In the search for factors contributing to the increasing burden of chronic diseases in developed countries, research interest has focused on nutrition from as early as fetal life. Fetal nutrition may influence the subsequent risk of chronic childhood and adult diseases, among them CHD, hypertension and non-insulin-dependent diabetes (Godfrey & Barker, Reference Godfrey and Barker2000). Unbalanced food intake together with metabolic changes occurring during pregnancy may influence the mother's health in increasing the risk of obesity, which may be a risk factor for metabolic conditions including gestational diabetes mellitus and hypertension (Pallard et al. Reference Pallard, Herranz, Garcia-Ingelmo, Grande, Martin-Vaquero, Janez and Gonzalez1999; Vasan et al. Reference Vasan, Larson, Leip, Kannel and Levy2001; Verma et al. Reference Verma, Boney, Tucker and Vohr2002), pregnancy weight increase being the strongest predictor of sustained weight retention (Rössner & Öhlin, Reference Rössner and Öhlin1995; Gunderson et al. Reference Gunderson, Abrams and Selvin2000).
Despite the importance of maternal nutrition on fetal well-being, only limited data are available on nutrient intake and nutrient requirements during pregnancy, and prospective studies, in particular, are lacking. Thus recommendations for dietary intake and even for weight gain during pregnancy are inconsistent (Abrams et al. Reference Abrams, Altman and Pickett2000) and there are no specific dietary recommendations for women with allergic disease. Mothers with atopic disease may restrict their diet due to symptoms of the disease (Hoppu et al. Reference Hoppu, Kalliomäki and Isolauri2000) and additionally they may modify their diet to reduce the risk of the disease in the infant (Sicherer, Reference Sicherer2002). Likewise, irrespective of the mother's own atopic disease status, manipulation of the maternal diet has been a common practice in an attempt to reduce the risk of disease in the infant (Arvola & Holmberg-Marttila, Reference Arvola and Holmberg-Marttila1999). This may, however, result in an unbalanced diet and even increase the risk of atopic disease in the child, as was shown in a study where maternal high intake of SFA during breast-feeding was associated with atopic sensitization of the infant (Hoppu et al. Reference Hoppu, Kalliomäki and Isolauri2000).
Dietary counselling of pregnant and breast-feeding mothers would be of importance in effecting nutrient intake potentially beneficial to both the mother and the child. To implement such counselling for populations of pregnant women, further information is needed on both the requirements of nutrients during pregnancy and the effects of counselling on the intake of foods and nutrients. The present study was undertaken to assess the impact of dietary counselling on dietary intake in a prospective cohort study of pregnant women. The counselling was designed to modify dietary intake to that recommended at the time of study initiation (Nordic Nutrition Recommendations, NNR; Nordic Working Group on Diet & Nutrition, 1996), with a particular focus on the amount and type of fat in the diet, and to ensure the growth and development of the fetus.
Subjects and methods
Subjects and study design
The study population comprised pregnant women participating in a prospective ongoing mother and infant nutrition and probiotic study. Women were recruited at their first visit to a maternal welfare clinic in the city of Turku and neighbouring areas in South-West Finland. The subjects were healthy and had no chronic or metabolic diseases, although those with an atopic disease were included. At entry they were randomized to a dietary intervention group receiving dietary counselling and food products and a control group. A total of 231 pregnant women were recruited, 215 of whom attended all study visits at each trimester of pregnancy. Of these subjects, 140 were randomized to the dietary intervention and sixty-nine to the control group. Additionally, the women in the intervention group were randomized in a double-blind manner to receive either probiotic or placebo capsules (which do not contain any nutrients) and the controls received placebo. The reasons for discontinuing the study were: miscarriage (n 4); moving to another town (n 1); diseases not related to pregnancy (migraine, mental problems; n 4); no particular reason (n 7). One woman in the first and the second and four women in the third trimester of pregnancy failed to complete food records and were excluded, leaving a total of 209 women in the final analysis. Written informed consent was obtained from the women, and the study was approved by the Ethical Committee of the Hospital District of South-West Finland.
Clinical evaluation
Height at the first research visit and weight and blood pressure at each visit were measured by a nurse. Weight prior to pregnancy was self-reported and used for calculation of pre-pregnancy BMI as weight (kg) divided by the square of the height (m2). Women were classified according to BMI (World Health Organization, 1988) as underweight (BMI < 20·0 kg/m2), normal weight (20·0 kg/m2 ≤ BMI < 25·0 kg/m2), overweight (25·0 kg/m2 ≤ BMI < 30·0 kg/m2) or obese (BMI >30 kg/m2). Total gestational weight gain was calculated by subtracting self-reported pre-pregnancy weight from the weight recorded at the prenatal visit or at hospital within one week before delivery. Energy requirements were estimated using pre-pregnancy weight, a physical activity level of 1·56 and 285 kcal being added during the second and third trimester of pregnancy (World Health Organization, 1985). Information on age, smoking, parity, education and all episodes of aerobic physical activity exceeding 30 min a time were obtained by interview. Birth data on the infants were obtained from hospital records.
Atopic disease in the pregnant women was defined as a history of self-reported atopic eczema, allergic rhinitis, asthma or adverse reactions to foods together with a positive skin prick test result measured at the last trimester of pregnancy, as described previously (Kalliomäki et al. Reference Kalliomäki, Salminen, Arvilommi, Kero, Koskinen and Isolauri2001; Laitinen et al. Reference Laitinen, Sallinen, Linderborg and Isolauri2006).
Dietary counselling and food products
At each study visit women in the intervention group were given detailed dietary counselling by a nutritionist aiming to modify dietary intake to comply with that recommended for pregnant women at the time of initiation of the study in 2002 (NNR; Nordic Working Group on Diet & Nutrition, 1996). Specifically, dietary counselling focused on the amount and the type of fat and the amount of fibre in the diet. The subjects were encouraged to increase their consumption of vegetables, fruits and wholegrain bread and cereals, to consume leaner meat products, low-fat cheese and milk products, and to use vegetable oil or soft margarine as a spread and in food preparation. Fish was recommended as one of the main meals twice a week. Practical dietary advice was given, adjusted to the women's current dietary habits and food diary analysis. Achievement of the recommended diet was supported by providing the mothers with conventional food products with favourable fat (e.g. low erucic acid rapeseed oil-based spreads and salad dressing) and fibre content (e.g. fibre-enriched pasta, breakfast muesli and porridge cereals) for use at home. The consumption of specified amounts of the food products was advised, but adjustment for current dietary habits was allowed. The recommended amounts of foods were planned to provide 19 g of MUFA and 19 g of PUFA. This, combined with the advised consumption of other foods, would result in MUFA contributing 10–15 % of energy intake (% energy), PUFA 5–10 % energy and SFA 10 % energy or less. Total intake of fat would be 30 % energy, carbohydrates 55–60 % energy and protein 10–15 % energy. Compliance with consuming the food products was evaluated by comparing their consumption with those advised immediately before study visits assessed by 3-day food records and overall use during the 12-week period between the study visits by interview.
Food records
Food and nutrient intakes were assessed using 3-day, including one weekend day, food records with household measures at each trimester of pregnancy. The subjects were given personal and written instructions on recording, and the records were reviewed for completeness and accuracy by a nutritionist with the aid of a portion picture booklet. If needed, missing portion sizes and food descriptions were added after discussions with the women, and the type, brand and preparation method of all foods used were recorded. Daily energy and nutrient intakes were calculated using the Micro-Nutrica® computerized program version 2.5 (Research Centre of the Social Insurance Institution, Turku, Finland), which uses the Food and Nutrient Database of the Social Insurance Institution and is continuously updated with data on commercial foods. Both groups received written feedback of the calculation and the intervention group also received advice on how to alter the diet to conform better to the recommended diet. Data on use of vitamin and mineral supplements were obtained by interview and the intakes of nutrients from the supplements were calculated separately.
Statistics
Results are presented as mean and standard deviation (sd) or mean with 95 % CI or proportions of the cohort. For baseline characteristics, the group means were compared by independent-samples t test for continuous variables tested for normal distribution by the Shapiro–Wilk test, and by Pearson χ2 test or Fisher's exact test for dichotomous variables. Pearson's correlation coefficient analysis was used to evaluate the association between energy intake and total gestational weight gain. Repeated-measures ANOVA was performed to establish any significant difference between intervention and control groups for dietary variable, and also by time (over the pregnancy), and time × group interactions for estimated energy requirement, blood pressure and physical activity. Huynh–Feldt adjustment was performed if the Maucly's Test of Sphericity P value was less than 0·05. Differences between the groups at each trimester were further examined by calculating 95 % CI. Significant time × group interactions were further examined by reanalysing the effect of the time factor within the intervention or control group with repeated-measures ANOVA to establish whether changes during pregnancy differed between women receiving and women not receiving dietary counselling. When significant time × group interactions were detected, 95 % CI were calculated for mean differences between trimesters to ascertain the timing of the change. The effects of women's pre-pregnancy BMI and episodes of aerobic physical activity on energy intake were evaluated by adding them to the analysis as a covariate. The impact of atopic disease on dietary intake was evaluated by independent-samples t test at first study visit as dietary counselling did not influence differently women with or without atopic disease. The level of significance used was P < 0·05. All statistical analyses were performed with the Statistical Package for the Social Sciences version 12.0.1 (SPSS Inc., Chicago, IL, USA).
Results
Subject characteristics
The baseline characteristics of the women in the intervention and control groups were similar (Table 1). The women attended study visits at each trimester of pregnancy, the first at a mean of 14 (median 14, range 7·4–7·7), the second at 24 (median 24, range 20·1–27·3) and the third at 34 (median 34, range 30·1–37·1) weeks of gestation. Of the women, 12 % were underweight, 61 % normal weight, 21 % overweight and 7 % obese as assessed by pre-pregnancy BMI (P = 0·98 between the groups). A positive skin prick test result was detected in 58 % of the women. The most common positive skin prick test reactions were for birch (69 % of the positive test results), cat (68 %), alder (64 %), grasses (61 %) and dog (50 %). A positive skin prick test result for foods was detected in 12 % of the women, the most common of which were for peanut (13 % of the positive test results), hazelnut (10 %), potato (7 %), carrot (6 %) and cod (3 %). Altogether, 55 % (n 75) of the women in the intervention and 56 % (n 38) in the control group (P = 0·48 between the groups) had atopic disease, defined as a history of self-reported atopic eczema, allergic rhinitis, asthma or adverse reaction to foods together with a positive skin prick test result.
Table 1 Baseline characteristics of the women in the intervention (n 140) and the control (n 69) groups(Values are means with their standard deviation or number and percentage)

* Independent-samples t test for continuous variables and χ2 test or Fisher's exact test for dichotomous variables.
Evolution of pregnancy and physical activity
During the course of the study, 207 women delivered healthy singleton infants and two delivered twins. The mean gestational length was 40·0 (sd 1·3) weeks (P = 0·76 between the groups) and the total gestational weight gain was 14·9 (sd 4·9) kg (P = 0·86 between the groups). The mean birth weight of the infants was 3547 (sd 438) g (P = 0·20 between the groups), length 51 (sd 2) cm (P = 0·61 between the groups) and head circumference 35 (sd 1) cm (P = 0·14 between the groups).
The women in both groups were normotensive as evaluated by mean baseline blood pressure measurements (Table 1). In evaluation of the evolution of blood pressure during pregnancy, both systolic [1·7 mmHg (95 % CI − 3·2, − 0·2) from the first to the third trimester; P = 0·02 time effect] and diastolic [1·8 mmHg (95 % CI − 3·1, − 0·6) from the first to the second trimester; P = 0·005 time effect] blood pressure decreased in all women (group effect P = 0·23 for systolic and P = 0·93 for diastolic blood pressure).
In the intervention group there were 1·9 (95 % CI 1·6, 2·2) weekly episodes of aerobic physical activity exceeding 30 min a time in the first, 1·4 (95 % CI 1·1, 1·7) in the second and 1·6 (95 % CI 1·2, 2·0) in the third trimester of pregnancy; in the control group the corresponding frequencies were 2·2 (95 % CI 0·9, 2·1), 1·5 (95 % CI 1·7, 2·8) and 1·0 (95 % CI 0·5, 1·5) (P < 0·001 time effect, P = 0·03 interaction time by group, P = 0·84 group effect). Aerobic physical activity decreased by 1·3 (95 % CI − 2·0, − 0·7) episodes per week by the third trimester of pregnancy in the control group only, while no change was observed in the intervention group.
Impact of atopic disease on dietary intake
When evaluating the impact of atopic disease on dietary intake before the onset of intervention, the intake of MUFA was 2·3 g (95 % CI 0·1, 4·4; P = 0·039) and that of vitamin E 0·9 mg (95 % CI 0·7, 1·8; P = 0·034) and consumption of margarines 3·9 g (95 % CI 1·5, 6·3; P = 0·001) higher in women with atopic disease compared to those without. No differences in intakes of other foods or nutrients between women with and without atopic disease were detected (data not shown).
Compliance in consumption of the food products provided
According to the interviews, the proportion of women who consumed the food products provided for each 12-week period between study visits ranged from 68 % to 100 % depending on the product (Table 2). However, as assessed by 3-day food records filled in immediately before the study visits, fewer women (39–81 %) had, except for spreads, consumed the provided food products. In addition to the habitual intake, the products provided 6·9 g (95 % CI 5·9, 7·9) MUFA and 2·9 g (95 % CI 2·3, 2·9) PUFA in the second trimester and 6·9 g (95 % CI 6·1, 7·8) MUFA and 2·6 g (95 % CI 2·3, 2·9) PUFA in the third trimester of pregnancy, this being 36 % and 14 %, respectively, of the counselled intake for both trimesters. However, for evaluation of compliance, the intake of MUFA and PUFA is likely to be underestimated as the consumption of rapeseed oil incorporated in food recipes could not be calculated separately.
Table 2 Proportion (%) of women consuming the provided food products during the 12-week period between study visits obtained by interview and from 3-day food records in the intervention group

* χ2 test between methods of estimating food product consumption.
† Low erucic acid rapeseed oil-based soft margarines and soft cheese.
Impact of dietary intervention on dietary intake
Consumption of foods in the intervention and control groups is shown in Table 3. Changes in food consumption during pregnancy were detected only in the intervention group, and were attributable to higher consumption of vegetables by 23 g (95 % CI 1·4, 44·7), fruits by 59 g (95 % CI 28·1, 89·9), soft margarines by 2·9 g (95 % CI 1·0, 4·7) and vegetable oils by 2·6 g (95 % CI 0·9, 4·3), and lower consumption of butter by 3·0 g (95 % CI − 4·0, − 2·0) in the intervention group during the course of pregnancy. Although no differences were observed between the groups in consumption of fish, meat or cheese over the pregnancy, in the third trimester the intervention group consumed 9·2 g (95 % CI 0·5, 18·0) more fish and 15·0 g (95 % CI − 29·5, − 0·3) less meat and 13·0 g (95 % CI − 21·3, − 5·4) less cheese than the control group.
Table 3 Daily consumption of foods (g) in the intervention (n 140) and the control (n 69) groups in each trimester of pregnancy (Mean values and 95 % CI)
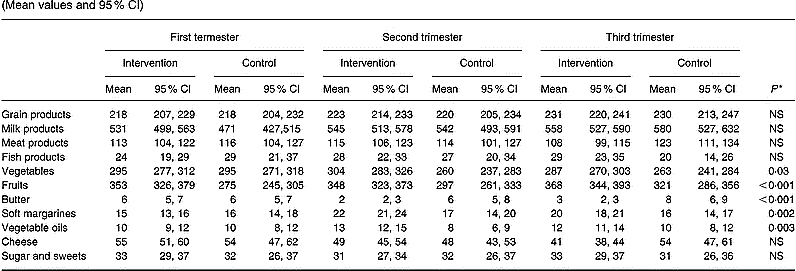
* Repeated-measures ANOVA group effect.
The mean daily intake of energy as a proportion of the estimated energy requirement was 89 % (95 % CI 86, 93) and 85 % (95 % CI 80, 90) in the first, 80 % (95 % CI 77, 88) and 76 % (95 % CI 72, 81) in the second, and 78 % (95 % CI 75, 81) and 75 % (95 % CI 80, 84) in the third trimester of pregnancy in the intervention and the control groups, respectively (P = 0·29 group effect). Independent of the group, the intake of energy as a proportion of estimated requirement decreased from the first to the third trimester of pregnancy by 9·5 % (95 % CI − 12·3, − 6·8; P < 0·001 time effect). Total weight gain during pregnancy was positively associated with energy intake in the first (R 0·15, P = 0·035, n 201) and the second (R 0·15, P = 0·035, n 201) but not in the third trimester (R 0·11, P = 0·118, n 201). Episodes of aerobic physical activity and pre-pregnancy BMI did not affect the energy intake during pregnancy.
Intakes of energy and energy-yielding nutrients in the intervention and control groups at each trimester of pregnancy are presented in Table 4. The distinction between the groups in the overall intakes of energy-yielding nutrients during pregnancy was attributable to a higher intake of PUFA by 1·3 g (95 % CI 0·2, 2·4) and by 0·5 % energy (95 % CI 0·1, 0·8) and to a lower intake of SFA by 0·8 % energy (95 % CI − 1·4, − 0·4) in the intervention compared to the control group (Fig. 1). In addition, the intake of dietary fibre was higher by 1·8 g (95 % CI 0·1, 3·4) in the intervention group than in the controls. Although no difference was observed between women receiving and not receiving dietary counselling in MUFA intake, over the pregnancy the intake was 4·7 g (95 % CI 2·1, 7·4) and 1·6 % energy (95 % CI 0·8, 2·4) higher in the second trimester of pregnancy in the intervention compared to the control group.
Table 4 Daily dietary intakes of energy, energy-yielding nutrients and dietary fibre in the intervention (n 140) and the control (n 69) group in each trimester of pregnancy* (Mean values and 95 % CI)
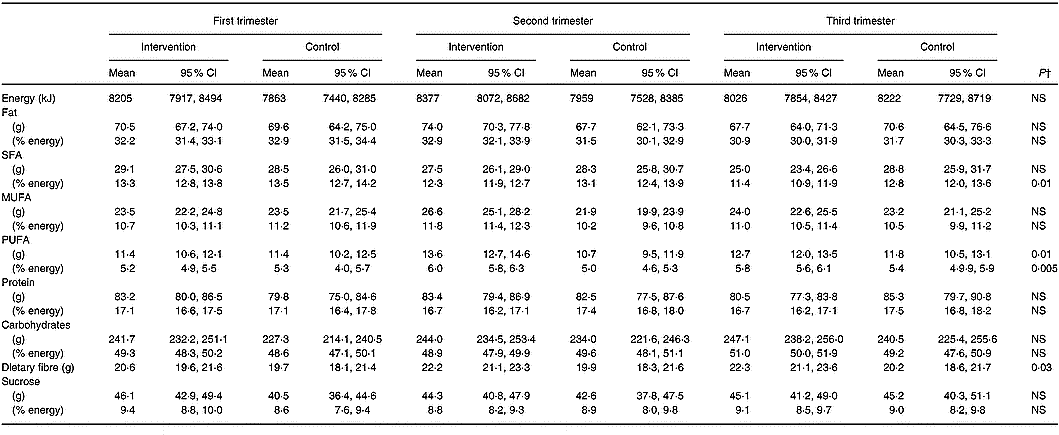
* Nordic Nutrition Recommendations (NNR) for pregnant women 2004 (Becker et al. Reference Becker, Lyhne and Pedersen2004). Recommended intake for fat 30 % energy, protein 15 % energy, carbohydrates 55 % energy, SFA 10 % energy, MUFA 10–15 % energy and PUFA 5–10 % energy.
† Repeated-measures ANOVA group effect.

Fig. 1 Intake of (A) SFA, (B) MUFA and (C) PUFA as a proportion of energy intake in the intervention (▲) and the control (■) group in each trimester of pregnancy.
The daily intakes of vitamins and minerals from diet, and total intake derived from diet and supplements, are shown in Table 5. Altogether, 96 % of women had used at least one dietary supplement during pregnancy (P = 0·34 between the groups), the use being 68 % in the first, 82 % in the second and 89 % in the third trimester (P < 0·001 between trimesters). Vitamin D (89 % of the women), iron (73 %), combination preparations comprising several vitamins and minerals (68 %) and calcium (47 %) were the supplements used most frequently. Additionally, assessed separately for wintertime (October to March), when vitamin D supplementation is recommended, 87 % of the women had used this supplement.
Table 5 Daily dietary and total intakes of vitamins and minerals in the intervention and the control group in each trimester of pregnancy* (Mean values and 95 % CI)
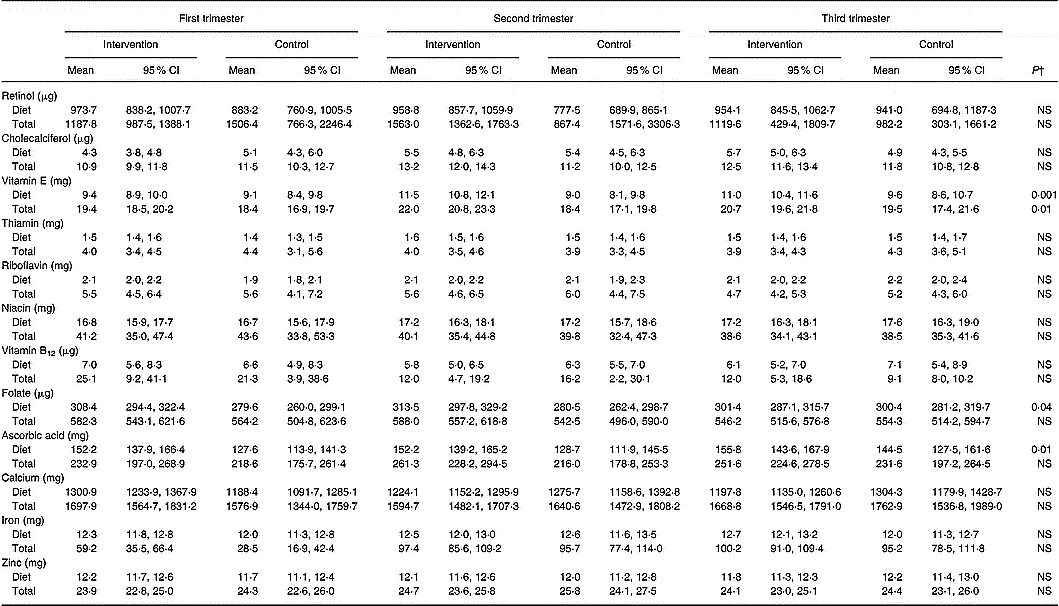
* Nordic Nutrition Recommendations (NNR) for pregnant women 2004 (Becker et al. Reference Becker, Lyhne and Pedersen2004). Recommended intake for retinol 800 μg, cholecalciferol 10 μg, vitamin E 10 mg, thiamin 1·5 mg, riboflavin 1·6 mg, niacin 17 mg, vitamin B12 2·0 μg, folate 500 μg, ascorbic acid 85 mg, calcium 900 mg, zinc 9 mg.
† Repeated-measures ANOVA group effect.
When the intakes of nutrients as a proportion of the recommended intake were compared between the groups, the intakes of vitamin E (55 % and 53 % in the first, 67 % and 53 % in the second and 65 % and 57 % in the third trimester of pregnancy in the intervention and control groups, respectively, P = 0·001 group effect), folate (62 % and 56 % in the first, 63 % and 56 % in the second and 60 % and 60 % in the third trimester of pregnancy, respectively, P = 0·04 group effect) and ascorbic acid (179 % and 150 % in the first, 179 % and 151 % in the second and 183 % and 170 % in the third trimester of pregnancy, respectively, P = 0·01 group effect) were higher in the intervention group compared to the control group, while no difference emerged between the groups for other nutrients (data not shown). Comparably, dietary intake of vitamin E was 1·4 mg (95 % CI 0·6, 2·2), folate 20·9 μg (95 % CI 0·8, 41·0) and ascorbic acid 19·8 mg (95 % CI 3·5, 36·0) and total intake of vitamin E was 2·1 mg (95 % CI 0·5, 3·8) higher in the intervention group compared to the controls.
Discussion
The present study is the first to apply a combined approach of dietary counselling and provision of food products for use at home in pregnant women in an attempt to encourage changes in food intake and consequently in nutrient intake. Dietary counselling resulted in increased consumption of vegetables, fruits, soft margarines and vegetable oils and decreased consumption of butter, and consequently resulted in higher intakes of PUFA, dietary fibre, vitamin E and ascorbic acid and folate and lower SFA intake in women receiving dietary counselling compared to those not receiving counselling. These changes have been associated with health benefits with the potential to counteract lifestyle-related chronic conditions such as CVD and diabetes (World Health Organization, 2003). With respect to pregnancy, an additional advantage may be achieved because the mother's dietary habits are known to influence the health and well-being of both the mother (Gunderson et al. Reference Gunderson, Abrams and Selvin2000; Vasan et al. Reference Vasan, Larson, Leip, Kannel and Levy2001) and the fetus or child (Godfrey & Barker, Reference Godfrey and Barker2000), which we are further evaluating in an ongoing study.
The mean overall weight gain during pregnancy was 14·9 kg and the total weight gain during pregnancy was associated with energy intake, suggesting an adequate energy intake by the pregnant women, However, the intake of energy remained less than the estimated requirements, as observed previously (Rogers et al. Reference Rogers and Emmett1998; Borah Giddens et al. Reference Borah Giddens, Krug, Tsang, Guo, Miodovnik and Prada2000; Swensen et al. Reference Swensen, Harnack and Ross2001; Turner et al. Reference Turner, Langkamp-Henken, Littell, Lukowski and Suarez2003). The discrepancy between intake and estimated energy requirements has been explained by a decrease in physical activity towards the end of pregnancy (Dufour et al. Reference Dufour, Reina and Spurr1999; Kopp-Hoolihan et al. Reference Kopp-Hoolihan, van Loan, Wong and King1999), which was not, however, consistently shown in our study. Another explanation may be an adaptation towards conserving energy, as energy balance studies have found a maintenance of weight gain during pregnancy, despite higher energy expenditure in comparison to energy intake (Kopp-Hoolihan et al. Reference Kopp-Hoolihan, van Loan, Wong and King1999). Supporting previous observations (Dufour et al. Reference Dufour, Reina and Spurr1999; Kopp-Hoolihan et al. Reference Kopp-Hoolihan, van Loan, Wong and King1999; King, Reference King2000), our study also questions the validity of using the current estimated energy requirements during pregnancy, which seem to be too high. Concomitantly, the limitations of the food record method used for the analysis of dietary intake need to be taken into consideration, as underestimation of energy intake or inaccurate reporting may occur (Caan et al. Reference Caan, Ballard-Barbash and Slattery2004), possibly influencing the reported energy intake.
The mean intake of most nutrients was in accordance with the dietary recommendations, except that the intakes of vitamins D and E and folate were less than recommended, as was also observed previously (Erkkola et al. Reference Erkkola, Karppinen, Järvinen, Knip and Virtanen1998; Rogers et al. Reference Rogers and Emmett1998; Swensen et al. Reference Swensen, Harnack and Ross2001; Pick et al. Reference Pick, Edwards, Moreau and Ryan2005). Low intake of these vitamins during pregnancy is of concern in view of their health-related effects. Low intakes of folate and vitamin E have been associated with an increased risk of neural tube defect in the fetus (Scholl & Johnson, Reference Scholl and Johnson2000) and with hypertensive disorders in the mother (Rumbold et al. Reference Rumbold, Maats and Crowther2005). However, the higher intakes of folate and vitamin E in the intervention group in the present study suggest that it may be possible to increase the intake of potentially critical nutrients during pregnancy by means of dietary counselling. For vitamin D, notwithstanding the low dietary intake, the recommendation for pregnant women to use vitamin D supplements during wintertime (10 μg daily from October to March) was achieved. Of the all women, 87 % had used the supplements, which is considerably more than the 33 % observed previously (Erkkola et al. Reference Erkkola, Karppinen, Järvinen, Knip and Virtanen1998) and speaks in favour of repeated dietary counselling as carried out in the present study. Despite adequate mean dietary intakes of most nutrients in comparison to dietary recommendations, the majority of the women (96 %) had used vitamin and mineral supplements at some stage of pregnancy, and despite counselling, which endeavoured to satisfy the demand for nutrients by diet, the use of supplements did not differ between women receiving dietary counselling or not. This probably reflects women's concern over the well-being of the fetus and of themselves, which is generally thought to be improved by supplement use. However, the use of supplements during pregnancy may not be without risk, and while the long-term effects on pregnant women and the fetus remain unresolved, previous supplementation studies in patients with chronic diseases and in healthy people have shown alarming effects (Bjekovic et al. Reference Bjekovic, Nikolova, Simonett and Gluud2004; Miller et al. Reference Miller, Pastor-Barriuso, Dalal, Riemersma, Appel and Guallar2004).
The recommended intakes of MUFA and PUFA were achieved and, additionally, although higher than recommended, the intake of SFA was lowered in the intervention group. Compared to a previous national study with Finnish women of child-bearing age, the intakes of SFA were considerable lower and the intakes of MUFA and PUFA were higher (Reinivuo et al. Reference Reinivuo, Männistö, Tapanainen, Pakkala, Männistö, Ovaskainen and Valsta2003). The difference in SFA intake between women receiving and women not receiving dietary counselling was, however, smaller than that observed previously in women with gestational diabetes mellitus who received dietary advice (Gillen & Tapsell, Reference Gillen and Tapsell2004). Increased motivation to alter dietary habits because of pregnancy-related complications possibly augmented the effect of counselling compared to healthy women in the present study. The longer duration of the study might have affected women's motivation to maintain the recommended diet and consumption of food products provided, as suggested by the improvements in the dietary intake from the first to the second trimester and a tendency to reach the baseline by the third trimester of pregnancy. All pregnant women participating in the study also attended communal well-women clinics, where they might have received dietary counselling by nurses, and thus might have already altered their dietary habits before entry to the study. For ethical reasons and to improve compliance in food record keeping, women in the control group also received feedback on their food records, which may have affected the dietary intake. Nevertheless, bearing in mind the presented limitations of the study, the focused dietary counselling supported by food products led to favourable changes in dietary intake.
Women with atopic disease may run a risk of nutritional inadequacy as they may limit their diet due to symptoms of the disease. In the present study atopic disease had no adverse impact on dietary intake, in contrast to previous observations, in which the intake of ascorbic acid was lower (Hoppu et al. Reference Hoppu, Kalliomäki and Isolauri2000) and the intakes of total fat and SFA were higher in women with atopic disease compared to those without (Hoppu et al. Reference Hoppu, Kalliomäki and Isolauri2000; Solvoll et al. Reference Solvoll, Soyland, Sandstad and Drevon2000; Trak-Fellermeier et al. Reference Trak-Fellermeier, Brasche, Winkler, Koletzko and Heinrich2004). The positive skin prick test results for foods in the present study were mainly for nuts and also for potatoes and carrots, which are usually tolerated cooked, and not for nutritionally important foods, such as grain products or milk. Additionally, the present results indicate that food cannot be used in manipulating symptoms of atopic disease as commonly as thought and may also reflect the awareness of mothers following a balanced diet during pregnancy.
The present study shows that the novel approach of dietary counselling combined with food products can improve the achievement of a dietary intake conforming with that recommended. Dietary counselling during pregnancy, if applied in a larger health-care setting, may reduce pregnancy-related disorders, promote the growth and development of the fetus and child and result in long-term health benefits in both. Furthermore, dietary counselling has been shown to be a cost-effective method in health care to prevent and provide care in lifestyle-related diseases (Franz et al. Reference Franz, Splett, Monk, Barry, McClain, Weaver, Upham, Bergenstal and Mazze1995; Delahanty et al. Reference Delahanty, Sonnenberg, Hayden and Nathan2001; Murray et al. Reference Murray, Lauer, Hutubessy, Niessen, Tomijima, Rodgers, Lawes and Evans2003). An advantage may be achieved by developing counselling practices in health care (Huurre et al. Reference Huurre, Laitinen, Hoppu and Isolauriin press), thus providing a foundation for continuing healthy eating habits extending to the postpartum period, an aspect that we are exploring in an ongoing study. In conclusion, the findings of the present study suggest that dietary counselling of pregnant women, combined with provision of appropriate food products, is of importance in modifying food and nutrient intake with potential health benefits, presenting a challenge to food manufacturers in providing supporting products.
Acknowledgements
We thank the women who participated in the present study, Ulla-Maija Eriksson, Johanna Hvitfelt-Koskelainen, Sari Laksio and Sanna Hirvonen for participating in study visits, and Robert MacGilleon for revision of the English text. We gratefully acknowledged financial support from the Academy of Finland, the Social Insurance Institution of Finland, the Sigrid Juselius Foundation and the Juho Vainio Foundation, and provision of food products by Raisio plc.








