To the Editor—The genus Cryptococcus spp comprises ~70 species,Reference Smith, Sehring, Chambers and Patel1 of which the C. neoformans–C. gattii complex are considered pathogenic. However, non-neoformans cryptococci, such as Cryptococcus laurentii and Cryptococcus albidus, are emerging as opportunistic pathogens, causing disease in patients with impaired cell-mediated immunity (eg, HIV-infected patients or those with hematologic malignancies), in those using steroids or chemotherapeutic agents, and in those with invasive devices.Reference Khawcharoenporn, Apisarnthanarak and Mundy2 Cryptococcus laurentii has been isolated from several sources such as Norway spruce trees (Picea abies) and trembling aspen trees (Populus tremuloides), bird droppings, and perihospital areas.Reference Ferreira-Paim, Ferreira and Andrade-Silva3 Human infections due to C. laurentii have been described as causing fungemia, central nervous system infection, skin and soft-tissue infections, and pneumonia, among others.Reference Khawcharoenporn, Apisarnthanarak and Mundy2 The finding of yeasts in a urine culture of asymptomatic patients is considered colonization. Recent guidelines suggest that the treatment of asymptomatic candiduria consists of eliminating risk factors such as indwelling catheters and witholding treatment except in patients with risk factors for dissemination (eg, neutropenic patients, very-low-birth-weight infants, and prior to urologic procedures).Reference Pappas, Kauffman and Andes4 Regarding non-neoformans cryptococcuria, the information is lacking; therefore, we aimed to describe a case series about the clinical characteristics and outcomes of hospitalized patients with in-hospital isolation of C. laurentii from urine cultures.
We performed a retrospective case series in Dr. Alfredo Badallo General Hospital in Mexico. We included all hospitalized patients with at least a urine culture positive for C. laurentii from 2015 to 2018, identified by Vitek 2 and phenotypic tests. Clinical data and outcomes were extracted from the medical records. The follow-up after the isolation of C. laurentii was between 7 and 210 days, and the data were reported with descriptive statistics. The study was approved by our institutional review board.
In total, 10 patients were identified with cryptococcuria by C. laurentii, of which 4 were men and 6 were women. Half of these patients were older than 70 years. The patients had several comorbidities, with a median Charlson index of 3 (range, 1–5) (Table 1).
Table 1. Demographic Data, Clinical Characteristics, and Outcomes of Patients With Urinary Cryptococcus laurentii
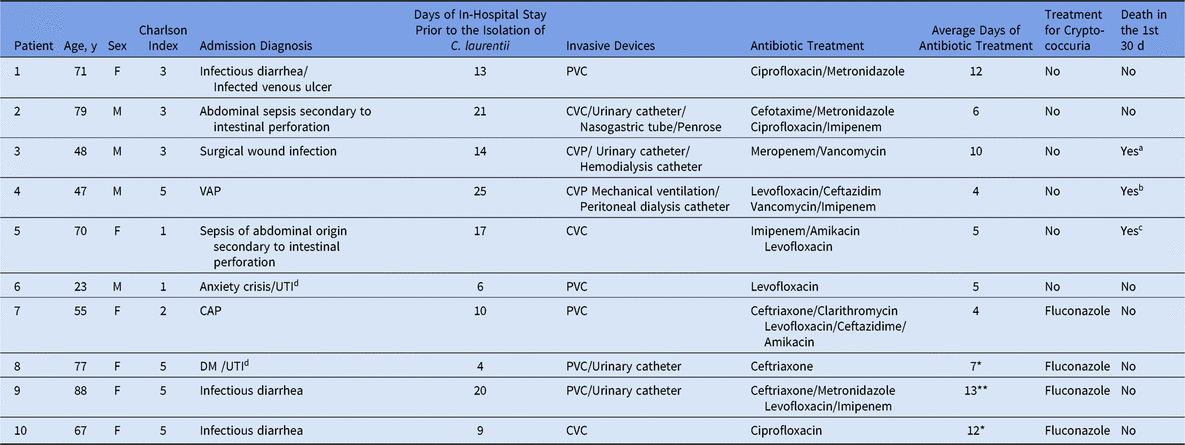
Note. M, male; F, female; PVC, peripheral venous catheter; CVC, central venous catheter; UTI, urinary tract infection; DM2, type-2 diabetes mellitus 2; CAP, community-acquired pneumonia; VAP; ventilator-associated pneumonia.
* Patients with negative controls after treatment.
** Patient with change of treatment after a new positive culture for C. laurentii after treatment, with negative control after second treatment.
a Death at 30 d in a subsequent hospitalization due to septic shock secondary to community-associated pneumonia.
b Death after 7 d in the same hospitalization due to septic shock secondary to Pneumonia associated with mechanical ventilation.
c Death at 7 d in the same hospitalization due to septic shock secondary to sepsis of abdominal origin.
d Patients who were admitted with a clinical data of urinary tract infection without positive cultures.
Six patients had a history of previous hospitalizations within the prior year. During their last hospitalization, the average length of stay was 9 days (range, 3–18), and these prior admissions were associated with their underlying comorbidities. An infectious diagnosis was the reason for admission in all 10 patients (100%), and the patients received antibiotic therapy with a range of 2–3 antibiotics per patient, with an average of 8 days of use (range, 4–13 days) (Table 1). The antibiotics mainly used were levofloxacin, imipenem, ciprofloxacin, ceftriaxone, and metronidazole.
Six patients were cultured from other areas; 2 had negative blood cultures, and 1 had a S. epidermidis blood isolate. Three patients with skin and soft-tissue cultures had S. epidermidis, Escherichia coli, and Acinetobacter baumanni, respectively. No other microorganisms were found in urine or blood with C. laurentii.
Clinicians started treatment with fluconazole in 4 patients; urine cultures were negative in 2 of these patients after treatment. Recurrent funguria with C. laurentii occurred in 1 patient, and therapy with itraconazole was started without follow-up urine cultures. Of the 4 patients treated with fluconazole, 3 survived free of symptoms, and 1 died of complications of liver cirrhosis in a subsequent hospitalization 7 months later. The other 6 patients did not receive treatment for C. laurentii; 2 of these died during the same admission, and another died within the first 30 days during a subsequent hospitalization. The other 3 patients survived.
This case series includes patients with urinary C. laurentii of nosocomial origin, and our patients had had antecedent hospitalizations. Remarkably, a high proportion were older patients, and all had received broad-spectrum antimicrobial therapy before the isolation of C. laurentii.
Isolates of C. laurentii of nosocomial origin have been reported in a recent molecular study, in which 26% were of common origin showing the same haplotype.Reference Ferreira-Paim, Ferreira and Andrade-Silva3 C. laurentii, as a nosocomial infection, has already been described. A systematic review of non-neoformans cryptococci demonstrated a significant association between the use of invasive devices and C. laurentii bloodstream infection (aOR, 8.7; 95% CI, 1.48–82.9).Reference Khawcharoenporn, Apisarnthanarak and Mundy2 A recent case report of a patient requiring invasive mechanical ventilation with a long hospital stay, central venous catheter use, and prolonged duration of broad-spectrum antibiotics including echinocandin, developed a C. laurenttii bloodstream infection.Reference Smith, Sehring, Chambers and Patel1 Another case report described a cystic fibrosis patient with a prior C. laurentii paranasal sinus colonization who developed pneumonia and disseminated cryptococcosis after sinus surgery.Reference Warren, Franco-Palacios, King and Shlobin5
In our case series, the clinicians considered treating the cryptococcuria in some patients; however, the sample size did not allow us to reach conclusions regarding whether treatment of asymptomatic urinary C. laurentii was beneficial; none of these patients developed cryptococcal disease without treatment. On the other hand, the treatment of C. laurentii infection (eg, fungemia or central nervous system infection) does require urgent treatment, mainly with amphotericin B because since C. laurenti has shown resistance to azoles in up to 50% of tested strains.Reference Bernal-Martinez, Gomez-Lopez and Castelli6,Reference Arendrup, Boekhout, Akova, Meis, Cornely and Lortholary7
In conclusion, C. laurentii is an opportunistic pathogen of immunosuppressed or severely ill hospitalized patients, and a critical risk factor is the previous use of antibiotic therapy. However, the isolation of urinary C. laurentii in the correct clinical setting may be nonsignificant. What to do in patients at risk, such as neutropenic patients, and patients before urologic instrumentation who have a urine culture positive for C. laurentii, has not yet been determined.
Acknowledgments
We would like to thank the microbiology laboratory and the medical education department from The Hospital General de Zona 27.
Financial support
No financial support was provided.
Conflicts of interest
The authors declare no conflict of interests.



