The advent of molecular diagnostics with the potential for clinical genetic testing in psychiatry has introduced the possibility of providing personalised treatments for patients with schizophrenia. Reference Ozomaro, Wahlestedt and Nemeroff1 Through selection of effective and well-tolerated treatments, such advances promise to significantly reduce patient, family and economic burden of illness. Pharmacogenomic studies of response to antipsychotic medications have been largely hampered, however, by the complex genetic aetiology of schizophrenia. Reference Ozomaro, Wahlestedt and Nemeroff1,Reference Costain and Bassett2 Although initial studies have suggested common genetic variants that may mediate response to antipsychotic treatments, small effect sizes and difficulties in replicating across patient groups limit clinical utility. Reference Ozomaro, Wahlestedt and Nemeroff1,Reference Arranz, Rivera and Munro3,Reference Fleeman, Dundar, Dickson, Jorgensen, Pushpakom and McLeod4 As an alternative, the well-established association between schizophrenia and 22q11.2 deletion syndrome (22q11.2DS) provides an opportunity to investigate the utility of a molecular diagnosis in studying treatment response in schizophrenia. The hemizygous 22q11.2 deletions associated with 22q11.2DS are found in up to 1 of every 100 individuals with schizophrenia, 5–Reference Bassett, Scherer and Brzustowicz7 representing the most highly replicated molecular subtype of schizophrenia to date. Reference Bassett, Scherer and Brzustowicz7–Reference Bassett and Chow9 Individuals with 22q11.2DS are clinically identifiable (for example other major features include dysmorphic features, congenital heart defects and hypernasal speech) and standard testing for the 22q11.2 deletion is available at clinical laboratories. Reference Bassett, McDonald-McGinn, Devriendt, Digilio, Goldenberg and Habel10 The schizophrenia of 22q11.2DS (22q11.2DS-schizophrenia) is essentially indistinguishable from the heterogeneous forms of schizophrenia found in the general population with respect to prodrome, age at onset, and core signs and symptoms. Reference Bassett, Chow, AbdelMalik, Gheorghiu, Husted and Weksberg8,Reference Bassett and Chow9,Reference van Amelsvoort, Henry, Morris, Owen, Linszen and Murphy11–Reference Stoddard, Niendam, Hendren, Carter and Simon14 Although previous case reports describe some challenges, Reference Krahn, Maraganore and Michels15–Reference Yacoub and Aybar21 standard antipsychotic management and clinical practice guidelines for schizophrenia are currently recommended for patients with 22q11.2DS-schizophrenia. Reference Bassett, McDonald-McGinn, Devriendt, Digilio, Goldenberg and Habel10 Clozapine is an effective atypical antipsychotic with a low risk of extrapyramidal side-effects; rare but serious side-effects, however, largely restrict its use to patients whose condition is treatment resistant. 22–Reference Meltzer, Alphs, Green, Altamura, Anand and Bertoldi24 In this study, we compared the response and safety of clozapine treatment in individuals with 22q11.2DS-schizophrenia (22q11.2DS group) and idiopathic schizophrenia (idiopathic group), where there was no 22q11.2 deletion or other clinically pathogenic copy number variation. We used a long-term observational retrospective design (median >5 years) including comprehensive medical chart reviews and standard assessment methods. Reference Guy25
Method
Participants
The initial 22q11.2DS cohort comprised 184 Canadian adults with 22q11.2DS. Participants were ascertained through adult congenital cardiac, psychiatric and genetic services using active screening and/or clinical referrals. Reference Fung, Chow, Webb, Gatzoulis and Bassett26,Reference Bassett, Costain, Fung, Russell, Pierce and Kapadia27 Of the 66 with schizophrenia, we identified 21 (31.8%) unrelated individuals who had been treated with clozapine, of these 1 patient was deceased but had ample data available for study. We excluded one individual who received two test doses of clozapine (total 18.75 mg) over a 2-day period immediately before death secondary to cardiovascular failure that was unrelated to clozapine initiation. Reference Butcher, Kiehl, Hazrati, Chow, Rogaeva and Lang28 This yielded a sample of 20 patients with 22q11.2DS molecularly confirmed to have a chromosome 22q11.2 deletion using standard clinical genetic testing (n = 19 by fluorescence in situ hybridisation and probe from the deletion region, n = 1 by clinical microarray). Reference Bassett, Chow, Husted, Weksberg, Caluseriu and Webb29,Reference Bassett, Marshall, Lionel, Chow and Scherer30
The idiopathic group was ascertained from a Canadian community mental health sample, primarily recruited from a single clinic, who had direct clinical assessments for potential genetic syndromic features using a standardised protocol and high-resolution genome-wide research microarrays. Reference Fung, Chow, Webb, Gatzoulis and Bassett26,Reference Costain, Lionel, Merico, Forsythe, Russell and Lowther31 Of 362 individuals, 42 (11.6%) were identified as ever having had a trial of clozapine, 31 of whom were potentially eligible for the current study (8 had no microarray results at study initiation and 3 were deceased; these 11 excluded individuals appeared comparable with those studied, for example, predominantly White men, median age at onset 22 years; clozapine initiated at a median of 31 years). A further 11 were excluded: 4 had a large (>500 kb) rare copy number variation (1q21.1 duplication, 8p23.1 deletion, 15q11–15q13 duplication, 16p11.2 duplication) independently deemed ‘pathogenic’ by two clinical cytogenetic laboratory directors using standard criteria, Reference Costain, Lionel, Merico, Forsythe, Russell and Lowther31,Reference Kearney, Thorland, Brown, Quintero-Rivera and South32 5 had insufficient medical records, 1 was related to another patient and 1 had syndromic characteristics. Reference Fung, Chow, Webb, Gatzoulis and Bassett26,Reference Costain, Lionel, Merico, Forsythe, Russell and Lowther31 This yielded a sample of 20 unrelated individuals in the idiopathic group.
All participants met DSM-IV criteria 33 for schizophrenia or schizoaffective disorder (n = 1 with schizoaffective disorder in the 22q11.2DS group, n = 2 in the idiopathic group). Reference Bassett, Caluseriu, Weksberg, Young and Chow34 Age at onset was defined as the age at first treatment of psychosis. Intellectual disability was determined for the 22q11.2DS group using DSM-IV criteria. Reference Chow, Watson, Young and Bassett12,33 None of the idiopathic group had a paediatric diagnosis of intellectual disability (Table 1); 11 had a history of non-specific intellectual difficulties or had failed at least one grade. Informed consent was obtained in writing, and the study was approved by local research ethics boards.
Table 1 Demographics and clinical characteristics and response to clozapine of 20 adults with 22q11.2 deletion syndrome (22q11.2DS) schizophrenia and 20 with idiopathic schizophrenia
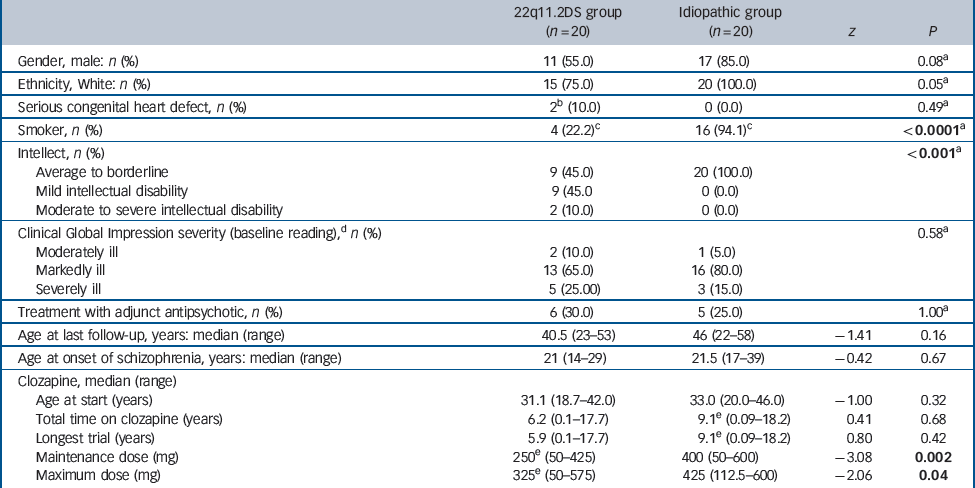
| 22q11.2DS group (n = 20) |
Idiopathic group (n = 20) |
z | P | |
|---|---|---|---|---|
| Gender, male: n (%) | 11 (55.0) | 17 (85.0) | 0.08Footnote a | |
| Ethnicity, White: n (%) | 15 (75.0) | 20 (100.0) | 0.05Footnote a | |
| Serious congenital heart defect, n (%) | 2Footnote b (10.0) | 0 (0.0) | 0.49Footnote a | |
| Smoker, n (%) | 4 (22.2)Footnote c | 16 (94.1)Footnote c | <0.0001Footnote a | |
| Intellect, n (%) | <0.001Footnote a | |||
| Average to borderline | 9 (45.0) | 20 (100.0) | ||
| Mild intellectual disability | 9 (45.0 | 0 (0.0) | ||
| Moderate to severe intellectual disability | 2 (10.0) | 0 (0.0) | ||
| Clinical Global Impression severity (baseline reading),Footnote d n (%) | 0.58Footnote a | |||
| Moderately ill | 2 (10.0) | 1 (5.0) | ||
| Markedly ill | 13 (65.0) | 16 (80.0) | ||
| Severely ill | 5 (25.00) | 3 (15.0) | ||
| Treatment with adjunct antipsychotic, n (%) | 6 (30.0) | 5 (25.0) | 1.00Footnote a | |
| Age at last follow-up, years: median (range) | 40.5 (23–53) | 46 (22–58) | –1.41 | 0.16 |
| Age at onset of schizophrenia, years: median (range) | 21 (14–29) | 21.5 (17–39) | –0.42 | 0.67 |
| Clozapine, median (range) | ||||
| Age at start (years) | 31.1 (18.7–42.0) | 33.0 (20.0–46.0) | –1.00 | 0.32 |
| Total time on clozapine (years) | 6.2 (0.1–17.7) | 9.1Footnote e (0.09–18.2) | 0.41 | 0.68 |
| Longest trial (years) | 5.9 (0.1–17.7) | 9.1Footnote e (0.09–18.2) | 0.80 | 0.42 |
| Maintenance dose (mg) | 250Footnote e (50–425) | 400 (50–600) | –3.08 | 0.002 |
| Maximum dose (mg) | 325Footnote e (50–575) | 425 (112.5–600) | –2.06 | 0.04 |
a. Fisher’s exact test.
b. Tetralogy of Fallot (n = 1); interrupted aortic arch type B (n = 1).
c. Lifetime history: 22q11.2DS group, n = 18; idiopathic group, n = 17.
d. Moderate interrater reliability (intraclass correlation coefficient (ICC) = 0.49).
e. n = 19.
Assessment of clozapine efficacy and safety
To assess clozapine-related changes in psychiatric behaviours and functioning and adverse events, we used comprehensive lifetime clinical summaries compiled for each patient using medical records, extensive clinical histories and semi-structured interviews. Patients and/or a caregiver or individual well-acquainted with the patient were contacted by telephone or in person for follow-up regarding the patient’s experiences with clozapine. We recorded: demographic data, psychiatric diagnoses, symptoms and hospital admissions, proxy measures of functioning (such as housing, employment, relationships, financial support), comorbid medical conditions, smoking history, medications and doses and adverse effects. Updated (to 2013) clinical information was available for the majority (n = 35, 87.5%) of the 40 participants. One patient had died in the 22q11.2DS group and two individuals in each group were unavailable for follow-up.
The clozapine maintenance dose was defined as the most recent dose for patients still on clozapine. For patients who had discontinued clozapine, the last dose before tapering for cessation began, or before medication non-adherence was clearly documented, was considered the maintenance dose. For patients with more than one clozapine trial, the maintenance dose was determined for the trial of longest duration. Adjunct antipsychotics used during clozapine treatment at the maintenance dose were recorded at the most recent dose. The highest clozapine dose was recorded for the patient’s lifetime experience with clozapine. Four patients in the 22q11.2DS group were prescribed clozapine by study authors (A.S.B. (n = 2), W.L.A.F. (n = 1) and E.W.C.C. (n = 1)).
The Clinical Global Impression (CGI) Scale was used to retrospectively evaluate mental illness severity and improvement following a clozapine trial, masked to group status. Reference Guy25 For CGI evaluation, the 40 comprehensive clinical summaries were prepared using identical formatting and careful masking to 22q11.2 deletion status, including the removal of all 22q11.2DS-associated phenotypes (for example intellectual disabilities, congenital defects, speech therapy, hypocalcaemia, etc.). The trial of longest duration was evaluated for patients with more than one clozapine trial. The CGI – Severity (CGI-S) scale was used to rate the severity of the patient’s mental illness from one (normal) to seven (among the most severely ill) at the time of clozapine initiation. The CGI – Improvement (CGI-I) scale was used to assess total improvement compared with baseline, with one being very much improved, two much improved, three minimally improved, four no change, and five to seven representing minimally, much and very much worse, respectively. The CGI scales were completed by two psychiatrists (W.L.A.F., A.S.B.) for all 40 patients in randomised order and assessed for interrater reliability using intraclass correlation coefficients (ICC). Reference Shrout and Fleiss35 The CGI raters had no involvement in the patient chart reviews, data extraction and patient/caregiver follow-up contacts conducted for the purposes of this study.
The average number of psychiatric hospitalisations per year was calculated for each person while on and off clozapine. Both psychiatric-related visits to the emergency room and hospital admissions were classified as a psychiatric hospitalisation. Time off clozapine was considered the time from first psychiatric hospitalisation until the start of the clozapine trial, and summed with other time periods off clozapine for patients with more than one trial or where the medication had been discontinued permanently. For patients where the precise start or end date of clozapine was unclear, the midpoint date between the last confirmed dates on or off clozapine was used.
Statistical analyses
To compare demographic and clinical variables, we used Fisher’s exact tests for independent categorical variables and the non-parametric Mann-Whitney U-test for independent continuous variables to limit bias from non-normal distributions. McNemar’s exact test and the Wilcoxon signed rank sum test were used to assess paired categorical and continuous variables, respectively. Odds ratios (ORs) for adverse events were calculated using Cochran-Mantel-Haenszel statistics. A 0.5 zero-cell correction was used, as necessary. Intraclass correlation coefficients were calculated using a two-way mixed model Reference Shrout and Fleiss35 using the ‘INTRACC’ macro developed for SAS by Hamer. Reference Hamer36 An ICC equal to zero represents interrater agreement equivalent to that expected by chance, whereas one represents perfect agreement. The following ICC interpretation scale was used: Reference Landis and Koch37 poor to fair (≤0.40), moderate (0.41–0.60), excellent (0.61–0.80) and almost perfect (0.81–1). All statistical analyses were two-tailed and performed with SAS version 9.2 software with statistical significance defined as P<0.05.
Results
Demographic and clinical characteristics are presented in Table 1. The 22q11.2DS group had significantly lower intellectual level and fewer smokers, and non-significantly fewer White participants and more women. All but 3 of the 40 patients were classified as markedly or severely ill at the time of initiation of a clozapine trial (Table 1). The median number of antipsychotics (treatment with a different antipsychotic medication and/or the addition of an adjunct antipsychotic) prior to clozapine initiation was high in both groups (22q11.2DS group, 5, range 2–10; idiopathic group, 4.5, range 1–21; z = −0.62, P = 0.54).
Dosing and therapeutic response
The median maintenance clozapine dose was significantly lower in the 22q11.2DS group (250 mg, range 50–425 mg, n = 19) compared with the idiopathic group (400 mg, range 50–600 mg, n = 20, z = −3.08, P = 0.002; Fig. 1). The median dose of clozapine of the four smokers in the 22q11.2DS group was 268.75 mg. There were no significant differences between groups in the total time spent on clozapine, the length of the longest clozapine trial (median >5 years for both groups) or frequency of adjunct antipsychotic treatment at maintenance dose (Table 1). Adjunct antipsychotic treatments for six individuals in the 22q11.2DS group were: chlorpromazine (50 mg) with levomepromazine (15 mg), flupentixol (4.5 mg), haloperidol (2.5 mg), quetiapine (37.5 mg) and risperidone (n = 2, 0.5 mg and 4 mg). For five individuals in the idiopathic group, adjunct antipsychotics were: flupentixol decanoate injection (150 mg/ml every 4 weeks), perphenazine (48 mg), risperidone (2 mg) and olanzapine (n = 2 10 mg and 20 mg).
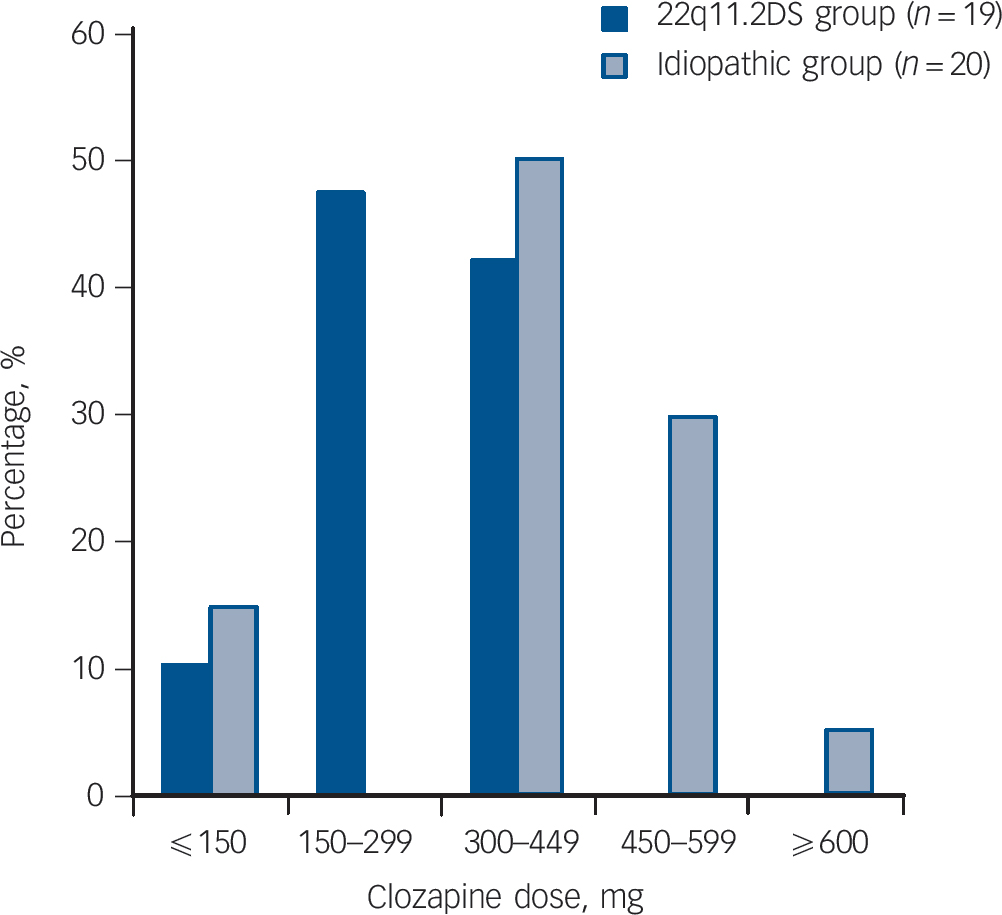
Fig. 1 Patients with 22q11.2 deletion syndrome (22q11.2DS) schizophrenia are maintained on a significantly lower therapeutic dose of clozapine than patients with idiopathic schizophrenia (P = 0.002).
The two 22q11.2DS-schizophrenia patients with doses ≤150 mg had been maintained in this range for at least 1 year; one was receiving adjunct antipsychotic flupentixol (4.5 mg) treatment. Of the three patients in the idiopathic group on a clozapine dose ≤150 mg, one was maintained on this dose for 4 years; the other two discontinued within 9 and 28 weeks, respectively (the latter on adjunct antipsychotic perphenazine (48 mg) treatment) and were not titrated above this low dose range.
The 22q11.2DS and idiopathic groups responded well to clozapine treatment with the majority of both groups rated as much or very much improved (Fig. 2). There was similar improvement (Fisher’s exact test, P = 0.33) as rated using the CGI-I with excellent interrater reliability (intraclass correlation coefficient (ICC) = 0.65). For those maintained on clozapine for at least 12 consecutive weeks, the median number of psychiatric hospitalisations standardised per year was significantly reduced for the 22q11.2DS group (n = 18; median, 0.0, range 0.0–1.90) compared with other antipsychotics (median, 0.73, range 0.15–6.35, Signed-Rank Test Statistic (S)6 = 66.5, P = 0.002). Results were similar in the idiopathic group (n = 17; median, 0.09, range 0.0–4.15 on clozapine; 1.09, range 0.23–2.03 on other treatment; S = 44.5, P = 0.04). There was no significant between-group difference in reduction of psychiatric hospitalisations (z = 0.02, P = 0.99).

Fig. 2 Clinical Global Impression – Improvement scale ratings for patients with 22q11.2 deletion syndrome (22q11.2DS) schizophrenia and idiopathic schizophrenia.
Therapeutic response to clozapine of patients in the 22q11.2DS group is similar to those in the idiopathic group (P = 0.33). One of the two patients with no change corresponds to a patient in the idiopathic group with a clozapine dose ≤150 mg who discontinued within 28 weeks of treatment.
Adverse effects
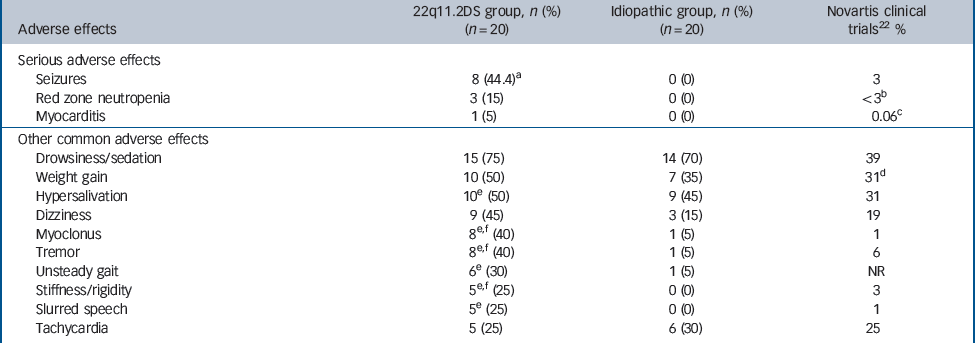
Common side-effects of clozapine, including drowsiness/sedation, weight gain and hypersalivation were reported in both groups in similar proportions (Table 2). However, even though clozapine treatment was at a higher dose and for a longer duration in the idiopathic group overall than the 22q11.2DS group, the latter had a higher prevalence of uncommon side-effects. Half of the 22q11.2DS group (n = 10) experienced at least one serious adverse event compared with none of the idiopathic group (gender-adjusted OR = 16.5, 95% CI 1.8–149.8), comprising myocarditis (n = 1), severe neutropenia (n = 3) and seizures (n = 8).
Table 2 Serious and/or common (≥25%) clozapine treatment-emergent adverse effects in adults with 22q11.2 deletion syndrome (22q11.2DS) schizophrenia compared with idiopathic schizophrenia
| Adverse effects | 22q11.2DS group,
n (%) (n = 20) |
Idiopathic group,
n (%) (n = 20) |
Novartis clinical trials 22 % |
|---|---|---|---|
| Serious adverse effects | |||
| Seizures | 8 (44.4)Footnote a | 0 (0) | 3 |
| Red zone neutropenia | 3 (15) | 0 (0) | <3Footnote b |
| Myocarditis | 1 (5) | 0 (0) | 0.06Footnote c |
| Other common adverse effects | |||
| Drowsiness/sedation | 15 (75) | 14 (70) | 39 |
| Weight gain | 10 (50) | 7 (35) | 31Footnote d |
| Hypersalivation | 10Footnote e (50) | 9 (45) | 31 |
| Dizziness | 9 (45) | 3 (15) | 19 |
| Myoclonus | 8 Footnote e,Footnote f (40) | 1 (5) | 1 |
| Tremor | 8 Footnote e,Footnote f (40) | 1 (5) | 6 |
| Unsteady gait | 6Footnote e (30) | 1 (5) | NR |
| Stiffness/rigidity | 5 Footnote e,Footnote f (25) | 0 (0) | 3 |
| Slurred speech | 5Footnote e (25) | 0 (0) | 1 |
| Tachycardia | 5 (25) | 6 (30) | 25 |
NR, not reported.
a. Excludes two patients in the 22q11.2DS group who had a prior diagnosis of epilepsy.
b. Includes leucopaenia/decreased white blood cell count/neutropenia.
c. Post-marketing surveillance in Canada. 22
d. InterCePT study. Reference Meltzer, Alphs, Green, Altamura, Anand and Bertoldi24
e. Includes one patient diagnosed with Parkinson’s disease during the clozapine trial.
f. Includes pre-existing conditions that worsened on clozapine (myoclonus, n = 1; stiffness/rigidity, n = 2; tremor, n = 3).
One (5%) patient in the 22q11.2DS group (a 25-year-old White man) with no congenital heart defect developed myocarditis approximately 3 weeks after starting on clozapine (Table 2). Adjunct antipsychotic treatment included chlorpromazine and levomepromazine. A cardiologist diagnosed myocarditis following admission to hospital with dilated, akinetic cardiomyopathy and congestive heart failure with a 2-day history of chest tightness. The patient’s symptoms resolved rapidly with discontinuation of clozapine (250 mg). A viral cause was considered unlikely and thus the discharge diagnosis was clozapine-associated myocarditis.
There were three confirmed cases of severe ‘red zone’ neutropenia (15%), defined as a white blood count (WBC) <2.0 × 109/L or absolute neutrophil count (ANC) <1.5 × 109/L reported in mandatory monitoring, in White female patients aged 26 (Patient 1), 37 (Patient 2), and 39 (Patient 3) years with 22q11.2DS (Table 2). None were treated with other medications clearly associated with agranulocytosis (for example carbamazepine). 22,Reference de Leon, Santoro, D'Arrigo and Spina38 Documented concomitant psychotropic medications at time of neutropenia included lamotrigine and clobazam for Patient 1, valproate for Patient 2, and lorazepam (as necessary) for Patient 3. Although there are rare case reports of neutropenia and agranulocytosis in patients taking valproate, Reference Vesta and Medina39,Reference Hsu, Tseng, Wang and Wang40 we note that Patient 2 received valproate both before and after clozapine with no neutropenia, and neutropenia resolved following discontinuation of clozapine.
The records available for Patient 1 and 3 indicated that baseline WBC prior to clozapine initiation (4.8 × 109/L and 5.6 × 109/L, respectively) were within standard laboratory norms and consistent with baseline WBC results for the overall 22q11.2DS group (mean 5.8 × 109/L, s.d. = 1.5 × 109/L, n = 17). The mean baseline WBC results for the 22q11.2DS group was lower than that reported previously for 11 309 individuals monitored by the Clozaril Patient Management System (8.3 × 109/L, s.d. = 2.7 × 109/L; t = –7.12, d.f. = 16, P<0.0001). Reference Alvir, Lieberman, Safferman, Schwimmer and Schaaf41 Notably, one of the three ‘red zone’ participants in the 22q11.2DS group (baseline WBC 5.6 × 109/L) exhibited persistent neutropenia on clozapine but was able to be successfully maintained on it for 8 years with an approved lowered ‘red zone’ alert zone. Such an
alert, accompanied by a sore throat and fever, eventually led to clozapine discontinuation. The two other patients demonstrated further severe neutropenia episodes following a second trial of clozapine.
Seizures and other neurological side-effects
Eight (44.4%, n = 4 male) of 18 patients in the 22q11.2DS group experienced at least one seizure on clozapine (Table 2). Two patients in the 22q11.2DS group who had a prior diagnosis of epilepsy (one with seizures on clozapine), were not included in the analysis. No patients in the idiopathic group had a seizure on clozapine. The occurrence of seizures in the 22q11.2DS group was significantly higher than reported for 1743 individuals during clinical testing of clozapine 42 (n = 61, OR = 22.1, 95% CI 8.4–57.9). In the 22q11.2DS group, non-significantly fewer (n = 3, 16.6%) had seizures when they were treated with other atypical antipsychotics compared with clozapine (S = 3.57, P = 0.059). Three individuals had one or two seizures each on a different atypical antipsychotic (risperidone with loxapine, quetiapine with low-dose levomepromazine, and olanzapine), two of whom also had seizure(s) on clozapine.
Seizures on clozapine were typically primarily or secondarily generalised tonic-clonic seizures (electroencephalogram (EEG) findings, online Table DS1). One patient also had complex partial and myoclonic seizures. Seizures occurred at relatively low doses (n = 7, median 300, range 100–425 mg; online Table DS1). Five (62.5%) of the eight individuals in the 22q11.2DS group who had seizures on clozapine had no prior history of seizures. Three had a prior history of one to two seizures each (primarily or secondarily generalised tonic-clonic seizures, one focal motor seizure) while on other antipsychotic medications. In two of these patients, the seizures occurred during antipsychotic management changes (changing from loxapine to risperidone, and on increase in loxapine dose).
Seizures during clozapine treatment appeared unlikely to be influenced by adjunct antipsychotic treatment; the majority of patients (n = 7, 87.5%) had at least one seizure while on clozapine alone. Of the only two individuals who had a seizure while receiving adjunctive treatment (olanzapine, haloperidol), one also had multiple other seizures on clozapine when not on another antipsychotic. This patient was noted to have borderline-low calcium at the time of one of these other seizures. The second patient had a single seizure on clozapine and haloperidol, likely also in the context of hypocalcaemia that had been diagnosed 11 days prior to seizure. Another patient who had two seizures on clozapine had hypocalcaemia diagnosed 4 weeks prior to seizures.
The seizures of seven (87.5%) individuals who were continued on, or later retried on, clozapine were stabilised through both anticonvulsant treatments and management of hypocalcaemia with vitamin D and calcium supplements. In one notable patient where clozapine had originally been discontinued because of seizures, hypocalcaemia was detected after the trial was discontinued and corrected with supplements. This occurred following diagnosis of 22q11.2DS and routine 22q11.2DS-indicated screening of ionised calcium levels. Reference Bassett, McDonald-McGinn, Devriendt, Digilio, Goldenberg and Habel10 Clozapine was then restarted following prophylactic treatment with an anticonvulsant (gabapentin) for seizure protection. This patient has subsequently been successfully treated with clozapine and concomitant anticonvulsant medications, and vitamin D and calcium supplements for 9 years. Another individual with treated hypocalcaemia and no prior history of seizures was started prophylactically on valproic acid at clozapine initiation to reduce seizure risk and has been successfully managed for 5 years with no seizures.
Other neurological side-effects that tend to be rarely associated with clozapine, 22 including myoclonus, tremor, unsteady gait, rigidity and slurred speech appeared to be relatively common in the 22q11.2DS group (Table 2). Eight (40%, n = 5 male) patients in the 22q11.2DS group but just one woman in the idiopathic group had myoclonus on clozapine (gender-adjusted OR = 4.7, 95% CI 0.6–36.2). Myoclonus was defined by either explicit report of ‘myoclonic jerks’ (n = 5) or ‘myoclonus’ (n = 2) or description of abnormal movements consistent with myoclonic episodes (n = 2; ‘jerky movements and twitching’, ‘jerking of limbs’). Two other patients in the 22q11.2DS group developed abnormal involuntary movements (‘periodic orofacial movements’ and ‘head-tilting’). Six of the individuals with myoclonus in the 22q11.2DS group had a seizure history (online Table DS1). Less frequent clozapine treatment-emergent side-effects, including other neurological symptoms, are described in online Table DS2.
Discontinuation and retrials of clozapine
At the last follow-up, the majority of patients were still being treated with clozapine (n = 13, 65% in each group). Clozapine was discontinued on initial trial in the 22q11.2DS group more commonly (n = 12, 60.0%), but non-significantly, than in the idiopathic group (n = 9, 45.0%, χ2 = 0.90, P = 0.34), and for different reasons, including rare serious side-effects (Table 3). Eight of these 12 (66.7%) patients in the 22q11.2DS group had a retrial of clozapine. Five (62.5%) retrials were successful with clinical improvement and no further clozapine discontinuations (Table 3). The other three patients had second discontinuations because of recurrent ‘red zone’ neutropenia (n = 2) or Parkinson’s disease (n = 1). This last patient was given a third trial of clozapine (maximum dose, 300 mg) upon relapse of psychosis but this was also discontinued, related to concerns about worsening Parkinsonism. In contrast, the reasons for discontinuation in the idiopathic group were primarily somatic complaints (Table 3). In three (33.3%) patients with idiopathic schizophrenia, there were retrials of clozapine; after which two showed clinical improvement.
Table 3 Reasons reported for initial discontinuation of clozapine treatment and success of clozapine retrials in adults with 22q11.2 deletion syndrome (22q11.2DS) schizophrenia compared with idiopathic schizophrenia

| Reason | n |
|---|---|
| 22q11.2DS schizophrenia group | 12 |
| Serious adverse effects | 6 |
| Red zone neutropenia | 3 |
| Seizures | 2Footnote a |
| Myocarditis | 1 |
| Insufficient clinical improvementFootnote b | 2Footnote a |
| Non-adherence/weight gain | 1 |
| Tachycardia/hypertension | 1Footnote a |
| Tachycardia/venipuncture difficulties | 1Footnote a |
| Venipuncture difficulties | 1Footnote a |
| Idiopathic schizophrenia group | 9 |
| Non-adherence | 2Footnote a |
| Unknown | 2 |
| Sedation | 1 |
| Sedation/unsteadiness | 1Footnote a |
| Heartburn | 1 |
| Other gastrointestinal complaints | 1 |
| Venipuncture difficulties/‘feeling funny’ | 1 |
a. Denotes successful retrial (n = 1) each: showed clinical improvement with retrial of clozapine and has had no further discontinuations.
b. One patient responded well to clozapine before the development of Parkinson’s disease and subsequent treatment changes (for example clozapine dose reduction to 100 mg with patient non-adherence and trials with adjunctive antipsychotics and electroconvulsive treatment). The other patient was treated with clozapine before discontinuing at approximately 6 months (maintenance dose 275 mg) and had a successful clozapine retrial (maintenance dose 300 mg).
Discussion
The results of this study demonstrate the potential for clinical utility and personalised psychiatric care with a molecular diagnosis of schizophrenia. Although adults with 22q11.2DS-schizophrenia responded as well to clozapine as those with idiopathic forms of schizophrenia, including significantly reduced hospitalisations, there were clinically relevant differences. Whereas a significantly lower dose was needed to achieve clinical improvement, there was a significantly higher proportion with a serious side-effect in the 22q11.2DS group (seizures, severe ‘red zone’ neutropenia and myocarditis).
Notably, our findings of low clozapine doses and serious side-effects are consistent with previous case reports of clozapine treatment in individuals with 22q11.2DS. Reference Krahn, Maraganore and Michels15–Reference Le Page19,Reference Yacoub and Aybar21,Reference Praharaj, Sarkar and Sinha43 Reports of seven patients show a low median therapeutic dose of clozapine (200 mg, range 75–350 mg). Reference Krahn, Maraganore and Michels15–Reference Gladston and Clarke18,Reference Yacoub and Aybar21,Reference Praharaj, Sarkar and Sinha43 Seizures were reported in four patients, Reference Krahn, Maraganore and Michels15,Reference Usiskin, Nicolson, Krasnewich, Yan, Lenane and Wudarsky17,Reference Gladston and Clarke18,Reference Yacoub and Aybar21 and agranulocytosis in another. Reference Le Page19 The results suggest that although patients with this underrecognised subtype account for up to 1% of all patients with schizophrenia 5–Reference Bassett, Scherer and Brzustowicz7 and have demonstrable efficacy of clozapine, they may be disproportionately represented in those with a rare serious adverse event on clozapine. Clinical testing for 22q11.2 deletions thus has significant implications in providing personalised management of schizophrenia.
Clinical implications
Studies of clozapine in schizophrenia consistently demonstrate its significant therapeutic benefits, including substantial clinical improvements and reductions in admissions to hospital. 22,Reference Meltzer, Alphs, Green, Altamura, Anand and Bertoldi24 Findings from the current study indicate that clozapine treatment response in patients with 22q11.2DS-schizophrenia is at least as good as in other forms of schizophrenia. Importantly, despite the increased risk of serious side-effects, the majority of patients with 22q11.2DS-schizophrenia in this study currently remain well treated with clozapine. Our experience and that of others with similar clinical situations indicates that a ‘start low, go slow’ approach in the context of dose and dose titration increases the likelihood of successful outcome. Reference Yacoub and Aybar21 In addition, careful monitoring, and prophylactic anticonvulsant medication and calcium and vitamin D supplementation may specifically help reduce associated side-effects in the context of the lowered seizure threshold of 22q11.2DS. Reference Bassett, McDonald-McGinn, Devriendt, Digilio, Goldenberg and Habel10 Importantly, for patients in this study, changes such as these in management strategies largely made it possible for them to continue on clozapine, or have a retrial. Although we observed that seizures occurred in more patients with 22q11.2DS-schizophrenia during clozapine treatment than when they were treated with any other atypical antipsychotic, this result did not reach statistical significance. Studies of the neurological side-effects of other antipsychotic medications are needed for 22q11.2DS.
Standard blood monitoring protocols were sufficient to detect neutropenia, despite the increased risk observed in the 22q11.2DS group; notably, no patients progressed to agranulocytosis. The lower baseline WBC reported here and previously Reference Bassett, McDonald-McGinn, Devriendt, Digilio, Goldenberg and Habel10,Reference Lazier, Chow, AbdelMalik, Scutt, Weksberg and Bassett44 in patients with 22q11.2DS-schizophrenia may mediate a lowered threshold to this rare side-effect of clozapine. Larger studies with additional reports of individuals with 22q11.2DS-schizophrenia treated with clozapine are needed to clarify the potential association of the 22q11.2 deletion with increased risk of severe neutropenia and the very rare occurrence of myocarditis.
In addition to seizures, the risk of other neurological abnormalities may also be elevated in 22q11.2DS-schizophrenia. Reference Bassett, McDonald-McGinn, Devriendt, Digilio, Goldenberg and Habel10 Myoclonus has been reported previously in two women patients with 22q11.2DS treated with clozapine, Reference Gothelf, Frisch, Munitz, Rockah, Laufer and Mozes16,Reference Gladston and Clarke18 although in one patient myoclonic jerks were attributed to valproate. Reference Gothelf, Frisch, Munitz, Rockah, Laufer and Mozes16 Given that individuals with 22q11.2DS are susceptible to early-onset Parkinson’s disease, Reference Butcher, Kiehl, Hazrati, Chow, Rogaeva and Lang28 the emergence or worsening of motor symptoms such as tremor and rigidity, observed in some patients in this study, could indicate a progression of neurodegenerative disease or the unmasking of a vulnerable nigrostriatal system by any antipsychotic treatment. Reference Tinazzi, Cipriani, Matinella, Cannas, Solla and Nicoletti45 Interestingly, there is one prior report of an individual with 22q11.2DS with early-onset Parkinsonism whose muscle rigidity dramatically worsened following a change to clozapine from fluphenazine treatment. Reference Krahn, Maraganore and Michels15 Further studies are warranted to examine the side-effects of clozapine and other antipsychotic medications on motor functioning in adults with 22q11.2DS, especially given clozapine’s low binding affinity for dopamine receptors. 22
Study strengths and limitations
To our knowledge, this is the first study to demonstrate that treatment outcomes and management can show clinically relevant differences in a genetic subtype of schizophrenia. We had access to a sample of adults with 22q11.2DS-schizophrenia treated with clozapine with extensive lifetime medical records available and a matched comparison group molecularly screened to exclude pathogenic copy number variations. Replication in independent samples of well-characterised adults using comparable methods and ideally a prospective design with long-term follow-up would be desirable. However, to our knowledge, our cohort remains the largest available (in this age range) on clozapine with long-term phenotypic data. The aetiological homogeneity of 22q11.2DS-schizophrenia likely provided increased power to detect significant differences Reference Chow, Watson, Young and Bassett12 in our modest sample size.
The main limitations of the current study are the retrospective, naturalistic design and unavoidable restrictions of available data. The frequency of side-effects, especially those that are mild and less likely to be documented in clinical records, may be underestimated. The possible effects of polypharmacy, often necessary in managing adults with 22q11.2DS-schizophrenia, Reference Bassett, McDonald-McGinn, Devriendt, Digilio, Goldenberg and Habel10,Reference Fung, Butcher, Costain, Andrade, Boot and Chow46 in mediating side-effect risks during clozapine treatment requires additional study. We note that there were no serious side-effects in the idiopathic group, which demonstrated similar rates of antipsychotic polypharmacy. Clozapine was the only medication common to the individuals with 22q11.2DS-schizophrenia who developed serious side-effects.
Further studies will also be necessary to elucidate the mechanism underlying the increased risk of rare, serious side-effects in 22q11.2DS-schizophrenia. Our sample was under-powered to detect differences with small effect-sizes between groups. We did adjust for possible gender effects on side-effect risks (for example all three patients in the 22q11.2DS group who developed severe neutropenia were women) but not for ethnicity, given that all individuals in the 22q11.2DS group who experienced serious side-effects were White (except two patients with seizures) and this would therefore not be expected to weaken the findings. However, other possible differences would include genome-wide variants modifying drug response, such as CYP enzyme genotypes, or non-genetic factors (such as smoking). The latter two factors may affect clozapine metabolism but are unlikely to have an impact on serious side-effects that are not dose-dependent (for example myocarditis and severe neutropenia) in people with 22q11.2DS-schizophrenia. Reference Ozomaro, Wahlestedt and Nemeroff1,22 They could, however, mediate seizure risk. Other factors suggested to affect generally elevated seizure risk in 22q11.2DS include rare abnormalities of cortical development (such as polymicrogyria, periventricular nodular heterotopia, cortical dysplasia). Reference Bassett, McDonald-McGinn, Devriendt, Digilio, Goldenberg and Habel10,Reference Fung, Butcher, Costain, Andrade, Boot and Chow46 Other large copy number variations do not appear to be a major factor in 22q11.2DS. Reference Bassett, Marshall, Lionel, Chow and Scherer30
In conclusion, the results suggest that the 22q11.2 deletion confers increased sensitivity to clozapine dosage and rare, serious side-effects. The findings provide proof-of-principle of personalised medicine in psychiatry and evidence of the utility of clinical genetic testing in schizophrenia. Molecular diagnosis coupled with targeted management strategies could reduce adverse events and discontinuation rates of clozapine. Patients with 22q11.2DS could represent an identifiable and more genetically homogenous population to investigate the molecular mechanisms mediating psychotropic treatment response and toxicity. Importantly, individuals with 22q11.2 deletions may account disproportionately for reports of rare, serious side-effects associated with clozapine. Additional reports are needed. Studies of 22q11.2 deletions and other high penetrance genetic variants associated with schizophrenia promise to aid in our understanding of the complex aetiology of schizophrenia and treatment response.
Funding
This work was supported by the Canadian Institutes of Health Research (A.S.B., MOP #97800, MOP #111238), Canada Research Chairs program (A.S.B.), a Canadian Institutes of Health Research Frederick Banting and Charles Best Canada Graduate Scholarship (N.J.B.), a Brain Canada Mental Health Training Award (N.J.B.) and a Brain and Behavior Research Foundation (NARSAD) grant (D.M.A.).
Acknowledgements
The authors thank the patients and their families for their participation, students and research assistants from the Clinical Genetics Research Program who assisted with data collection, especially Laura Slade, Evelyn Cheung and Harikesh Wong, and Greg Costain for critical discussions of the manuscript.








eLetters
No eLetters have been published for this article.