficiency after 21 d were calculated and recorded for all the groups as indicated in Table 4. Statistical analysis revealed that mixed groups had a significant (P ≤ 0·05) effect on feed intake and feed efficiency ratio, while a non-significant (P>0·05) effect was found on initial body weight, final body weight and gain in weight compared to single groups. However, after 3 weeks of treatment, final body weight (216·83 (sem 6·08) g) of the normal diet control group (NDCtrl) and the HDCap91 group was lower in comparison to other groups (219·67 (sem 7·15) to 230·67 (sem 7·50) g) and did not differ significantly (P>0·05). The hypercholesterolaemic diet control (HDCtrl) group animals, which were fed on a cholesterol-enriched diet, showed a relatively higher body weight (238·33 (sem 9·22) g) in comparison to other experimental groups. The highest body weight gain was recorded in the HDCtrl group (90·67 (sem 2·43) g), while the body weight gain of the NDCtrl group was slightly lower than that of the other groups and did not differ significantly (P>0·05). Similarly, feed intake was higher in the HDCtrl group (174·35 (sem 2·78) g) than in probiotic treatment groups and the NDCtrl group. The feed efficiency in rats fed HD91 (54·42 (sem 5·20) was greater than the HDCtrl (52·84 (sem 1·33)), NDCtrl (51·49 (sem 2·38)), HDCap91 (46·89 (sem 1·86)) and HD21 (41·99 (sem 1·50)) groups.
Table 4 Body weight, weight gain and feed efficiency of rats fed control and experimental diets
(Mean values with their standard errors, n 6)
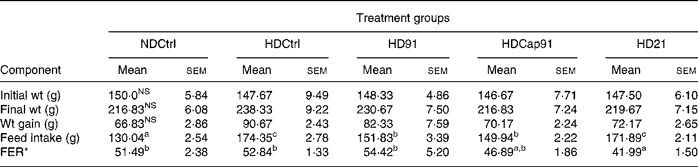
NDCtrl, normal diet control; HDCtrl, hypercholesterolaemic diet control; HD91, HD containing L. plantarum Lp91; HDCap91, HD containing microencapsulated L. plantarum Lp91; HD21, HD containing L. plantarum Lp21; FER, feed efficiency ratio.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
NS P>0·05.
* FER = wt gain (g)/feed intake (g) × 100.
Plasma lipid profile
The effect of dietary treatments on plasma lipid profile (plasma total cholesterol, HDL-cholesterol, LDL-cholesterol, VLDL-cholesterol, TAG and AI) has been recorded in Table 5 and Fig. 2. Total plasma cholesterol values for each dietary treatment group were recorded as 768·29 (sem 41·01), 1037 (sem 39·9), 795·7 (sem 75·9), 874·1 (sem 59·2) and 881·3 (sem 62·2) mg/l for the NDCtrl, HDCtrl, HD91, HDCap91 and HD21 dietary groups, respectively. Groups HD91, HDCap91 and HD21 showed decreases in plasma cholesterol levels of 23·26, 15·71 and 15·01 %, respectively, compared with the HDCtrl group after 21 d of dietary treatment. These three groups also demonstrated a lowering of LDL-cholesterol at a significant level. LDL-cholesterol values for each group were recorded as 293·7 (sem 46·7), 607·8 (sem 50·7), 376·1 (sem 83·2), 466·7 (sem 51·2) and 477·6 (sem 72·5) mg/l for the NDCtrl, HDCtrl, HD91, HDCap91 and HD21 groups, respectively. The reduction in LDL-cholesterol was 38·13, 23·22 and 21·42 % in the HD91, HDCap91 and HD21 groups, respectively, while HDL-cholesterol showed the opposite trend. HDL-cholesterol values for all the five groups were recorded as 317·8 (sem 31), 202·3 (sem 11·5), 240·6 (sem 13·5), 223·1 (sem 14·2) and 218 (sem 10·1) mg/l, respectively, and the corresponding increases for HDL-cholesterol in the probiotic treatment groups, i.e. HD91, HDCap91 and HD21, were 18·94, 10·30 and 7·78 %, respectively. On the basis of statistical analysis, it was found that plasma HDL-cholesterol concentrations differed significantly among all the experimental groups (P ≤ 0·05). Similarly, plasma TAG and VLDL-cholesterol concentrations also differed significantly (P ≤ 0·05) among all the groups throughout the experiment. After dietary treatment, TAG and VLDL-cholesterol levels in the HD91, HDCap91 and HD21 groups were reduced by 21·09, 18·77 and 18·17 %, respectively. The AI of the dietary treatment groups of rats also differed significantly (P ≤ 0·05). The AI of treatment groups increased sharply after feeding on the hypercholesterolaemic diet in comparison to that recorded with the NDCtrl group (1·514 (sem 0·232)). However, the AI of the probiotic treatment groups was found to decrease after 21 d of treatment as compared with the HDCtrl group (4·247 (sem 0·458)). The maximum decline in AI was recorded in the HD91 group (2·415 (sem 0·472)) in comparison to the HDCap91 (2·937 (sem 0·203)) and HD21 groups (3·104 (sem 0·364)).
Table 5 Plasma total cholesterol, TAG, HDL-, LDL- and VLDL-cholesterol concentrations (mg/l) in rats fed experimental diets
(Mean values with their standard errors)

AI, atherogenic index; NDCtrl, normal diet control; HDCtrl, hypercholesterolaemic diet control; HD91, HD containing Lactobacillus plantarum Lp91; HDCap91, HD containing microencapsulated L. plantarum Lp91; HD21, HD containing L. plantarum Lp21.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* AI = (total cholesterol − HDL-cholesterol)/HDL-cholesterol.

Fig. 2 Percentage decrease in plasma lipids (total cholesterol (![]() ), TAG (
), TAG (![]() ), HDL (
), HDL (![]() )-, LDL (
)-, LDL (![]() )- and VLDL (
)- and VLDL (![]() )-cholesterol) of dietary treatment groups. NDCtrl, normal diet control; HDCtrl, hypercholesterolaemic diet control; HD91, HD containing Lactobacillus plantarum Lp91; HDCap91, HD containing microencapsulated L. plantarum Lp91; HD21, HD containing L. plantarum Lp21.
)-cholesterol) of dietary treatment groups. NDCtrl, normal diet control; HDCtrl, hypercholesterolaemic diet control; HD91, HD containing Lactobacillus plantarum Lp91; HDCap91, HD containing microencapsulated L. plantarum Lp91; HD21, HD containing L. plantarum Lp21.
Faecal cholic acid excretion
As shown in Fig. 3, faecal cholic acid levels differed significantly among the various groups (P ≤ 0·05). Supplementation of the HD with Bsh-positive L. plantarum Lp91 and Lp21 led to considerable increases in the excretion of cholic acid in the faeces. After 21 d of feeding, the maximum faecal cholic acid excretion (5·489 (sem 0·09) μmol/g faeces) was recorded in the HDCap91 group, whereas in the other probiotic treatment groups HD91 and HD21, the corresponding increase in faecal cholic acid was 5·084 (sem 0·09) and 5·183 (sem 0·13) μmol/g, respectively. However, in animals fed on the normal diet, the faecal cholic acid content was found to be 1·084 (sem 0·09) μmol/g, which was lower than the HDCtrl group (1·703 (sem 0·04) μmol/g).

Fig. 3 Faecal cholic acid concentration for rats fed experimental diets. NDCtrl, normal diet control; HDCtrl, hypercholesterolaemic diet control; HD91, HD containing Lactobacillus plantarum Lp91; HDCap91, HD containing microencapsulated L. plantarum Lp91; HD21, HD containing L. plantarum Lp21.
Faecal microbial analysis
The overall mean ( ± sem) faecal bacterial counts at 0, 7, 14, 21 and 28 d obtained from different groups have been recorded in Table 6. Rats supplemented with the probiotic treatment diets showed relatively higher total bacterial counts (8·991–9·629 cfu log10/g) in the faecal samples as compared to the control groups. However, total bacterial counts decreased progressively in all the treatment groups during the post-treatment period (28 d). Although the presence of faecal lactobacilli was consistently recorded in all the groups with some variation (P ≤ 0·05), the rats fed on Lactobacillus strains as a dietary adjunct maintained a high level of lactobacilli in the faecal samples throughout the experiment compared with control groups. The maximum faecal Lactobacillus count was observed in HDCap91 group rats compared with HD91 and HD21 group rats. After the post-treatment period (28 d), faecal lactobacilli counts were significantly higher in rats fed on L. plantarum Lp91 and Lp21 than those in the HDCtrl and NDCtrl groups. Total lactobacilli ranged from 8·782 to 9·717 cfu log10/g of faeces in all the experimental groups. These findings clearly indicate that feeding of Bsh-active lactobacilli as a dietary adjunct resulted in a more stable shift of the intestinal lactobacilli population than feeding of the high-cholesterol and normal diet alone. However, discontinuation of Lactobacillus feeding in all probiotic treatment groups resulted in a consistent decrease in faecal lactobacilli. Rats supplemented with the diet containing L. plantarum Lp21 showed a marginal decrease in faecal E. coli counts compared with the HDCtrl group during the dietary treatment and post-treatment period. Total coliforms ranged from 7·707 to 7·966 cfu log10/g among all the treatment groups.
Table 6 Effect of probiotic feeding on faecal bacterial population
(Mean values with their standard errors, n 6)

CFU, colony-forming unit; NDCtrl, normal diet control; HDCtrl, hypercholesterolaemic diet control; HD91, HD containing Lactobacillus plantarum Lp91; HDCap91, HD containing microencapsulated L. plantarum Lp91; HD21, HD containing L. plantarum Lp21.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
NS P>0·05.
Discussion
It is now well documented that hypercholesterolaemic conditions are the predisposing factors associated with an increased risk of CHD. Lowering of total cholesterol and LDL-cholesterol in the serum/plasma of hypercholesterolaemic patients reduces the incidence of CVD. One of the possible strategies that could be promising and cost effective in lowering cholesterol levels in serum is by modulating diets through probiotic interventions. This approach could be very effective in the management of CVD, including strokes. Probiotic lactobacilli are considered as normal components of the intestinal microflora in both humans and animals, and have been invariably associated with various health-promoting functions. It is in this context that probiotics are gaining prominence and are used as dietary adjuncts in the development of novel probiotic foods for health applications. Probiotic bacteria are mostly delivered in a food system and must be acid and bile tolerant to survive in the human gastrointestinal tract. The transit time from entrance to release from the stomach has been estimated to be approximately 90 min, with further digestive processes requiring longer residence time(Reference Berada, Lemeland and Laroch44). Stresses to organisms begin in the stomach with a pH between 1·5 and 3·0, and in the upper intestine that contains bile(Reference Corzo and Gilliland45, Reference Lankaputhra and Shah46). The survival and tolerance of probiotic cultures are considered essential for expressing their physiological functions optimally(Reference Usman47). The present study was initially aimed at investigating the role of two Bsh-positive L. plantarum strains Lp91 and Lp21 in their survival under high bile concentration along with cholesterol-lowering ability under in vitro conditions before assessing their functional efficacy in vivo in a rat model. Strains of lactobacilli used in the present study showed varying levels of viability at pH 2·5 and 2 % bile salts after 3 h. Many authors have also investigated the effect of bile on survival of lactic acid bacteria with varying degree of survivability/inhibition(Reference Kim, Ren and Dunn48–Reference Rönkä, Malinen and Saarela50).
In the present study, all the three Lactobacillus strains could deconjugate and hydrolyse glycocholic acid and assimilated cholesterol micelles in their cellular membrane along with co-precipitation of cholesterol in the presence of deconjugated cholic acid. There was a good correlation between bile salt deconjugation and Bsh activity in the three Lactobacillus strains. The present results in this regard are in agreement with those of Klaver & van der Meer(Reference Klaver and van der Meer18) who theorised that while bile salts were deconjugated and the pH of the fermentation media was dropped due to natural acid production by culture, cholesterol micelles destabilised and cholesterol co-precipitated with free bile acids. Although the precise mechanism of the cholesterol-lowering activity of lactic acid bacteria has not yet been worked out completely, probiotics may alter serum cholesterol by two possible mechanisms: (1) directly binding dietary cholesterol into the small intestine before cholesterol can be absorbed into the body(Reference Gilliland and Walker51–Reference Pereira and Gibson53) and (2) bile salt deconjugation by Bsh to produce free bile acids(Reference Gilliland and Speck17, Reference Park, Jong and Young23, Reference Liong and Shah35). Free bile acids thus formed by the deconjugation of conjugated bile salts are less soluble and are less likely to be reabsorbed by the intestinal lumen compared to bile salts, and are lost from the human body through faeces(Reference Center and Micheal54, Reference Begley, Hill and Gahan55). This could lead to a higher metabolism of cholesterol and, subsequently, could result in the reduction of serum cholesterol(Reference Reynier, Montet and Gerolami21). However, the development of any therapy, even those using biological products, requires methodological research of the potential side effects to ensure their safety for health applications. Since large amounts of deconjugated bile salts may have undesirable effects for the human host, concern may arise over the safety of administering a Bsh-active probiotic strain. However, the positive feature that goes in their favour is that bacterial genera that would most likely to be used as probiotics (Lactobacillus and Bifidobacterium) are not capable of dehydroxylating deconjugated bile salts as reported by Takahashi & Morotomi(Reference Takahashi and Morotomi56), Ahn et al. (Reference Ahn, Kim and Lim34) and Begley et al. (Reference Begley, Hill and Gahan55), and hence the majority of the breakdown products of Bsh activity by probiotic strain may be precipitated and excreted out in the faeces.
The Bsh activity of probiotics has often been correlated with their in vivo cholesterol-lowering effect(Reference De Smet, De Boever and Verstraete10, Reference Ha, Cho and Chai22, Reference Park, Jong and Young23). To establish the role of Bsh in cholesterol lowering, the effect of potential Bsh-producing L. plantarum Lp91 strain in its microencapsulated and non-encapsulated form was examined in the present study in a rat model along with L. plantarum Lp21 (non-capsulated). During the last decade, many studies have been conducted on experimental animal models and human subjects to establish the cholesterol-reducing ability of probiotic cultures, i.e. Lactobacillus spp. and Bifidobacterium spp.(Reference Usman and Hosono27, Reference Kawase, Hashimoto and Hosoda57). However, there are only very few specific reports available on the effects of Bsh-positive L. plantarum on serum lipid profiles in either animal models or human subjects(Reference Ha, Cho and Chai22). In the present study, experiments were designed specifically to elucidate the role of Bsh in cholesterol lowering in SD male rats. The present study shows that Bsh-active L. plantarum strains could reduce plasma total cholesterol and LDL-cholesterol in rats fed a diet high in cholesterol. Supplementation of the diet with L. plantarum Lp91 (HD91) resulted in a significant reduction in plasma total cholesterol, LDL-cholesterol and TAG by 23·26, 38·13 and 21·09 %, and a significant enhancement of 18·94 % in HDL-cholesterol in hypercholesterolaemic rats. Similarly, plasma total cholesterol, LDL-cholesterol and TAG values obtained from HDCap91 and HD21 probiotic treatment groups were also significantly lower than for the hypercholesterolaemic control group, and HDL-cholesterol values of these groups were higher than in the hypercholesterolaemic control group. Although encapsulated Lp91 did reduce total cholesterol, LDL-cholesterol and TAG along with increased HDL-cholesterol in plasma, these values were on the lower side than those recorded with Lp91 as such. This variability in results with regard to Lp91 under encapsulated v. non-encapsulated conditions could be attributed to the release of fewer cells in a free form from the encapsulated preparation in the lower gut, thereby restricting the availability of Bsh for bile acid deconjugation and also due to the loss of encapsulated Lp91 in faeces. Nguyen et al. (Reference Nguyen, Kang and Lee31) investigated the hypocholesterolaemic effects of Bsh-active L. plantarum PH04 in a mouse model, and recorded 7 and 10 % reduction in serum cholesterol and TAG. In a similar study, Ha et al. (Reference Ha, Cho and Chai22) used a Bsh-producing L. plantarum strain, which had been isolated from human faeces, to reduce serum cholesterol levels in SD male rats, and recorded 27·7 and 28·66 % reductions in total cholesterol and LDL-cholesterol. The AI for the probiotic treatment groups decreased significantly (P ≤ 0·05) when compared with the hypercholesterolaemic control group. These observations are consistent with the findings of previous reports carried out on similar lines(Reference Kawase, Hashimoto and Hosoda57), indicating a significant decrease in AI of probiotic treatment groups. De Smet et al. (Reference De Smet, De Boever and Verstraete10) had reported the use of enhanced Bsh activity of the L. reuteri strain to reduce serum cholesterol levels in pigs fed on a high-cholesterol diet. However, only a few reports are available on the use of the Bsh-active L. plantarum strain(Reference De Smet, Van Hoorde and De Saeyer9, Reference Ha, Cho and Chai22) to reduce serum cholesterol levels in rats or other animal models. The use of microencapsulated Bsh-active L. plantarum in reducing plasma cholesterol levels in rats had never been reported to the best of our knowledge. This is perhaps the first study wherein the anti-hypercholesterolaemic effect of microencapsulated L. plantarum Lp91 has been demonstrated in rats fed on a HD. However, the in vitro bile acid deconjugation ability of isogenic microencapsulated L. plantarum 80 strain was studied, and it was found that microencapsulated L. plantarum 80 cells could efficiently break down glycodeoxycholic acid and taurodeoxycholic acid(Reference Jones, Chen and Ouyang58). It was shown in the present study that faecal cholic acid excretion increased throughout the experimental period in the probiotic treatment groups, while significantly higher excretion was observed in groups fed on diets containing Bsh-active L. plantarum strains than control groups of rats (Fig. 3). Higher excretion of cholic acid through faeces in the HDCap91, HD91 and HD21 groups indicated that changes in bile salt metabolism had been brought about by ingested Bsh-active L. plantarum strains. This clearly points to a shift towards a more Bsh-active Lactobacillus population in the rat gut, which might influence the bile salt metabolism to reduce the amount of bile salts available for resorption. Consequently, the feedback inhibition mechanism of bile salt synthesis is decreased, and the synthesis of bile salts from cholesterol increased, yielding reduced serum cholesterol levels.
Although the effects of probiotic lactobacilli on different bacterial counts in faeces had been investigated previously by different groups(Reference Usman and Hosono27, Reference Liong and Shah29, Reference Akalin, Gonc and Duzel59), the literature specifically on the effect of Bsh-active Lactobacillus strains on faecal bacterial counts in animal models is quite scanty. In this investigation, we had specifically examined the effects of experimental diets containing potentially Bsh-active Lp91 and Lp21 strains on faecal bacterial population. Surviving passage through the gastrointestinal tract is considered to be important for probiotics to function effectively in the intestine(Reference Gilliland and Walker51). In the present study, faecal microbial analysis revealed significantly higher faecal lactobacilli counts in probiotic treatment groups compared to that with the control groups. This could be attributed to their ability to survive at low pH and high bile concentration as described previously in in vitro experiments. Occurrence of high lactobacilli counts during the post-treatment period (28 d) in groups fed on Bsh-active L. plantarum strains clearly suggests possible involvement towards the contribution of Bsh in gut colonisation as reported previously by Dussurget et al. (Reference Dussurget, Cabanes and Dehoux60). However, no significant effect on faecal coliforms and E. coli counts was recorded in groups fed on L. plantarum strains, while a significant decrease in faecal E. coli and coliform counts has been reported previously due to probiotic feeding in rats(Reference Usman and Hosono27, Reference Liong and Shah29). The outcome of the present study demonstrates that consumption of L. plantarum Lp91 as a probiotic dietary adjunct might be useful in reducing human serum cholesterol level. However, well-designed placebo-controlled clinical trials need to be conducted to validate the efficacy and safety of the strain and its use in the management of high cholesterol in humans. The alteration in bile salt metabolism through enhanced bile salt hydrolase activity might also affect cholesterol lowering more directly by influencing its solubility and intestinal absorption(Reference Reynier, Montet and Gerolami21, Reference Barr, Russ and Eder61) and by enhanced faecal excretion of deconjugated bile acids(Reference Park, Jong and Young23). Based on the 1–2 rule(Reference Begley, Hill and Gahan55, Reference Frick, Elo and Haapa62), which states that a 1 % reduction in serum cholesterol causes a 2 % lowering of the risk for CHD, a significant positive effect for patients suffering from elevated cholesterol might be obtained by ingesting lactobacilli to improve hydrolysis of bile salts. In order to further elucidate the precise role of Bsh in cholesterol lowering, our next target is to assess the effect of microencapsulated recombinant Bsh and Lactobacillus clone in animal models for establishing its bioactive functionality, which forms the subject of a separate communication.
Acknowledgements
The present research was financially supported by the Department of Biotechnology, India. The authors greatly appreciate the financial support received from the Indian Council of Agricultural Research (ICAR, India) in terms of providing fellowship to the first author of the paper to carry out his doctoral programme. R. K., S. G. and V. K. B. conceived, conceptualised and supervised the present study; R. K. performed the experiments; R. K., S. G. and V. K. B. performed the analysis and interpreted the data; S. G. and V. K. B. contributed reagents/materials/analysis tools; R. K., S. G. and V. K. B. were involved in the preparation of the manuscript and have no conflict of interest.











