For decades, CVD has been accounting for the main death rate in different countries with upper-middle income or high income(1). In Germany, about 40 % (approximately 140 000) of deaths were attributed to CVD events in 2017(Reference Timmis, Townsend and Gale2). Evidence from clinical studies suggested that dyslipidaemia, which is mainly characterised by elevated levels of LDL-cholesterol, TAG or total cholesterol (TC), is the important predictive factor for CVD(Reference Zhao, Liu and Xie3–Reference Hohmann, Cramer and Michalsen5). Generally, reducing LDL-cholesterol concentration is the primary target for individuals who have high risk of arteriosclerotic CVD in clinics, and statin is the preferred drug(Reference Rabar, Harker and O’Flynn6,7) . However, for patients at the early stage of dyslipidaemia, drug therapy seems not so necessary; on the other hand, dietary habits and lifestyle modification may play primary roles in reducing the risk of CVD with different kinds of mechanisms(Reference Georgousopoulou, Panagiotakos and Pitsavos8,Reference Rivellese9) , being also consistent with American Heart Association guidelines on the management of blood cholesterol(Reference Grundy, Stone and Bailey10). For that reason, it has been attracting researchers’ attention to seek an alternative therapy like transforming the diet pattern to improve abnormal lipids.
β-Glucan, a kind of dietary fibre, can be found not only in the cell wall of certain micro-organisms but also in protists like mushrooms and grains. In fact, there are two different linkages that exist in β-glucan: mixed β-(1,6) and β-(1,3) glucosidic linkages, derive from yeast and mushrooms, which are insoluble; another one is β-(1,3/1,4)-D-linked glucose units originating from oat and wheat, which is soluble(Reference Vetvicka and Vetvickova11–Reference Lazaridou and Biliaderis13). In general, most of the biological effects of yeast β-glucan have been focused on enhancing immunity(Reference Novak and Vetvicka14,Reference Vetvicka, Richter and Svozil15) ; on the other hand, the majority of reports about the health benefits of β-glucan obtained from grains are focused on the modulation of blood lipids, and part of mushrooms were included(Reference Ferguson, Stojanovski and MacDonald-Wicks16–Reference Smith, Queenan and Fulcher18). Since Groot reported the cholesterol-lowering effect of β-glucan for the first time(Reference Groot, Pikaar and Luyken19), a quantity of studies with further objectives have been performed to explore the biological efficacies of β-glucan. A recent review by Sima et al. (Reference Sima, Vannucci and Vetvicka20) summarised the relationship between β-glucan and cholesterol and listed the clinical evidence in detail. Official institutions like the Food and Drug Administration (FDA) recommended individuals obtaining beneficial effects by supplementing more than 3 g oat β-glucan each day. To date, robust evidence from clinical studies and meta-analysis has confirmed the cholesterol-lowering effects of β-glucan(Reference Whitehead, Beck and Tosh21–Reference Keenan, Goulson and Shamliyan25); of those, however, participants included in trials involved populations without any restriction on blood lipids, either in healthy individuals, hypercholesterolaemic patients or a mixture of both; in other words, effects of β-glucan on lipids in mildly hypercholesterolaemic individuals remained inconclusive. Therefore, illuminating the benefits of β-glucan for a population with marginal high cholesterol may be better to reflect its primary application value. In addition, although β-glucan with different molecular weights (MW) was assessed in trials(Reference Wang, Harding and Eck26), few studies have paid attention to the effects of delivering matrices where β-glucan will be incorporated into, which may execute a minor discrepancy on blood lipids to some extent.
Hence, to further investigate the potential effects of β-glucan in mildly hypercholesterolaemic individuals, we deemed it necessary to collect a quantitative synthesis of evidence and make a meta-analysis to evaluate the effect in a mildly hypercholesterolaemic population.
Methods
This meta-analysis was conducted in accordance with The Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines(Reference Moher, Liberati and Tetzlaff27). In addition, there was no necessity for ethical approval since all trials included in this meta-analysis were published officially.
Literature search strategy
Potential literature was identified through a systematic and comprehensive search in June 2019 in the following four electronic databases without language restriction: Web of Science, PubMed, Scopus and Cochrane Library. We employed a combination of MESH terms and keywords for searching (‘Hypercholesterolemia’ or ‘Hyperlipidemias’ and ‘beta-Glucans’ or ‘Glucans ’ or ‘β-glucan’). References in papers were viewed as well so that any relevant studies were not missed. Data extraction was performed by two independent reviewers (D. X. and H. L.). Our study was limited only to randomised controlled trials, and any disagreements were resolved either by consultation together or by the third author (H. X.).
Inclusion and exclusion criteria
To do the meta-analysis, selected studies must meet the following criteria: (1) study design has to be randomised controlled trial which evaluated the effects of β-glucan on blood lipids, (2) the level of fasting serum TC or LDL-cholesterol was between 5·0 and 8·0 mmol/l or 2·7 and 5·0 mmol/l, respectively(Reference Thandapilly, Ndou and Wang28), (3) inclusion of an appropriate control diet and (4) contain data with available mean change from end point to baseline and any of sd, se or 95 % CI for blood lipids.
The exclusion criteria were listed as below: (1) research objectives were animal or cell, (2) secondary information like reviews, (3) articles without sufficient data (e.g. data were showed only by figures) or suitable control group and (4) reporting outcomes were which we have no interest.
Data extraction and methodological assessment
Detailed information of articles included in this meta-analysis was extracted as follows: first author and year, sample size (male/female), mean age, baseline of blood lipids, sources of glucan, delivering matrices, amount of glucan, duration, comparison group and study design. Besides, net mean change and standard deviation of TC, TAG, LDL-cholesterol and HDL-cholesterol in each group were also collected; for studies which did not show effective data directly, we calculated it according to the following methods: the net mean change of blood lipids was measured through subtracting the end and baseline values, we assumed the last one as the end value if there were end points more than one; for sd of the net change, formula was used as below:
for designing a correlation coefficient R 0·5 according to Higgins et al. (Reference Higgins, Altman and Gotzsche29). We divided the delivering matrices of β-glucan given to volunteers into three ways: incorporated into food like bread and biscuits, named ‘solid products’; dissolved in drink like milk and beverages, called ‘liquid products’; and the last one is a combination of both, which means volunteers consumed β-glucan derived from both ‘solid products’ and ‘liquid products’. Also, we regarded there was a parallel number of trials when involving multiple experimental groups in a single study. It is worth noting that we had converted mg/dl into mmol/l if necessary before the meta-analysis was performed; the conversion coefficient of TC, TAG, LDL-cholesterol and HDL-cholesterol is 0·0259, 0·0113, 0·0258 and 0·0258 for 1 mg/dl, respectively.
Quality assessment was examined based on the Cochrane Risk of Bias Tool(Reference Higgins, Altman and Gotzsche29), and the tool mainly covers seven validity questions: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias; each item was scored by high, unclear, low risk of bias for all included studies; upon evaluating above domains for each article, a figure of overall risk of bias was derived.
Statistical analysis
First of all, we have an assumption that randomisation could balance the baseline value between groups and effective value is estimated using the inverse-variance method(Reference Higgins and Green30). Heterogeneity within studies was assessed by means of the I 2 statistics, which range from 0 to 100 %. We considered I 2 of <25 and 50 % as a no obvious and a moderate heterogeneity, respectively, and an I 2 of >75 % suggests a high level of heterogeneity; based on this, a fixed effects model was applied if heterogeneity is <50 %; otherwise, the random effects model was used. In addition, we performed a sensitivity analysis and subgroup to investigate the sources of heterogeneity by omitting each study and then repeating the analysis, and the subgroup analysis was conducted by delivering matrices of β-glucan, sources of β-glucan, dose of daily intake, duration of intervention, mean age of participants, study design and controlled diets. Furthermore, a funnel plot and Egger’s regression were created to explore potential publication bias, and a ‘trim and fill’ analysis was to further observe the stability of results if there is any asymmetry in funnel plot(Reference Duval and Tweedie31). Finally, meta-regression with a restricted maximum likelihood method was performed to investigate association between blood lipids change and delivering matrices. All analyses were run in Stata version 12 with a 5 % level of significance.
Results
Search results and trial characteristics
A flow diagram for selected trials is shown in Fig. 1. A total of 1262 articles were identified initially with the search strategy from four databases, of which 1217 were removed after reviewing title and abstracts, including ninety-five duplications. And then, forty-five full-text trials were retrieved for further information; of those, twenty-four studies were excluded for the following reasons: ten for insufficient information, five for inappropriate control and nine for uninterested outcomes. Finally, twenty-one trials with enough data were selected in this meta-analysis.

Fig. 1. Flow diagram for selected trials. RCT, randomised controlled trail.
The present meta-analysis involved 1120 participants in total, ranging from sixteen to 155 for each study, of which the ratio of male:female is 50·8 to 49·2 %. The mean age was between 10·60 and 63·36 years approximately. Sixteen trials were interventions with β-glucan derived from oat, and the remaining were from barley. The mean intervention duration of included studies was 5·95 (sd 2·13) weeks with a daily intake amount of β-glucan varing from 1·45 to 11·2 g. All trials were randomised controlled trials with six cross-over designs and fifteen were performed as parallel studies. More detailed characteristics included in the meta-analysis are summarised in Table 1. Assessments of the risk of bias in included studies are shown in Table 2.
Table 1. Characteristics of included twenty-one trials for meta-analysis
(Mean values and standard deviations)
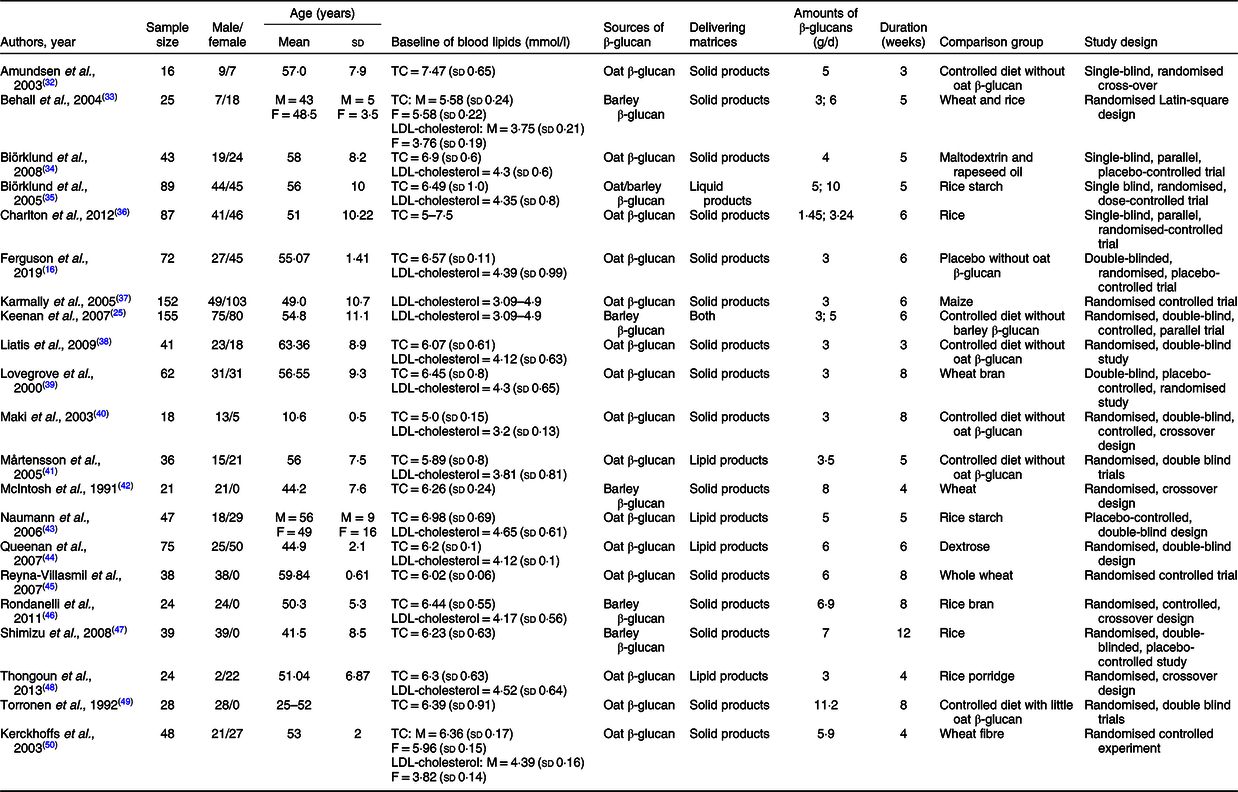
TC, total cholesterol; M, male; F, female.
Table 2. Quality assessments of included studies based on the Cochrane guidelines
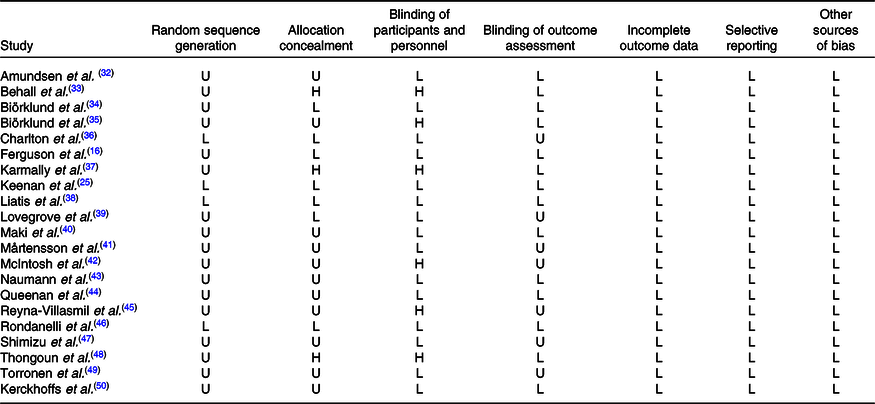
U, unclear risk of bias; L, low risk of bias; H, high risk of bias.
Overall and subgroup effects of β-glucan intake on fasting serum total cholesterol concentration in mildly hypercholesterolaemic individuals
Twenty-nine studies (based on twenty-one articles) were reported to calculate the pooled effect, resulting in a significant decrease on the mean difference of TC of −0·27 mmol/l (95 % CI −0·33, −0·21, P < 0·001) with a random effects analysis. Although substantial evidence of heterogeneity between studies was observed with an overall analysis (I 2 = 71·2 %, P < 0·001), TC reduced more (−0·46 mmol/l, 95 % CI −0·56, −0·35, P < 0·001) after intaking β-glucan with ‘both’ ways in subgroup of delivering matrices and accompanied with a lower heterogeneity (I 2 = 0 %, P = 0·461), which is shown in Fig. 2. More detailed subgroup analysis is summarised in Table 3.
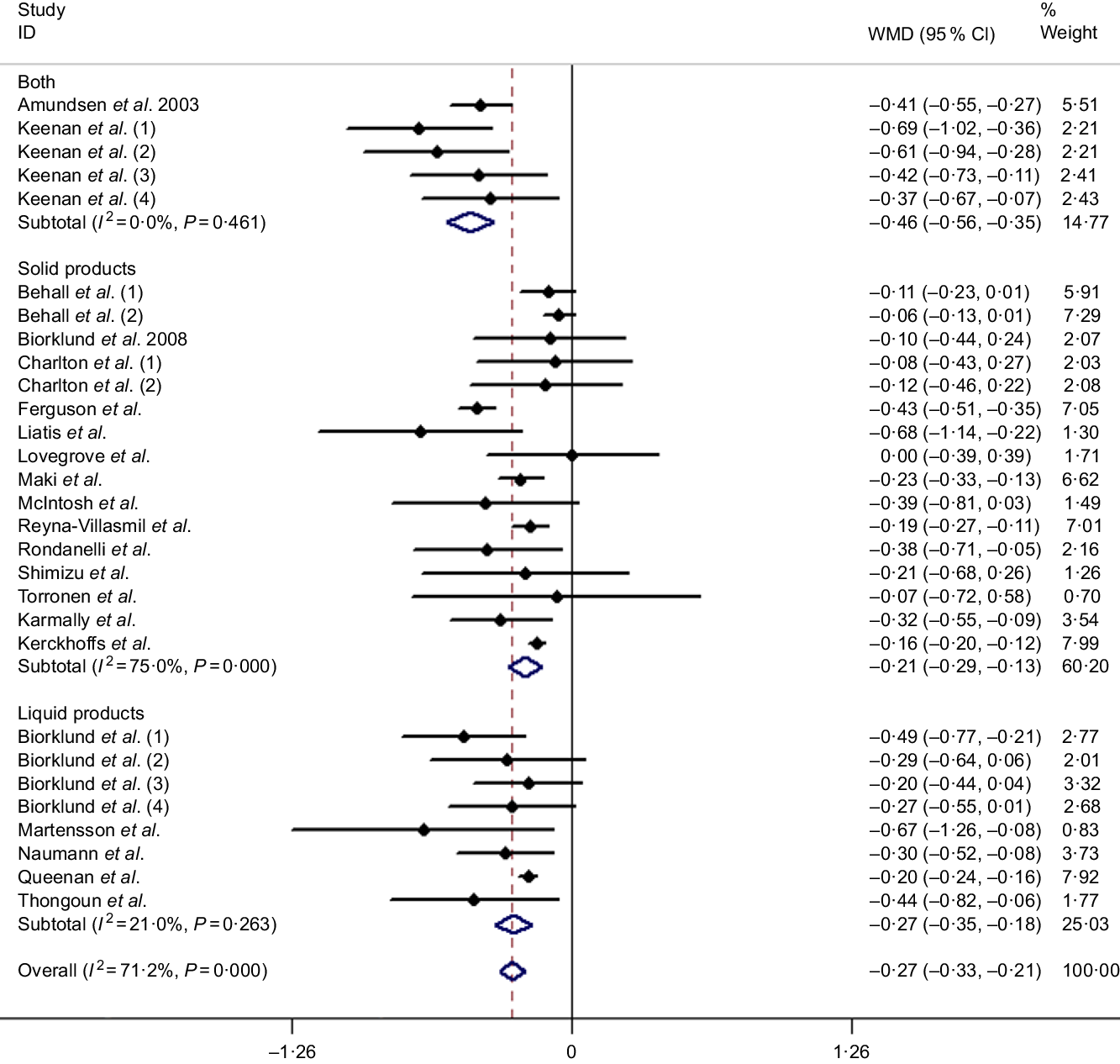
Fig. 2. Overall and subgroup effects of β-glucan intake on fasting serum total cholesterol concentration in mildly hypercholesterolaemic individuals. Weights are from random effects analysis. WMD, weighted mean difference.
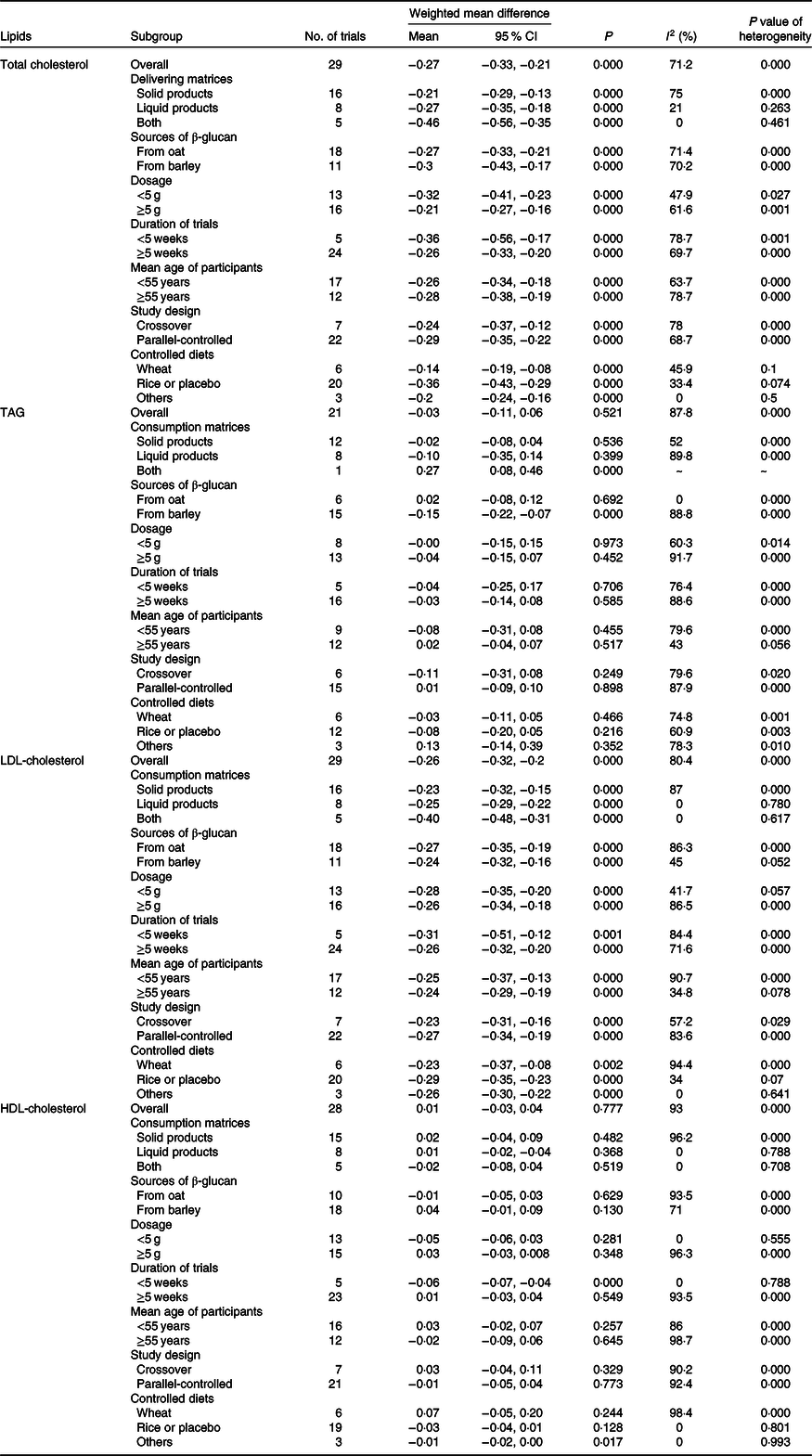
Table 3. Effects of β-glucan on blood lipids by delivering forms, sources of β-glucan, dosage, duration of trials, mean age of participants, study design and controlled diets
(Mean values and 95 % confidence intervals)
Overall and subgroup effects of β-glucan intake on fasting serum TAG concentration in mildly hypercholesterolaemic individuals
The meta-analysis consisted of twenty-one studies (including seventeen articles) demonstrated that the level of fasting serum TAG was not significantly changed by β-glucan consuming compared with control groups (weighted mean difference = −0·03 mmol/l, 95 % CI −0·11, 0·06, P = 0·521). There was still a serious heterogeneity before subgroup analysis (I 2 = 87·8 %, P < 0·001, Fig. 3). However, we observed a significant difference between TAG and β-glucan intake after adjusting delivering matrices and sources of β-glucan (see Table 3).

Fig. 3. Overall and subgroup effects of β-glucan intake on fasting serum TAG concentration in mildly hypercholesterolaemic individuals. Weights are from random effects analysis. WMD, weighted mean difference.
Overall and subgroup effects of β-glucan intake on fasting serum LDL-cholesterol concentration in mildly hypercholesterolaemic individuals
Results of the present study revealed that the LDL-cholesterol-lowering effect of β-glucan was significant overall with the pooled estimate of −0·26 mmol/l (95 % CI −0·32, −0·20, P < 0·001), which was conducted from the random effects model with heterogeneity I 2 = 80·4 % (P < 0·001). In addition, an outcome similar to TC was obtained when subgroup analysis was performed (Fig. 4). Table 3 shows the remaining results of subgroup analysis.
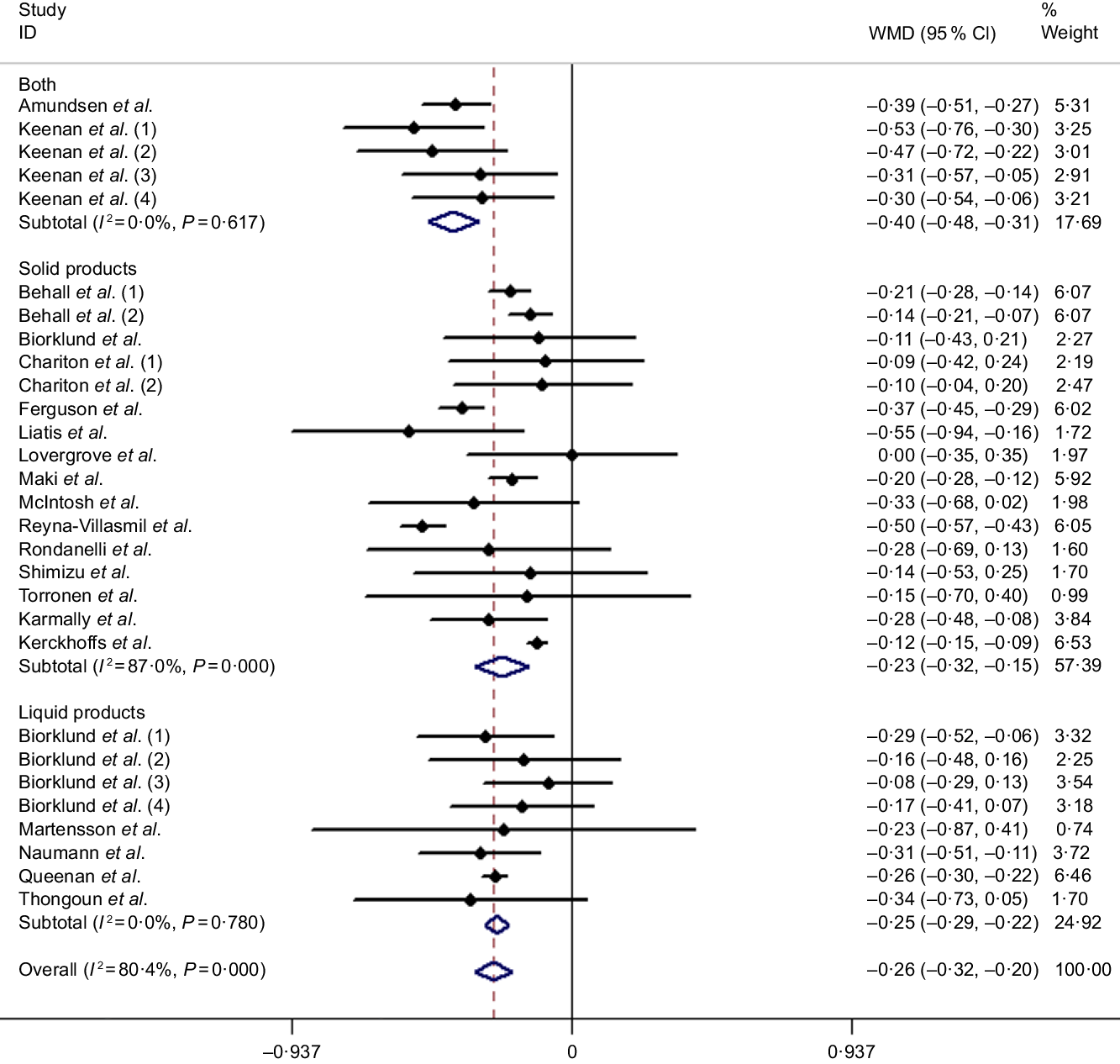
Fig. 4. Overall and subgroup effects of β-glucan intake on fasting serum LDL-cholesterol concentration in mildly hypercholesterolaemic individuals. Weights are from random effects analysis. WMD, weighted mean difference.
Overall and subgroup effects of β-glucan intake on fasting serum HDL-cholesterol concentration in mildly hypercholesterolaemic individuals
It is well known that HDL-cholesterol is regarded as a protective factor for CHD; the present meta-analysis including twenty-eight studies (based on twenty articles) was to explore the effect of β-glucan on fasting serum HDL-cholesterol concentration in mildly hypercholesterolaemic individuals. However, we did not identify any significant difference compared with control group from the random effects model (weighted mean difference = 0·01 mmol/l, 95 % CI −0·03, 0·04, P = 0·777), and heterogeneity of inter-study was I 2 = 93 %, P < 0·001 (Fig. 5). Nevertheless, we found several significant differences after conducting subgroup analysis by duration and controlled diets. The remaining results of subgroup analysis for HDL-cholesterol are found in Table 3.
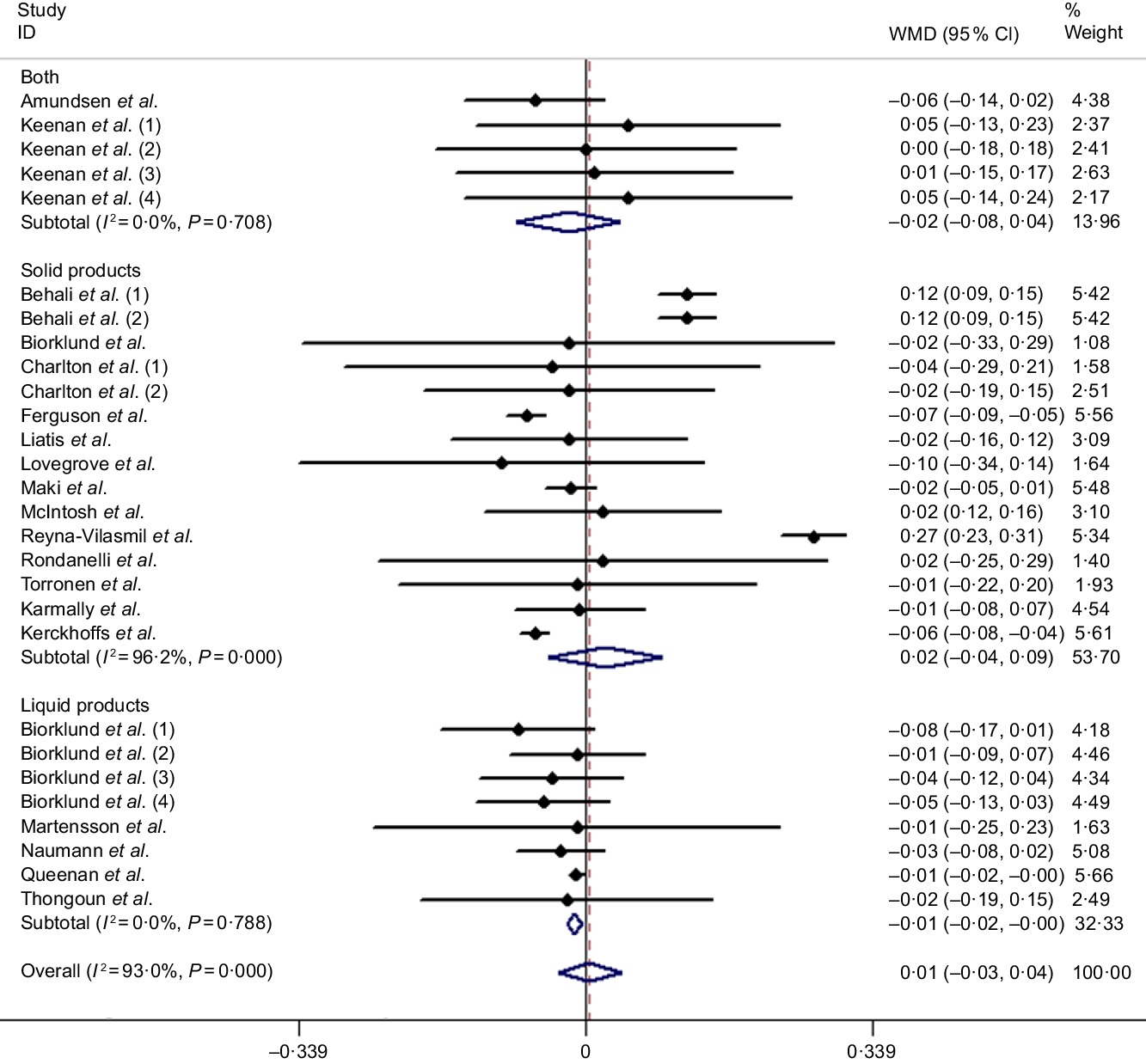
Fig. 5. Overall and subgroup effect of β-glucan intake on fasting serum HDL-cholesterol concentration in mildly hypercholesterolaemic individuals. Weights are from random effects analysis. WMD, weighted mean difference.
Sensitivity analysis
Sensitivity analysis was performed to assess the stability and credibility of pooled effect through sequentially removing each eligible study and then repeating the analysis, and results suggested that it could not change the overall estimate effects of β-glucan on blood lipids in mildly hypercholesterolaemic individuals.
Subgroup analysis
The effects of β-glucan intake on blood lipids in subgroup based on volunteers’ characteristics are summarised in Table 3. Generally speaking, there was no obvious difference from the overall effects even after adjusting delivering matrices, sources of β-glucan, dosage, durations of trials, mean age of participants, study design and controlled diets for TC and LDL-cholesterol. As for TAG and HDL-cholesterol, several distinctions were observed before and after subgroup analysis.
Publication bias
Funnel plots and Egger’s regression test of blood lipids were conducted to measure if there are potential publication biases, which are judged through symmetry of the funnel plots. For TAG, LDL-cholesterol and HDL-cholesterol, visual scanning of funnel plots suggested no asymmetry with Egger’s regression P values of 0·118, 0·259 and 0·64, respectively, while we observe there is minor asymmetry for TC with Egger’s regression P value of 0·019 (Fig. 6); consequently, we further performed the ‘trim and fill’ method to evaluate the robustness of the results in the presence of publication bias, and results show there is no change before and after adding the estimated missing literature, which suggesting that the results of TC are reliable.

Fig. 6. Funnel plots measuring publication bias and effect of β-glucan intake for (a) total cholesterol (TC) Egger’s test (P = 0·019), (b) TAG Egger’s test (P = 0·118), (c) LDL-cholesterol Egger’s test (P = 0·259) and (d) HDL-cholesterol Egger’s test (P = 0·64) in mildly hypercholesterolaemic individuals. WMD, weighted mean difference.
Meta-regression
To further explore the sources of heterogeneity between studies, meta-regression was performed to assess association between blood lipids changes and delivering matrices of β-glucan. Results indicated a significant inverse relationship among mean difference and delivering matrices of β-glucan for TC (slope = −0·12, 95 % CI −2·0, −0·04, P = 0·004), while little significant association was observed for LDL-cholesterol, TAG and HDL-cholesterol (slope = −0·07, 95 % CI −0·14, 0·007, P = 0·078; slope = 0·05, 95 % CI −0·1, 0·2, P = 0·525 and slope = −0·03, 95 % CI −0·08, 0·03, P = 0·311, respectively).
Discussion
The present meta-analysis of twenty-one trials involving 1120 participants revealed the beneficial properties of β-glucan on risk factors for CVD, consuming ≥3 g/d of β-glucan for a period of time, could significantly reduce the concentrate of fasting serum TC (−0·27 mmol/l) and LDL-cholesterol (−0·26 mmol/l) in mildly hypercholesterolaemic individuals, and subgroup analysis further suggested it appears that the effects in the consumption manner of combination with ‘solid products’ and ‘liquid products’ were greater than alone of either one. However, we did not notice significant effects on TAG and HDL-cholesterol level. Different delivering matrices and controlled diets may explain some unknown heterogeneity.
The most outcomes concluded from the present meta-analysis have some similarities with previous studies. Whitehead et al. and Talati et al. (Reference Whitehead, Beck and Tosh21,Reference Talati, Baker and Pabilonia51) reported significant decreases of TC and LDL-cholesterol through intaking barley and oat with reductions of −0·35, −0·30 mmol/l and −0·26, −0·25 mmol/l, respectively, and it seems that barley may have more strength in improving TC concentrate compared with oat; the rationale behind this may be owing to a higher content in β-glucan in barley under the same weight(Reference Havrlentova and Kraic52), which in accordance with our overall and sub-analysis results. In the meta-analysis conducted by Talati et al., the authors found that there was a significant decrease in TAG with barley-derived soluble fibre, which was the same with our subgroup analysis, even though inconsistent with the articles published before(Reference Holloender, Ross and Kristensen53,Reference Tiwari and Cummins54) , and the discrepancy may be related to the meta-analysis including small-size articles since executing a flow with strict inclusion criteria. Interestingly, a recent network meta-analysis consisted of a total of 3900 individuals conducted by Hui et al. (Reference Hui, Liu and Lang55) suggested, except oat and oat bran, barley and wheat show no remarkable association with either TC or LDL-cholesterol, and then we speculate a part of healthy volunteers were included in analysis and unconventional statistical methods may attribute to the surprising conclusions to some extent. More importantly, subgroup analysis in this meta-analysis indicated that β-glucan would have a slight adverse effect on HDL-cholesterol because the no. of size of trials ≤5 weeks was relatively small. Also we were unclear about the potential effects of controlled diet itself, it is possible that some potential confounding factors generated some confusions in the meta-analysis, but maybe it is credible for mildly hypercholesterolaemic individuals. Further studies with high quality would be necessary to understand the clear mechanisms.
The bioactivity of β-glucan relies on its physical structure like MW and three-dimensional conformation, which will influence the viscosity and solubility of β-glucan in turn(Reference Wang and Ellis56). However, there were also some distinctions in the extracting and processing, including the storage condition and distribution of wholegrain as bran and endosperm(Reference Butt, Tahir-Nadeem and Khan57,Reference Regand, Tosh and Wolever58) . It is reported that the cholesterol-lowering effect of β-glucan was mainly determined by its viscosity; when the high-molecular-weight β-glucan was ingested, a special microenvironment with high viscosity would be created in the small intestine, which will act as a physical barrier by preventing the absorption of cholesterol, promoting the excretion of bile acids which participate in the synthesis of cholesterol and reducing the reabsorption of bile acids existed in enterohepatic cycle(Reference Kerckhoffs, Hornstra and Mensink50,Reference Bashir and Choi59–Reference Gunness, Michiels and Vanhaecke62) . Besides above physical properties of β-glucan, mechanisms involved in biomolecule metabolism with body were also reported, which included the whole process of cholesterol synthesis, metabolism and transport(Reference Gil-Ramirez, Morales and Soler-Rivas63). Furthermore, β-glucan could be fermented by the colon microbiota and gives the end products of SCFA (mainly acetic, propionic and butyric acids), of which propionic acid plays a crucial role in hypocholesterolaemic activities with suppressing the activity of hydroxy methylglutaryl coenzyme A (HMG CoA), which is a rate-limiting enzyme during endogenous cholesterol synthesis(Reference Morrison and Preston64–Reference Ryan, Ross and Fitzgerald66). In vitro, β-glucan could also act as an inhibitor of HMG-CoA and resulting in impairment of cholesterol biosynthetic pathway(Reference Morales, Rutckeviski and Villalva67,Reference Morales, Smiderle and Villalva68) . Clinical trials and reviews suggested that supplement of β-glucan could stimulate to increase the abundance of certain beneficial gut microbiota, such as Bifidobacterium and Lactobacillus (Reference Jaskari, Kontula and Siitonen69,Reference Deehan, Duar and Armet70) , and these bacterial genera are known to predominately contain bile salt hydrolase-positive species, which could modulate the host bile acid pool signature through deconjugation of conjugated bile acid. Unconjugated bile acids have reduced micellular activity and therefore are less effective mediators of cholesterol absorption in the host relative to conjugated bile acids(Reference Jones, Begley and Hill71,Reference Liong and Shah72) . According to Wang et al.’s(Reference Wang, Ames and Tun73) report, β-glucan could alter the gut microbiota and the changes observed were positively associated with an improved CVD risk factor profile. Interestingly, the delivering food where β-glucan was incorporated into may have different effects on the efficacy of β-glucan, according to Kerckhoffs, drink with oat β-glucan appeared to be somewhat more effective than food like bread and cookies enriched with oat β-glucan, since the processing could reduce its MW to some extent(Reference Tosh, Brummer and Miller74), and beverages and liquid test meal may rank the best carrier to deliver β-glucan(Reference Ho, Matia-Merino and Huffman75). Our subgroup analysis further supported those ideas, and liquid meal enriched with β-glucan is better than food like bread and cookies; in this way, we observed a combination of ‘liquid’ and ‘solid’ food might be the most effective way to deliver β-glucan compared with isolate food or drink, although there is a modest heterogeneity.
In addition, trials included in this meta-analysis involve a variety of controlled diets, ranging from rice, maize, dextrose and even wheat, those may have crucial impacts on overall heterogeneity, especially wheat that have a low amount of β-glucan(Reference Pritchard, Lawrence and Larroque76), and subsequent subgroup analysis by controlled diets proved it. On the other hand, participants included in the meta-analysis were defined as mildly hypercholesterolaemic individuals. To our knowledge, this is the first meta-analysis to assess the effects of β-glucan on blood lipids in the population and have a similar result with previous studies.
There are several limitations which should be taken account to the meta-analysis: first, insufficient information in some included studies which we could not find a standardised method to measure the MW of β-glucan; as a result, a detailed description on the MW of β-glucan would not be obtained, even the area where the β-glucan was extracted from was not taken into consideration; all the above conditions may make a potential confound on our results. Second, even though subgroup analysis by forms of delivering matrices and controlled diets explains most of the heterogeneity, a modest heterogeneity was still observed in the analysis, of which we consider it may be inevitable due to studies with amounts of different aspects, such as countries and races where the trials were conducted, and definitions of mild hypercholesterolaemia. Third, we still found a slight asymmetry in funnel plots for TC, although a comprehensive and systematic search was performed to avoid publication bias, since unpublished trials with negative outcomes have always existed; nevertheless, the further method of ‘trim and fill’ has ensured the robustness of results for TC. Consequently, a greater number of high-quality trials with appropriate control are necessary to verify these results.
We conclude that β-glucan can reduce risk factors like TC and LDL-cholesterol for CVD in mildly hypercholesterolaemic individuals significantly. Furthermore, the food matrices of delivering β-glucan with a combination of both ‘liquid’ and ‘solid’ products were ranked as the best way to exert its beneficial properties, while ‘liquid’ product was ranked as the second, following with ‘solid’ product.
Acknowledgements
The authors thank each other for their support.
This work was supported by the National Natural Science Foundation of China (grant no. 81872618), Postgraduate Research & Practice Innovation Program of Jiangsu Province (grant no. KYCX19_0121) and the Scientific Research Foundation of Graduate School of Southeast University (grant no. YBPY1944). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
D. X. designed the study and wrote the paper, D. X. and H. L. searched and reviewed the relevant trials and collected the data. H. X. played a role as a consultant. D. P. helped employing search strategies. C. Y. and X. Y. performed statistical analysis. L. Y. and S. W. were responsible for the quality assessments for the studies. All authors reviewed the manuscript and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520001610












