Despite the endogenous synthesising capability of individuals, vitamin D (VD) deficiency is widespread globally(Reference Holick1). India is a tropical country with plenty of sunshine and the prevalence of VD deficiency, defined as serum 25 hydroxy vitamin D (25(OH)D) concentrations of < 20 ng/ml ranges from 70 to 100 %(Reference Marwaha, Tandon and Reddy2–Reference Gupta4). This magnitude of VD deficiency in India mandates food fortification, which is as yet not in practice(Reference Gupta4) However, for any effective implementation strategy, there is a need to assess the dose–response relationship between supplemented VD dose and serum 25(OH)D concentrations.
The recommended daily intake of oral VD in populations at risk of VD deficiency to achieve a serum 25(OH)D concentration of more than 20 ng/ml by the Indian Council of Medical Research (ICMR)(5), Indian Academy of Paediatrics (IAP)(Reference Khadilkar and Khadilkar6) and National Academy of Medicine (NAM)(Reference Ross, Taylor and Yaktine7) is 600 μg/d for ages ranging from 1 to 75 years. In contrast, the Endocrine Society (ES) recommends daily intake of 1000 μg/d for 1–18 years old and 1500–2000 μg for all men and women aged above 18 years including pregnant/lactating women, to keep serum 25(OH)D concentrations of > 30 ng/ml considered sufficient for overall good health(Reference Holick, Binkley and Bischoff-Ferrari8). The dilemma, whether serum concentration of 25(OH)D of > 30 ng/ml translates into non-skeletal benefits such as prevention of infections, non-communicable disease like insulin resistance, diabetes mellitus, CVD, psychiatric morbidities, cancer, etc.,(Reference Martineau, Jolliffe and Hooper9–Reference Holick11) due to its pleiotropic effects, continues to persist. The guidelines which suggest to raise serum 25(OH)D > 20 ng/ml for skeletal benefits are not backed by high-quality evidence and mostly involve studies in Western populations(Reference Ross, Taylor and Yaktine7,Reference Rosen, Abrams and Aloia12) .
In India, there is a lack of clear-cut data on the optimal dose/schedule of VD supplementation to achieve serum 25(OH)D > 20 ng/ml, owing to heterogeneity of the studies about age, duration (8 weeks to 1 year), doses (200 to 60 000 μg), sample size, level of serum 25(OH)D considered sufficient and populations studied(Reference Marwaha, Garg and Sethuraman13–Reference Marwaha, Dev and Mittal16). Hence, we undertook this randomised study to evaluate the comparative effectiveness of three different daily dosage regimens of oral cholecalciferol supplementation among themselves and with once-monthly schedule. The main objective was to find appropriate oral VD dose required to achieve VD sufficiency (serum 25(OH)D > 20 ng/ml) at the end of 36 weeks in VD-deficient Indian adults.
Material and methods
Trial design and oversight
A randomised parallel-group, active-controlled trial was conducted at the department of endocrinology at a tertiary care institution of northern India (Latitude: 32°44ʼN, Longitude: 74°54ʼE) from June 2019 to December 2020; however, subjects were recruited and first dose administered within 2 weeks from 1 June to 31 July 2019 and 1 April to 30 May 2020. The study was conducted according to 1975 Declaration of Helsinki. The trial was approved by the Institutional Ethics Committee and registered with Clinical Trial Registry of India (CTRI/2019/01/016855).
Participants
The study participants were invited through advertisements in pamphlets, print and electronic media, detailing the standard operating procedures of the study.
Inclusion and exclusion criteria
All healthy subjects (both males and females) in the age group of 18–60 years who signed an informed written consent were included. The enrolled participants were subjected to a questionnaire-based interview. Age, education level, sun exposure (duration of exposure and body surface area exposed to sunshine based on rule of nine formula)(Reference Moore, Waheed and Burns17), dietary consumption, history of any systemic disorders, medications, health supplements, etc., were recorded at screening. Participants with history of any chronic illness (diabetes mellitus, hypertension, stroke, malignancy, kidney, liver, heart bone or endocrine dysfunction, etc.) or drug intake in previous 2 months likely to affect Ca and VD metabolism were excluded. Other exclusion criteria included pregnancy, miscarriage or lactation. The participants having serum 25(OH)D concentrations above 20 ng/ml were excluded from the study.
Randomisation, allocation concealment and blinding
The subjects were randomised into one of the four groups (A: 600 μg/d; B: 1000 μg/d; C: 2000 μg/d; and D: 60 000 μg/month) in 1:1:1:1 ratio using computer-generated random number sequence. Subjects were provided an opaque and sealed envelope containing the random sequence number for the intervention. The envelopes were opened in a numerical sequence, as each new person entered into the trial. The trial was partially blinded. All the subjects and the investigators were blinded to three arms (daily doses of cholecalciferol); however, the monthly dosing schedule arm was open label. All subjects received the drug in the form of odourless, amber-coloured capsules packed as thirty capsules in coded unlabelled bottles with the exception that the subjects in the fourth arm received the drug as a single monthly capsule with similar attributes. The random sequence was deposited with central pharmacy until all the data were analysed. The cholecalciferol capsules were manufactured by a standard protocol by pharmaceutical company (USV Private Ltd), and the company had no role in data collection or randomisation. VD content of capsules was analysed independently by another laboratory to contain cholecalciferol within 90–110 % of labelled amount. The trial schedules as well as the results of the randomisation are shown in flow chart (Fig. 1).
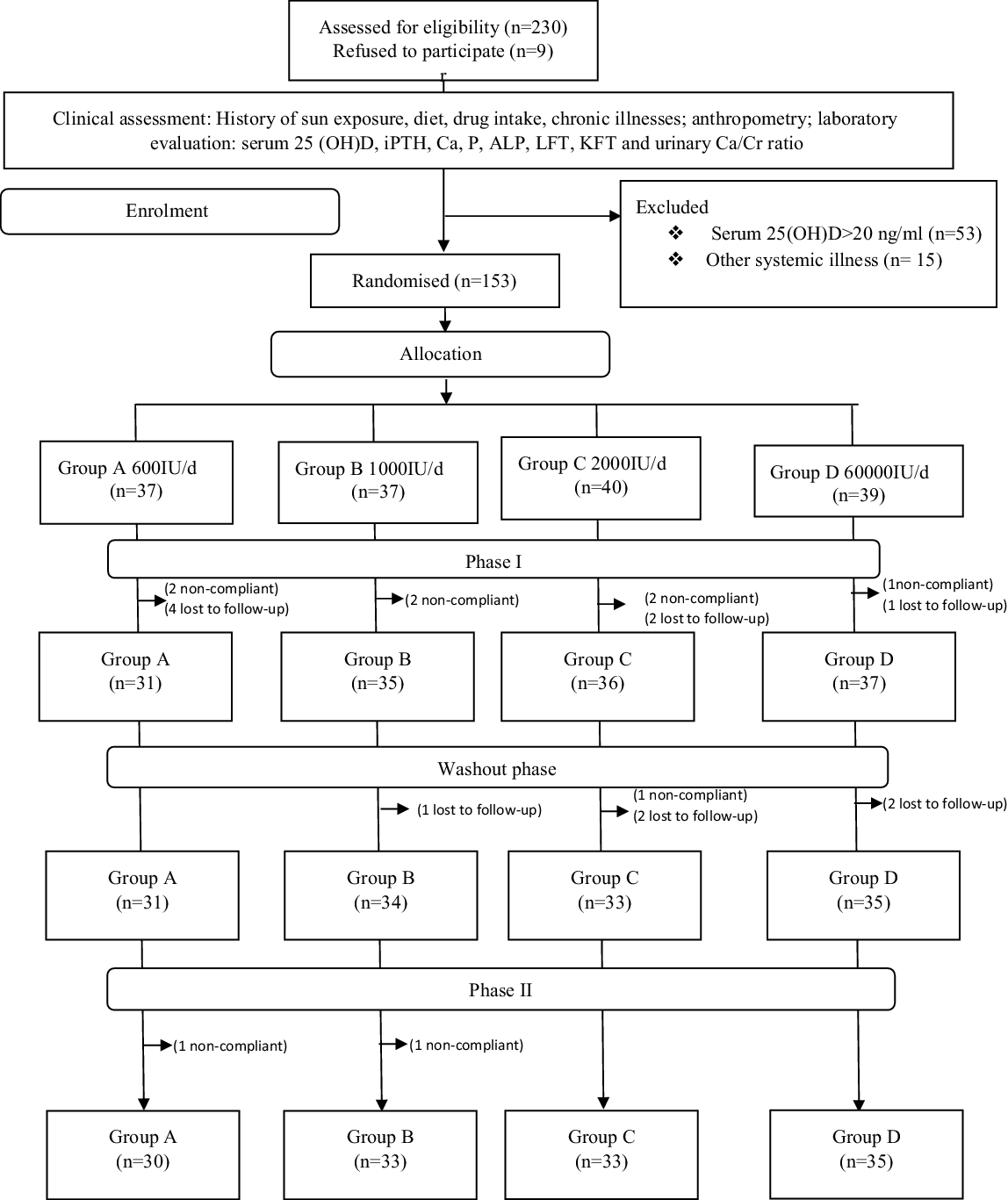
Fig. 1. Enrolment and randomisation. iPTH, intact parathyroid hormone; 25 (OH)D, 25-hydroxy vitamin D; ALP, alkaline phosphatase; LFT, liver function test; KFT, kidney function test.
Treatments and dosing schedule
The included subjects were supplemented with oral cholecalciferol doses as following: Group A: 600 μg/d; Group B: 1000 μg/d; Group C: 2000 μg/d; and Group D: 60 000 μg/month initially for 12 weeks (phase I). These subjects were then kept off supplementation for the next 12 weeks (13–24 weeks; washout phase). Subsequently, the subjects were once again supplemented with the same dose for further 12 weeks (i.e. 25 to 36 weeks; phase II). The objective of the washout phase was to assess the impact of off-supplementation period on serum 25(OH)D concentration and thereby know the periodicity of supplementation required. The duration was based on considering 2–3 weeks half-life of 25(OH)D in literature(Reference Jones, Assar and Harnpanich18). Subjects were instructed to take VD capsules at any time of day with a cup of non-fortified milk. For subjects taking once-monthly doses, capsules were given under supervision of the investigator. Subjects were advised to report any adverse reactions (pain abdomen, nausea, vomiting, dysuria, graveluria and hematuria) immediately either in person or telephonically to their treating physician-in-charge (SS and MAG), and if so, serum total Ca and urine (Ca/Cr was checked immediately. Otherwise, all subjects had periodic monitoring of serum total Ca and urine Ca/Cr at 12, 24 and 36 weeks. During the course of trial, only three subjects (two in Group C and one in Group D) had asymptomatic hypercalciuria which was managed by stoppage of oral cholecalciferol. Since hypercalciuria settled in all three subjects, supplementation was restarted in 1 week. Treatment adherence was ensured by sending SMS and/or telephone calls to all the subjects every 2 weeks. Among daily-dose arms, monthly pill count with return of empty bottles was enforced, while in the Group D (once-monthly dose) VD was administered monthly under direct supervision. During washout phase, no capsules were provided to the subjects and were emphasised not to take any VD or Ca supplements.
Clinical assessment
All the enrolled subjects were clinically examined by single clinician (SS) recording general physical examination including anthropometry, blood pressure, etc. Anthropometric measurements were performed using standard methodologies and instruments (SECA 13) as was for blood pressure (Omron HEM7120, Omron Corporation). Participants were advised to follow stable diet and exercise routine and were advised not to use sunscreens or any drug or additional health supplements likely to effect Ca or VD metabolism unless deemed necessary.
Laboratory evaluation
After an overnight (8–12 h) fast, about 10 ml of venous blood sample was drawn for measurement of serum total Ca, phosphorus (PO4), alkaline phosphatase (ALP), urea, Cr, albumin, 25(OH)D and intact parathyroid hormones (iPTH). The blood samples were collected in plain, ethylenediamine tetra acetic acid and fluoride vacutainers depending upon the assay, and the aliquots were transported as soon as possible in cold boxes to the lab. The samples were aliquoted for immediate biochemical estimations, while as for hormonal analysis samples were stored at −80°C until the assay. Serum total Ca, PO4, ALP, urea, Cr and albumin were measured using commercially available kits on an automated biochemistry analyzer (Response-910 Diasys Diagnostic systems). The serum 25(OH) D and iPTH were measured by electrochemiluminescence assay (COBAS e411, Roche Diagnostics Limited) with respective ranges as 3–100 ng/ml and 10–65 pg/ml. The external quality control for serum 25(OH) D assay in our laboratory is done by participating in Randox International Quality Assessment Scheme (RIQAS), with intra- and inter-assay CV for repeatability as 3·5 and 5 % for serum 25(OH)D and 2·4 and 3·6 % for serum iPTH, respectively. Similarly, the CV for precision was 3·8–8·9 % and 1·7 and 2·0 %, respectively, for serum 25(OH) D and iPTH. The accuracy of serum 25(OH)D assay in our laboratory is about 95 %. Spot urine samples were also collected for the Ca/Cr ratio (both serum Ca and Cr measured in mg) measured using an automated analyzer (Beckman Coulter, Inc.).
Follow-up
All participants were subjected to measurements of repeat serum Ca, PO4, ALP, 25(OH)D, iPTH and urinary Ca/Cr at each follow-up visit, that is, 12 weeks, 24 weeks and 36 weeks. The rate constant defined as change in serum 25(OH)D concentration per 100 μg ingestion was calculated as follows: serum 25(OH)D at 36 weeks minus serum 25(OH)D at baseline divided by total units of cholecalciferol ingested daily in μg multiplied by 100.
Outcomes
The primary outcome measure was percentage of subjects achieving VD sufficiency, that is, serum 25(OH)D > 20 ng/ml at the end of 36 weeks after supplementation with 400, 1000, and 2000 μg of VD daily and 60 000 μg of cholecalciferol monthly. The secondary outcome was the change in concentrations of serum 25(OH)D, iPTH, Ca, PO4, ALP and any adverse events (hypercalcemia, i.e. serum total Ca > 10·5 mg/dl, and hypercalciuria, i.e. spot urine Ca/Cr ratio > 0·2 mg/mg) at the end of 36 weeks.
Sample size calculation
A sample size of eighty-four subjects, twenty-two in each group, is adequate to detect a clinically important difference assuming the small effect size (partial eta squared = 0·03) in the assessment of a primary outcome serum 25(OH)D concentration assuming a correlation among the repeated measures of 0·5 and (non-sphericity correlation = 1) using repeated-measures ANOVA, within–between interaction at 90 % power and a 5 % level of significance. Considering a high attrition rate during the study either due to withdrawal or non-compliance, we enrolled 153 subjects in the study (Group A: n 37, Group B: n 37, Group C: n 40 and Group D: n 39).
Data analysis
All statistical analyses were performed using SPSS software, version 22 (SPSS Inc.). The normality of all the variables was tested using Kolmogorov–Smirnov test, and all the variables in four groups were found to be normally distributed. Baseline variables among different groups were presented as mean and standard deviation, if not stated otherwise. The missing data at different time points were handled using simulation-based multiple imputations method using regression analysis. Variables were summarised with repeated-measures ANOVA for comparisons within and between group effects on the key outcomes. The model include intervention group (four treatment groups) x time (four time points) as fixed factors for the outcome measures (serum 25(OH)D, serum iPTH, serum Ca, serum PO4, serum ALP and urine Ca/Cr ratio)). If there were significant interactions, the post hoc analysis was done using Tukey’s HSD (equal variances) or Games-Howell (unequal variances). The homogeneity of variance (sphericity) was checked by the Mauchly’s test of sphericity. The Greenhouse–Geisser correction done in case sphericity assumption was violated. We used a generalised linear model (binomial distribution) with the dependent variable as VD sufficiency (Yes/No) to find the difference in VD sufficiency status in different doses and different time points. Quoted P values are not adjusted for multiple comparisons. To preserve the original randomisation and to avoid the bias due to exclusion of patients, the data were analysed by intention-to-treat analysis. All the tests were conducted two-sided, and a P value of < 0·05 was considered statistically significant.
Results
Of total 230 subjects screened, nine refused to participate and 221 were clinically evaluated, out of which sixty-eight were excluded either because their serum 25(OH)D concentrations were > 20 ng/ml (n 53) or had systemic illnesses (n 15). The remaining 153 subjects were enrolled and randomised into four groups (Group A: n 37; Group B: n 37; Group C: n 40; and Group D: n 39) to receive oral cholecalciferol doses of 600 μg/d, 1000 μg/d, 2000 μg/d or 60 000 μg/month, respectively (Fig. 1). No significant difference was reported in age, clinical, anthropometric and biochemical characteristics at baseline (Table 1). Overall, in the screened population (n 221), 69·2 % of the subjects were VD-deficient. The average daily sun exposure of screened subjects was 40 ± 12 min with average body surface area exposed to sun was 8·75 %. During the course of trial, twelve subjects were lost in the follow-up and ten subjects were withdrawn from the final analysis due to non-compliance (Fig. 1).
Table 1. Demographic and baseline characteristics of study participants
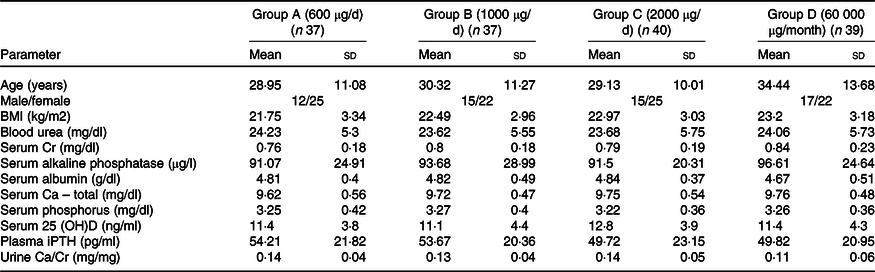
25 (OH)D, 25 hydroxy vitamin D; iPTH, intact parathyroid hormone.
One-way ANOVA test was performed to compare groups
A significant time–group interaction was found in serum 25(OH)D concentration. The Post hoc analysis revealed that serum 25(OH)D concentration increased significantly from baseline to 12 weeks (phase I of supplementation) in all the groups. At 24 weeks (end of washout phase), no change in serum 25(OH)D concentrations was observed compared with 12 weeks. A further increase in serum 25(OH)D concentration was observed at 36 weeks (end of phase II of supplementation) in all the groups (Table 2). Group C had a significantly higher increase in serum 25(OH)D concentration at 12 and 36 weeks compared with other groups (Table 2). The overall mean rate constant of study subjects was 1·7 ± 0·7 ng/ml/100 μg. At the end of the study, 65 %, 78 %, 95 % and 90 % of the subjects in Groups A, B, C and D, respectively, were VD-sufficient (Table 3 and online Supplementary Table 1). However, no difference was observed in Group C and Group D in proportion of subjects achieving VD sufficiency.
Table 2. Trajectory of bone mineral parameters with different oral cholecalciferol dosing regimen and schedules
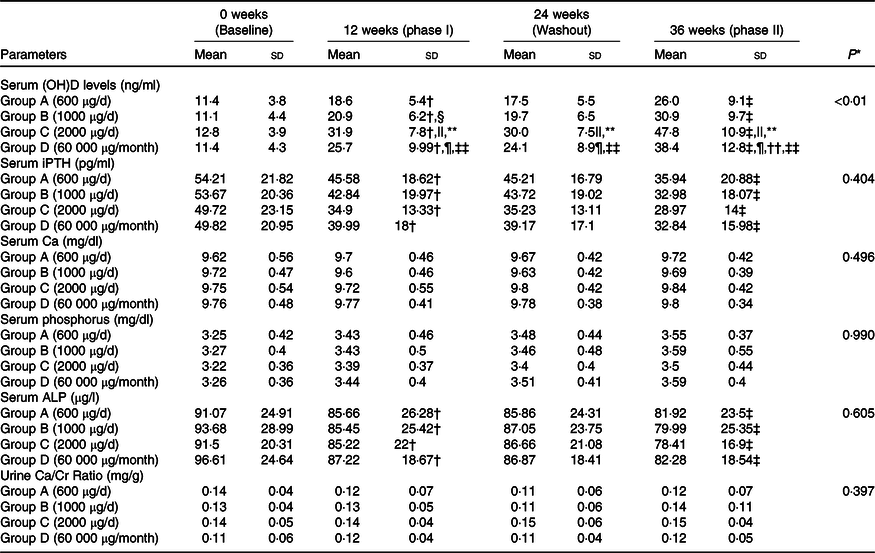
25 (OH)D, 25 hydroxy vitamin D; iPTH, intact parathyroid hormone; ALP, alkaline phosphatase.
* P value for time-by-group interactions; P < 0·05 statistically significant; analysed using repeated-measures ANOVA; P < 0·05 is considered as significant.
† P < 0·05, baseline v. phase I.
‡ P < 0·05, washout v. phase II.
§ Group B v. A.
|| Group C v. A.
¶ Group D v. A.
** Group B v. C.
†† Group B v. D.
‡‡ Group C v. D; intention-to-treat analysis.
Table 3. Proportion of subjects with sufficient serum 25 (OH)D at different phases of supplementation
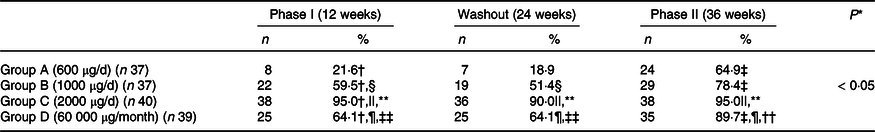
25 (OH)D, 25 hydroxy vitamin D.
* P value for group-by-time interactions.
† P < 0·05, baseline v. phase I.
‡ P < 0·05, washout v. phase II.
§ P < 0·05, Group A v. B.
|| P < 0·05, Group A v. C.
¶ P < 0·05, Group A v. D.
** P < 0·05, Group B v. C.
†† P < 0·05, Group B v. D.
‡‡ P < 0·05, Group C v. D; VD sufficient > 20 ng/ml; Wald test ; P < 0·05 is considered as significant.
Serum iPTH decreased significantly from baseline to 12 weeks, with no change during washout phase and a further significant decrease from 24 to 36 weeks, with no time–dose interactions. Similarly, no significant time–dose interactions were observed in serum Ca, PO4 and ALP or urine Ca/Cr ratio (Table 2). No major adverse events were recorded during this trial except asymptomatic hypercalciuria in three subjects (two subjects in Group C and one in Group D) at 12 weeks which subsided after stopping oral cholecalciferol for 1 week.
Discussion
The pursuit to arrive at an optimal daily dose of VD supplementation to prevent VD deficiency continues because of discrepant recommendations for adults by NAM, ICMR (600 μg/d) and ES (1500–2000 μg/d)(5,Reference Ross, Taylor and Yaktine7,Reference Holick, Binkley and Bischoff-Ferrari8) generating significant debate(Reference Rosen, Abrams and Aloia12,Reference Rosen, Abrams and Aloia12) . Among plethora of studies in literature, only few are comparable due to varying duration and doses of VD supplementation, ages and sex of subjects as well as cut-off levels taken to define sufficiency(Reference Goswami, Gupta and Ray15,Reference Sacheck, Van Rompay and Chomitz19–Reference Talwar, Aloia and Pollack24) . There are no studies reporting impact of daily oral cholecalciferol supplementation on serum 25(OH)D concentrations among Indian adults. In view of the above, we undertook this prospective randomised trial comparing three different daily oral doses v. monthly oral bolus of cholecalciferol among VD-deficient adults.
High prevalence (68·9 %) of VD deficiency in the present study with an average daily sun exposure of 40 ± 12 min over 8·75 % (0–14) body surface area reiterates the reported widespread nature of VD deficiency among Indians(Reference Marwaha, Tandon and Reddy2–Reference Gupta4). On daily oral supplementation with 600 and 1000 μg of cholecalciferol, 65 % and 78 % subjects achieved VD sufficiency, leaving a significant percent of population VD-deficient at the end of 36 weeks. These observations are in contrast to those reported by Gallagher et al. (Reference Gallagher, Sai and Templin21) and Lehman et al. (Reference Lehmann, Riedel and Hirche25), who demonstrated that supplementing with 800 μg/d oral cholecalciferol achieved VD sufficiency in approximately 94 % subjects. Both these studies were conducted in non-Asian subjects, and their baseline serum 25(OH)D was higher than the current study. In addition, the duration of treatment was 12 months in Gallagher et al. (Reference Gallagher, Sai and Templin21) as against 6 months in the current study. Likewise, Cashman et al. (Reference Cashman, Fitzgerald and Kiely26) in a meta-regression analysis of forty-four non-Asian studies also worked out a supplementation dose of 930 μg/d for achieving serum levels of 25(OH)D > 50 nmol/l or 20 ng/ml. Several studies suggested suboptimal efficacy of doses ranging from 400 to1000 μg daily among non-Indian adult subjects(Reference Sacheck, Van Rompay and Chomitz19,Reference Dawodu, Saadi and Bekdache20,Reference Ng, Scott and Drake23,Reference Talwar, Aloia and Pollack24,Reference Bonjour, Dontot-Payen and Rouy27) consistent with the results of the present study. In one of our studies, Indian children could achieve VD sufficiency in 71·5 % and 81·8 % with daily supplementation of 600 and 1000 μg for 6 months(Reference Marwaha, Garg and Sethuraman13). In most of these studies, baseline serum 25(OH)D was lower as well as duration of treatment was less than 6 months.
The major observation of the present study was the adequacy of 2000 μg in achieving VD sufficiency among 95 % subjects, consistent with other studies wherein VD was supplemented for 3 to 24 months among non-Asians adult subjects(Reference Ng, Scott and Drake23,Reference Talwar, Aloia and Pollack24,Reference De Niet, Coffiner and Da Silva28,Reference Diamond, Wong and Golombick29) . Few authors, however, did report suboptimal efficacy at equivalent or even higher than 2000 μg/d prescribed in this study, especially in pregnancy, lactation and patients with diabetes mellitus or fractures(Reference Dawodu, Saadi and Bekdache20,Reference Saadi, Dawodu and Afandi30–Reference Al-Shahwan, Al-Othman and Al-Daghri32) . The dose-dependent increase of 35·74 ng/ml in serum 25(OH)D concentration with 2000 daily μg in the current study was similar to what was observed in earlier study(Reference Shab-Bidar, Bours and Geusens31) but higher than that observed by Chandler et al. (19·2 ng/ml)(Reference Ng, Scott and Drake23) and Shieh et al. (13·8 ng/dl)(Reference Shieh, Ma and Chun33) likely because of higher BMI, higher baseline serum 25(OH)D and shorter duration of treatment in these studies.
The mean rate constant of 1·7 ± 0·7 ng/ml/100 μg in our study was higher than that reported by Holick M F et al. (Reference Holick, Binkley and Bischoff-Ferrari8) but lower (2 ng/ml/100 μg) than that reported by McKenna et al. (Reference McKenna and Murray34) in severely VD-deficient elderly subjects. This supports the fact that baseline 25(OH)D concentration determines the rate constant inversely. This lower rate constant may also be explained by altered VD metabolism in Asian/Indian population(Reference Awumey, Mitra and Hollis35) with documented increased 24-hydroxylase activity which may increase metabolism of 25(OH)D and decrease its concentrations.
VD sufficiency achieved in 90 % subjects with monthly dose of 60 000 μg is more or less similar (95 %) to that achieved with oral dose of 2000 μg/d, though with a higher increase in serum 25(OH)D concentration in Group C. This finding is supported by reports from Niet et al. in adults(Reference De Niet, Coffiner and Da Silva28) and Marwaha et al. (Reference Marwaha, Dev and Mittal16) among others(Reference Saadi, Dawodu and Afandi30,Reference Binkley, Gemar and Engelke36–Reference Ish-Shalom, Segal and Salganik38) who documented 100 % and 90 % subjects attaining serum 25(OH)D concentration > 20 ng/ml, respectively, with 60 000 μg and 50 000 μg oral monthly doses. These results assume significance in view of the fact that once-monthly cholecalciferol supplementation may be convenient for people and ensure better compliance.
The interesting point of this study was observing washout period from week 13 to 24 to observe time dependency of VD supplementation and impact of baseline concentration on its kinetics. The initial supplementation for 12 weeks increased serum 25(OH)D concentration significantly in all groups rendering about 22 %, 60 %, 95 % and 64% of VD sufficient in Groups A, B, C and D, respectively. However, stopping supplementation for 3 months did not change serum 25(OH)D concentration, contrary to our expectations. This may be probably due to half-life of VD and may suggest the interval of bolus supplementation. Further supplementation in phase II (week 25–36) lead to VD sufficiency in 65 %, 78 %, 95 % and 90 % in Groups A, B, C and D, respectively, at 36 weeks. Carefully observing dose–response VD trials reveal that doses about 1000 μg may achieve sufficiency if given for longer periods, while as higher doses > 1000 μg renders subjects VD sufficient in about 12 weeks(Reference Cashman, Fitzgerald and Kiely26,Reference Gallagher, Jindal and Smith39,Reference Shab-Bidar, Bours and Geusens40) . Thus, we can conclude that not only the dose but also the duration of treatment is also important while considering VD supplementation.
Though no time–dose interaction was observed in serum iPTH concentration during the trial period, serum iPTH concentration decreased significantly at 12 weeks in all groups with no change at 24 weeks but a further decrease at 36 weeks. Some studies(Reference Cashman, Wallace and Horigan22,Reference Bhagatwala, Zhu and Parikh41) have observed dose- and time-dependent decrease in serum PTH after VD supplementation, but the data suggest that the PTH decreasing tendency of VD pleatues about 1000 μg daily(Reference Bhagatwala, Zhu and Parikh41). Except that three subjects (two in Group C and one in Group D) developed transient hypercalciuria which settled after 1 week, no major side effects were reported. To the best of our knowledge, this is the first randomised study simultaneously evaluating efficacy of three oral daily doses and once-monthly bolus dose of cholecalciferol among VD-deficient Indian adults. The study shows that 2000 μg daily or 60 000 μg monthly doses of oral cholecalciferol achieve VD sufficiency in higher percentage of subjects (95 % v. 90 %) than 600 and 1000 μg daily with no major adverse events. Though 2000 μg daily cholecalciferol supplementation had a higher increase in serum 25(OH)D concentration, but once-monthly dose may have better compliance. The results may not, however, be generalisable to population with low prevalence of VD deficiency. However, certain limitations of this study are as follows: (a) inability to evaluate bone formation and resorption markers, (b) spot Ca/Cr ratio instead of 24-h urine Ca excretion was evaluated to gauge hypercalciuria and (c) lack of placebo group as it would provide absolute effect of supplementation on serum 25(OH)D concentration. However, it would have been unethical not to supplement subjects who were VD-deficient, (d) having one arm of study as open label (once monthly 60 000 μg), (e) confounding effect due to seasonal variation in sun exposure, but the recruitment window in our study was relatively narrow and we did not expect any gross differences in the results, (f) lack of standardised assessment of adverse events whereby some minor side effects were not captured, and (g) taking cholecalciferol capsules with cup of milk though not fortified with VD may have a confounding effect on 25(OH)D concentration, especially in Group D and during washout phase.
We conclude that while reiterating the high prevalence of VD deficiency among Indian adults, the study demonstrates that 2000 μg of oral cholecalciferol daily for 6 months is safe and efficacious among VD-deficient Indian adults. Administering this as a single monthly dose of 60 000 μg orally is a plausible option.
Acknowledgements
The authors acknowledge the critical review comments of Dr Ishaq Geer and USV India Private Limited, for providing customised (VD) formulation. The authors also acknowledge the Multi-Disciplinary Research Unit, SKIMS, Srinagar funded by the Department of Health Research, Govt of India, for providing necessary research facilities for carrying out this study.
The study was partly funded by Endocrine Society of India.
M. A. G., R. K. M., I. A. W. and R. A. M. designed the research; S. S., T. S., A. R. and I. A. W. conducted the study; M. A. G., M. S. B., T. S., A. R. W., S. S. and M. M. A. analysed the data; M. A. G., M. S. B., S. S., T. S., M. M. A. and R. A. M. wrote the manuscript; and M. A. G. was primarily responsible for final content. All authors have read and approved the final manuscript.
Authors disclose no conflict of interest except that customised (VD) formulation was provided by a pharmaceutical company (USV India Pvt Ltd).
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522002641







