Micronutrient intake at low level is essential for normal cellular metabolism; the role of vitamin C in collagen biosynthesis has been established for 70 years. Latterly, effects of micronutrients have been investigated using systems biology approaches(Reference Aldred, Sozzi, Mudway, Grant, Neubert, Kelly and Griffiths1, Reference Weissinger, Nguyen-Khoa and Fumeron2). However, limiting factors to the widespread application of proteomics in nutrition include the limited signal:noise ratio, where variability between subjects complicates the analysis of effects of a nutrient and highlights the need for experimental validation of potential biomarkers of nutrient effect.
In addition to the difficulty of inter-individual variability, the availability of material for study ex vivo is normally restricted to biological fluids such as plasma and cerebrospinal fluid(Reference Grant, Scheel-Toellner and Griffiths3) or less-invasive samples of tears, urine or saliva. Analysis of the plasma proteome is also complicated by the large dynamic range of protein concentrations, over ten to twelve orders of magnitude, which further compounds the difficulty in measuring low-frequency proteins(Reference Rose, Bougueleret and Baussant4, Reference Lescuyer, Hochstrasser and Rabilloud5). Plasma has an essential buffering role and may accumulate products secreted from living and dying cells, particularly if these products are ineffectively cleared or if they are produced in excess, for example by tumours(Reference Polley, Mulholland, Pin, Williams, Bradburn, Mills, Mathers and Johnson6). Approaches to improve the reproducibility of proteomic methods for application to plasma and other biological fluids have recently been investigated and strategies which reduce the variability imposed by depletion of abundant proteins have been described(Reference de Roos, Duthie and Polley7).
Proteomic technologies have been successfully applied to improve understanding of cellular effects of micronutrients in vitro and have led to novel hypotheses concerning pathways that may be regulated by micronutrients(Reference Aldred, Grant and Griffiths8). Peripheral blood mononuclear cells (PBMC) may constitute a useful surrogate model of cellular responses in vivo in which to validate markers of nutrient effect which have been determined in vitro by proteomics.
In addition to its role in collagen biosynthesis, vitamin C has variably been reported to improve immune function and decrease cardiovascular risk, possibly by interfering with reactive oxygen species signalling(Reference Li and Schellhorn9–Reference Douglas, Hemilä, Chalker and Treacy11). Up-regulation of collagen matrix production has been detected in SHSY5 cells(Reference Grant, Barber and Griffiths12) by proteomics, and using T cells exposed to vitamin C in vitro, we have previously demonstrated that vitamin C increases the expression of phosphatidylinositol transfer protein (PITP) within 5 min of ascorbic acid treatment, an observation which was confirmed by Western blotting(Reference Grant, Mistry, Lunec and Griffiths13). Proteins which showed a decrease in expression in this model included the caspase 3 recruitment domain, which if observed in vivo, may contribute to enhanced cell survival following vitamin C supplementation, and the K+ channel, which may affect physiological potassium homeostasis.
In order to evaluate whether a reductionist approach using in vitro proteomics can identify possible markers of nutrient effects in vivo, we have isolated PBMC from healthy volunteers taking part in a vitamin C supplementation study. The effect of vitamin C supplementation on PBMC apoptosis, plasma K+ levels and PBMC PITP expression was investigated pre-, during and post-supplementation. If it is possible to use proteomics to interrogate in vitro models we suggest that this may provide an efficient process to identify novel biomarkers of nutrient functional effect in vivo which can be further validated in intervention studies.
Experimental methods
The study of T cell protein expression was undertaken in a random subset of subjects (n 55) taking part in a larger trial (n 209)(Reference Lunec14). The subject recruitment protocol and study design for the larger trial is described later.
Intervention study design
The vitamin C intervention study was carried out according to strict protocols approved by Leicestershire, Rutland and Northamptonshire Ethics Committee, Leicestershire Partnership Trust Research Offices and University Hospitals of Leicester NHS Trust.
Subjects (209) were recruited on to the trial from the Leicestershire area following response to local advertisements. Eligibility of each subject was determined by completion of a confidential questionnaire outlining the inclusion and exclusion criteria of the study. The principal inclusion criteria were healthy individuals of 18 years or over who were not supplementing their diet with any mineral or vitamin and had no history of gastrointestinal irritation, thalassaemia or haemochromatosis. Exclusion criteria were set to include subjects who may have been at increased risk of adverse side-effects following supplementation with vitamin C, smokers who are known to have altered vitamin C status(Reference Northrop-Clewes and Thurnham15) and women who were or thought they might be pregnant. If subjects were found to be eligible for inclusion on to the trial, written and informed consent was obtained.
The intervention study was designed as a double-blind randomised controlled trial. Upon recruitment, each subject was randomly assigned to one of four study groups comprising group A (100 mg vitamin C/d), group B (500 mg vitamin C/d), group C (2000 mg vitamin C/d) and group D (placebo). At week 0, subjects were given sufficient supply of tablets for the remainder of the trial and received the appropriate dose of vitamin C by taking two tablets per day, one in the morning and one in the evening. Both vitamin C and placebo tablets were supplied by DHP pharma (Crickhowell, Powys, UK) and placebo was composed of microcrystalline cellulose.
At week 0, subjects were required to attend the phlebotomy clinic at the Clinical Research Unit, Leicester Royal Infirmary NHS Trust in order to provide a fasting (12 h overnight) blood sample (50 ml). Subjects returned at intervals of 1, 5 and 10 weeks to provide further blood samples.
Processing of blood samples
Each blood sample was collected into lithium/heparin vacutainers (Sarstedt, Leicester, UK) and an EDTA vacutainer and was stored on ice for a maximum of 2 h before separation of leucocytes or plasma, as described later.
Isolation of peripheral blood mononuclear cells from whole blood
PBMC were isolated from whole blood by density gradient centrifugation, which produced a high yield of mononuclear cells with minimal granulocyte contamination. Briefly, 1·5 ml Histopaque 1077 (Sigma-Aldrich, Poole, UK) was aliquotted into a 15 ml centrifuge tube and 10 ml of whole blood (diluted 1:1 with PBS) was carefully layered on top. Following centrifugation at 400 g for 30 min at 18°C, blood components were separated into four layers; the uppermost layer containing plasma was removed for vitamin C analysis and the second layer containing PBMC was collected. PBMC were subsequently washed twice with PBS and centrifuged at 700 g for 30 min at 18°C prior to storage at − 80°C.
Measurement of intracellular and plasma vitamin C
Intracellular vitamin C was determined in PBMC isolated from whole blood (4·5 ml) collected into EDTA. Vitamin C was stabilised by addition of an equal volume of 10 % metaphosphoric acid (Sigma-Aldrich) prior to storage at − 80°C.
HPLC analysis was undertaken using a Perkin Elmer isocratic LC pump (model 250) with 15 mmol/l phosphate buffer containing 9 % methanol, pH 6·0, ESA model 542 autosampler (ESA Analytical Ltd, Aylesbury, UK) and a Dionex UV detector, model UVD340U (Dionex Ltd, Camberley, UK). Samples were separated using a Luna 5 μm C18 (2) HPLC column (150 × 4·60 mm; Phenomenex, Macclesfield, UK) and collected using Chromeleon™ software, version 6.0 (Dionex Ltd). Linearity of standards (>99·9 % purity; Sigma-Aldrich) was achieved for concentrations up to 100 μmol/l vitamin C with sample intra- and inter-assay CV of < 10 %. Plasma and intracellular vitamin C concentration was calculated from a known standard curve; intracellular ascorbate concentrations were calculated relative to sample protein content (μmol/mg protein) as a direct reflection of cell number.
Phosphoinositol transfer protein expression analysis in peripheral blood mononuclear cells
For SDS–PAGE and Western blotting, randomised samples were selected for pooling according to supplementation dose and duration. Three independent pools of ten volunteers were collected for each dose and time-point (i.e. from 120 PBMC samples). Samples were lysed into hypotonic 2-amino-2-hydroxymethyl-1,3-propanediol (Tris) hydrochloride (100 μl; 40 mmol/l) homogenised and incubated (at room temperature with rotation, 30 min) in the presence of pan-protease inhibitors (1:100 Focus-Protease Arrest; Merck, Nottingham, UK).
Lysates were centrifuged at 13 000 g for 5 min to remove cellular debris prior to analysis of supernatant for protein content. Equal amounts (2 μg) of each of ten PBMC lysates were pooled and three pooled samples were electrophoresed and blotted as described later.
Western blotting for phosphoinositol transfer protein
Protein samples (20 μg) were separated by one-dimensional SDS–PAGE (12·5 % gel) and electroblotted on to polyvinylidene fluoride membrane(Reference Grant, Mistry, Lunec and Griffiths13). After electroblotting at 20 mA for 16 h for PITP, membranes were blocked in Tween 20 (1 %) Tris-buffered saline (TTBS) containing 3 % bovine serum albumin for 2 h. Membranes were incubated with either a mouse monoclonal antibody against PITP raised against native recombinant human PITP with no reported cross-reactivity (sc-13 569; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or mouse monoclonal antibody against actin which recognises β and γ actin in human samples (ab1081; Abcam, Cambridge, UK) overnight. The membranes were washed with TTBS (6 × 15 min) before incubation with a peroxidase conjugated secondary antibody (Sigma Aldrich) for 2 h (15:100 000, diluted in TTBS containing 0·3 % bovine serum albumin). Membranes were rinsed in Tris-buffered saline (6 × 15 min) and then a chemiluminescent substrate (ECL Plus, Amersham Biosciences, Little Chalfont, UK) was used to visualise detected proteins. The images were recorded using a GS 710 calibrated imaging densitometer (Biorad, Hercules, CA, USA). Bands were analysed and quantified using Scion software (National Insitutes of Health, Bethesda, MD, USA).
Caspase 3 activity
Caspase activity was determined individually in the leucocytes of the subset of volunteers randomly selected for PITP expression analysis. For this analysis, leucocytes were thawed into lysis buffer comprising 1 % Triton and 10 mmol/l dithiothreitol with 0·5 mmol/l phenylmethylsulphonyl fluoride. Protein concentration was again determined for each sample using the bicinchoninic acid assay(Reference Smith, Krohn and Hermanson16) and lysates were analysed for caspase 3-like activity against the synthetic substrate aspartate-glutamate-valine-aspartate (DEVD)-aminomethylcoumarin (25 μmol/l; Merck). After 1 h in the dark, DEVD-aminomethylcoumarin release was determined as fluorescence which was read at 460 nm following excitation at 380 nm(Reference Nicholson, Ambereen Ali and Thornberry17).
Blood potassium measurement
Analysis was carried out on plasma samples obtained from whole blood collected into Li/H and was undertaken at the Department of Chemical Pathology, Guy's and St Thomas' Hospital NHS Foundation Trust. All samples were assayed using a Roche Modular Analyser with the ISE1800 and P800 sections of the module.
Statistics
Potassium levels, PBMC protein and caspase 3 data are presented as the median value with 25th and 75th percentiles and sample range with statistical analysis using GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego, CA, USA).
Analysis was performed using a Friedman's test with post hoc analysis using the Dunn post test. Groups of data were evaluated statistically by paired comparison analysis, where differences were considered significant when P < 0·05.
Correlations were calculated with a Spearman correlation coefficient (two-tailed test of significance).
Results
Fig. 1 confirms that there was no significant difference in protein yield from the PBMC isolated from subjects receiving dietary vitamin C according to the dose of supplement given. However, there was a trend towards reduced protein yield after 1 week, which was seen in all samples irrespective of supplementation group and including placebo. After PBMC harvest, the cells were split into equal aliquots according to cell volume recovered rather than cell number. The lower protein yield suggests that the total PBMC recovery was reduced following the first sampling. In addition, we noted that protein yield from primary PBMC was significantly lower compared to cultured HSB-CCRF-2 cells.

Fig. 1 Protein recovery from snap-frozen peripheral blood mononuclear cells isolated from fifty-five healthy subjects at four time-points during vitamin C supplementation (A, placebo; B, 100 mg/d; C, 500 mg/d; D, 2 g/d). Cells were subsequently thawed into hypotonic lysis buffer in the presence of specific protease inhibitors and protein was determined by the bicinchoninic acid assay. Values are medians and inter-quartile ranges with complete data ranges depicted by vertical bars. There was no significant effect of vitamin C dose on protein yield at any time-point for any of the doses studied.
Figs. 2 and 3 illustrate that endogenous caspase 3 activity in PBMC isolated from the peripheral blood of subjects receiving vitamin C supplements is highly variable between individuals and that supplementation does not affect caspase 3 activity significantly at any dose or at any time-point during the intervention. There was a trend for increased caspase 3 activity after 1 week of supplementation with 2 g vitamin C/d and for a decrease in caspase 3 activity with 2 g ascorbate/d after 10 weeks.

Fig. 2 Lysates from peripheral blood mononuclear cells do not show increased caspase 3 activity following differing doses of vitamin C supplementation in healthy subjects (n 55) for 1 (A), 5 (B) and 10 (C) weeks. Cellular debris was removed by centrifugation and the supernatant incubated with aspartate-glutamate-valine-aspartate (DEVD)-aminomethylcoumarin as a synthetic caspase 3 substrate. Released fluorescence, indicative of caspase 3 activity, was measured at 460 nm following excitation at 380 nm and corrected for protein content. Values are medians and inter-quartile ranges with complete data ranges depicted by vertical bars. There was no significant effect of ascorbate dose on caspase 3 activity as analysed by one-way ANOVA followed by Dunnett's multiple comparison test.

Fig. 3 Lysates from peripheral blood mononuclear cells (n 55) do not show increased caspase 3 activity following vitamin C supplementation (A, placebo; B, 100 mg/d; C, 500 mg/d; D, 2 g/d) at any time-point up to 10 weeks of supplementation. Cellular debris was removed by centrifugation and the supernatant incubated with aspartate-glutamate-valine-aspartate (DEVD)-aminomethylcoumarin as a synthetic caspase 3 substrate. Released fluorescence, indicative of caspase 3 activity, was measured at 460 nm following excitation at 380 nm and corrected for protein content. The data are presented as the median, inter-quartile range and complete data range. There was no significant effect of ascorbate supplementation time on caspase 3 activity as analysed by one-way ANOVA followed by Dunnett's multiple comparison test.
To further investigate whether intracellular ascorbate status was related to caspase 3 activity in vivo, the two parameters were correlated using Spearman's rank method. From these data no relationship was found between cellular ascorbate and caspase 3 activity in the cell lysates (Fig. 4). The levels of measurable ascorbate were very tightly skewed towards the limit of detection for the vitamin C assay, whereas the caspase 3 activity levels that were recorded showed a closer distribution to normality.

Fig. 4 Caspase 3 activity in peripheral blood mononuclear cells isolated from subjects (n 55) post-vitamin C supplementation does not correlate with cellular ascorbate levels.
As a decrease in the K+ transporter was observed in T cells exposed to vitamin C in vitro, the effect of supplementation in vivo on plasma K+ levels was investigated. No significant effect of vitamin C dose or supplement duration was observed on blood K+ levels (Fig. 5).
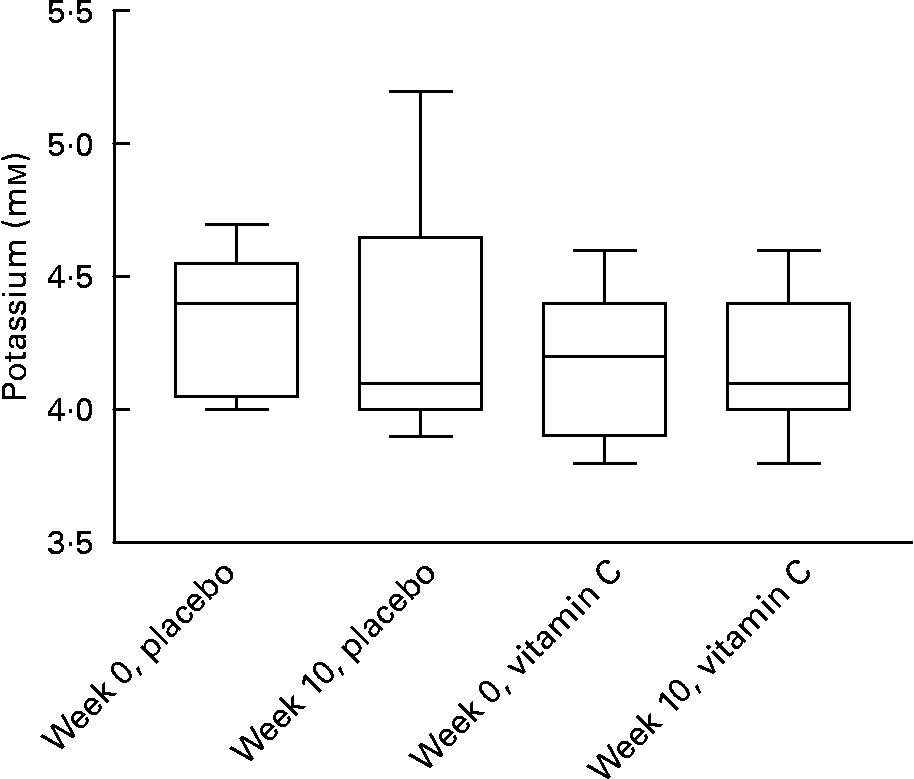
Fig. 5 Plasma potassium levels in healthy subjects are not affected by supplementation with vitamin C (2 g/d) for 10 weeks. Values are medians and inter-quartile ranges with complete data ranges depicted by vertical bars.
To confirm whether the in vitro CCRF model of ascorbate exposure was a model for the effects seen on the PBMC proteome in vivo, PITP was analysed in three different pools of ten subject PBMC proteomes under each intervention regimen and matched across all time-points, as described in the Experimental methods, by Western blotting. Fig. 6 (A) shows representative Western blots from one subject pool for each group, dose and time. Scanned images were quantitated using Scion software and PITP was expressed relative to actin for each of three sample pools under each of the four intervention regimens. Fig. 6 (B) demonstrates the linearity of actin band intensity relative to protein loading, up to 30 μg protein per lane and validates the use of normalisation methodology to adjust for errors in protein loading. Integration of band intensities confirmed that PITP was significantly elevated after 5 and 10 weeks of supplementation with vitamin C (2 g/d) compared with the same subjects at baseline (Fig. 6 (C)).

Fig. 6 (A) Phosphoinositol transfer protein (PITP) levels are increased following ascorbate supplementation (2 g/d) in healthy volunteers. Proteomes from three pools of ten independent volunteers in each of the supplementation groups were extracted and subjected to one-dimensional PAGE with Western blotting for PITP or actin. (B) Representative standard curve of peripheral blood mononuclear cell lysate protein loaded and actin band intensity (r 2 0·94). Different amounts of lysate (0, 10, 20 or 30 μg protein) were separated by SDS–PAGE, subject to Western blot for actin and the integral of the resultant bands were used to confirm linearity of band intensity. M, marker. (C) Blots were scanned and interrogated using Scion software and PITP band intensities are expressed relative to the actin band integral for each group at each dose. 0, 1, 5, 10 refer to weeks of intervention. Mean values were significantly different from those of week 0: *P < 0·05.
Discussion
In the present paper we have investigated whether our previous study of the effects of vitamin C on T cell protein expression in vitro is a predictor for changes in protein expression or function in vivo using plasma and PBMC isolated from supplemented individuals.
As expected, inter-individual variability proved to be high in spite of the population size; at the extreme, caspase 3 activity variance was four times the median recorded value. The outliers responsible for the extended whiskers to the box plots represent caspase activity in PBMC from individual subjects which was not sustained over time. It is not clear why such variation exists, although increased apoptosis of leucocytes is reported post-infection(Reference Pilione, Agosto, Kennett and Harvill18).
In vitro we showed a loss of the caspase 3 recruitment domain protein between 2 and 8 h, but an increase at 24 h which we considered may result in a protective effect of short-term exposure to ascorbic acid, but that prolonged exposure to high doses of ascorbic acid may prime the cell for apoptosis(Reference Grant, Mistry, Lunec and Griffiths13); induction of DNA damage in individuals supplemented with high doses of vitamin C has previously been shown by Anderson et al. (Reference Anderson, Phillips, Yu, Edwards, Ayesh and Butterworth19). In vitro, caspase 3 activity in HSB-CCRF-2 cells was significantly greater after culture with 10 μmol/l ascorbate than 1000 μmol/l ascorbate after 24 h(Reference Lunec14). Others have also shown using propidium iodide staining for detection of apoptosis, that incubation of primary PBMC ex vivo with 0·2 mg/ml vitamin C for 24 h caused a 39 % increase in the percentage of apoptotic cells, as compared to those kept at the same incubation conditions without vitamin C(Reference Bergman, Salman, Djaldetti, Fish, Punsky and Bessler20). In the context of determining apoptotic effects of vitamin C in vitro, there are several possible confounders which should be considered and are reviewed by Halliwell(Reference Halliwell21).
Blood potassium levels remained unaffected by vitamin C supplementation in vivo despite a decrease in K+ channel expression in T cells in vitro. The major organ responsible for maintaining blood potassium is the kidney and if a decrease of channel expression occurred in vivo on supplementation, a change in K+ transport into T cells is unlikely to affect overall homeostasis.
Our earlier in vitro proteomic studies identified PITP as a candidate marker of ascorbate effect which was mobilised within 5 min of exposure to ascorbate and was sensitive to ascorbate concentration. PITP are ubiquitous proteins that transport lipids, such as phosphatidylinositol and phosphatidylcholine, between membranes and thus have important roles in signalling(Reference Allen-Baume, Segui and Cockcroft22). Using pooled PBMC from three sets of ten independent donors at each dose and time-point, increased mobilisation of the signalling protein PITP was observed which is indicative of a vitamin C-dependent priming effect within the PBMC population in vivo (Reference Hay and Martin23). It is important to note that PITP expression is detectable at low levels in PBMC isolated from peripheral blood and this is consistent with a role in cell signalling and metabolism.
The normalisation of PITP protein expression level in PBMC to actin levels may be questioned owing to over-exposure of actin blots (a facet of the low expression levels of PITP relative to actin and the need to load greater sample volumes on gels in order to visualise both proteins on the same gel/blot). Over-exposed actin blots are included to illustrate the amount of cellular extract loaded for analysis of PITP, however, when actin was visualised after lower PBMC loading concentrations, PITP was below the level of detection in re-probed blots. Moreover, a standard curve of protein loaded against actin integral showed highly significant correlation (P < 0·0001). In the absence of normalisation to actin, the same trend of increasing PITP with vitamin C dose and duration of supplement is observed, however, there is large variability between different pools.
Pooling of samples was necessary as the median protein concentration of lysates was 2 mg/ml (Fig. 1), the concentration of protein required to be loaded on to SDS–PAGE for visualisation of PITP. To avoid the potential bias introduced by analysis of only those lysates of protein concentration >2 mg/ml, pooling was undertaken. There are clear disadvantages of such an approach, for example, only a subset of healthy subjects may show an increase in PITP and individual sample analysis offers further information on inter-individual variation as reported here for the caspase assay. Further studies are required to evaluate phosophoinositide metabolism in individual subjects following vitamin C supplementation in order to determine whether the changes in protein expression reported here have biological significance.
In conclusion, the present study has demonstrated that PITP is modulated by ascorbate in PBMC in vitro and in vivo. In contrast, functional changes in apoptosis or K+ homeostasis were not observed. Elevated expression of PITP suggests increased PBMC activation following exposure to ascorbate and confirms the value of in vitro modelling for identifying suitable biomarkers of effect or exposure in vivo. It also highlights the importance of verification of observations made in cellular systems using human intervention studies as false positives may emerge from in vitro studies alone.
In addition to complexity in the technology which creates ‘high dimensional’ data sets(Reference Aldred, Sozzi, Mudway, Grant, Neubert, Kelly and Griffiths1), the genetic variability in human studies adds further complications to the application of proteomics to nutritional intervention studies in vivo. One of the key issues in considering nutrition in healthy subjects is that homeostatic mechanisms which serve to maintain normal physiology are, by definition, likely to reduce any effects of diet on the proteome. By adopting an early biomarker search strategy using a proteomic approach in cell lines and then examining the potential for such markers to be affected in vivo, the analysis of human populations may be undertaken. Future advances in bioinformatics and multiple component analysis to enable analysis of large numbers of gel data sets (>100) each with up to 1000 spots per gel, will provide a route to work through such inter-individual variability and large sample sizes to define consistent patterns of effect without the need for sample pooling or reductionism(Reference Zhang, Lu and Shi24).
Acknowledgements
The authors gratefully acknowledge financial support from the Food Standards Agency, UK (TO1026). H. R. G. analysed protein, caspase activity, actin and was principal author of the manuscript. R. S. W. analysed PITP and measured cellular protein content. N. M. was responsible for subject recruitment, PBMC preparation and ascorbate analysis. M. M. G. developed the PITP WB assay and advised on methodology. J. L. was principal applicant on T01026, was responsible for project ethics and organised supplementation. R. J. B. was responsible for data collection and analysis. The authors declare no conflicts of interest.








