INTRODUCTION
Pertussis, commonly referred to as whooping cough, is a major cause of morbidity and mortality in children, and remains one of the leading aetiologies of vaccine-preventable death [Reference Zepp1]. The Pan American Health Organization/World Health Organization (PAHO/WHO) estimated that 50 million cases and 300 000 deaths occurred because of pertussis worldwide in 2012 [2]. The re-emergence of pertussis has been found in many countries [Reference Cherry3–Reference Mink7]. Vaccination remains the primary method of protecting susceptible populations. However, vaccine-acquired immunity weakens 5–10 years after vaccination, which can be a factor contributing to the occurrence of pertussis [8].
IgG antibodies against pertussis toxin (anti-PT IgG) are a specific indicator of disease prevalence in a population, and adolescents generally have higher anti-PT IgG levels and prevalence than children due to the decrease in vaccine-induced immunity [Reference Mossong9–Reference Greenberg11]. As such, adolescents are considered an important source of infection for infants who have not completed primary immunization [Reference Greenberg11]. The specific distribution of anti-PT IgG levels and prevalence in children and adolescents in China are unknown.
In China, a combined diphtheria, tetanus and whole-cell pertussis vaccine (DTwP) was introduced in 1978. However, in 2007, a combined diphtheria-tetanus-acellular pertussis vaccine (DTaP) was introduced because of the severe side-effects of whole-cell vaccines. Presently, DTaP vaccines are administered in the 3rd, 4th and 5th months of life. A booster dose is given to children aged 18–24 months. The immunization rate of three doses of DTaP vaccination in childhood has been reported as >90% since 2002 [12]. The immunization coverage of four doses was >99% in 2011 [Reference Qi13].
After adoption of the DTwP and DTaP vaccines, the incidence of pertussis decreased sharply from pre-vaccination era rates of 100–200 infections/100 000 people to <1 infection/100 000 people during the 1990s [Reference Ou14–Reference Yan16]. In recent years, the reported incidence of pertussis in China was ∼0·2 infection/100 000 people, and in Gaobeidian city, there has not been a single reported case of pertussis (Gaobeidian CDC, unpublished data) in almost 10 years. We suspect that reporting bias contributes to the discrepancy between the high worldwide pertussis prevalence and that in Gaobeidian city, according to previous studies [Reference Cherry3–Reference Mink7].
Gaobeidian city is located in the northern part of China, near Beijing. This city has ∼560 000 inhabitants, of which children and adolescents account for 9·33%. The vaccination programme used in Gaobeidian city is the same as the above-mentioned national programme. Since the replacement of DTwP in 2007, the immunization rate has been >97% (Gaobeidian CDC, unpublished data).
Here, we present a cross-sectional study that measured IgG antibodies against pertussis toxin (anti-PT IgG) in a single serum sample; anti-PT IgG is the most specific antigen for pertussis, as cross-reacting antigens have not been described [Reference Muller17]. Anti-PT IgG concentrations after the 18–24 months vaccine booster are supposed to be negligible at the age of 3 years [Reference Versteegh18, Reference Hallander19]. We focused on children and adolescents to elucidate the seroepidemiology of these age groups.
METHODS
Study design
Serum samples were collected between October 2012 and June 2013. Samples were residual specimens collected during regular physical examinations in Gaobeidian city, China, which would otherwise have been discarded. The serum samples of all students in secondary schools (aged 12–18 years) were collected, and the residual serum samples were tested in our laboratory. Samples were selected randomly from each school. Samples from very young children (aged ⩽6 years) and primary schoolchildren (aged 7–11 years) were collected from randomly selected kindergartens and primary schools in Gaobeidian city.
In this study, subjects were selected using stratified sampling, with the strata of gender, school and age. In the case of school sampling, the proportional stratified sampling method was used according to the size of the school. Individuals who had received pertussis vaccination within 1 year were excluded. In total, 1032 subjects (525 males, 507 females) from kindergartens, and primary and secondary schools, aged 3–18 years (mean 11·66 ± 3·6 years, median 13 years) were included in the analysis.
Serology
A commercial enzyme-linked immunosorbent assay (ELISA; Serion ELISA classic, Institut Virion/Serion GmbH, Germany), calibrated with international standard serum 06/140, was used to determine the IgG antibody levels against the Bordetella pertussis toxin (PT). The anti-PT IgG levels corresponding to negative, equivocal and positive samples were <40, 40–100 and >100 IU/ml, respectively, according to the manufacturer's protocol. However, 100 IU/ml was widely accepted as the criteria of recent infection, and the anti-PT IgG concentration could easily wane to 40 IU/ml within 1 year [Reference Versteegh18–Reference Duranoglu21]. The aim of the present study was to discover all infections in the population, including recent and previous infections. Thus, after comprehensive consideration, subjects with anti-PT IgG levels ⩾40 IU/ml were scored as positive (the positive rate was designated as seroprevalence), persons whose anti-PT IgG levels were ⩾40 IU/ml were identified as infections, while anti-PT IgG levels >100 IU/ml were considered to indicate recent infections.
Quality control
After the samples were tested, we randomly chose 5% of the samples for re-testing. Then, we calculated the coefficient of variation (CV) of the results for these re-tested samples. The results were only considered valid if the CV value of each sample was <15%, otherwise the test was repeated.
Statistical analysis
All data were recorded using Microsoft Excel 2007. Statistical analysis and graphs were produced in SPSS v. 16.0 (SPSS Inc., USA) and SAS v. 9.2 (SAS Institute Inc., USA). The prevalence of PT antibodies, geometric means and the corresponding 95% confidence intervals (CIs) were calculated. The prevalence rates were compared with each other using χ 2 test, and the levels of PT antibodies were compared by one-way analysis of variance (ANOVA) in SPSS for the different age groups. A value of P < 0·05 was considered statistically significant. Logistic regressions were conducted on secondary schools in SAS, with school as the dummy variable. Schools were sorted according to incidence; however, the two schools with the lowest incidence were merged as control groups because one of them did not have any positive cases. Regression was conducted to investigate the odds ratio (OR) between schools and control groups. Finally, the schools were divided into two groups: non-endemic or sporadic schools, and endemic schools.
Ethics statement
Approval for the serosurvey was obtained from the National Institute for Communicable Disease Control and Prevention. Informed consent was not obtained as the data were analysed anonymously. However, we did receive oral approval from to the Center of Disease Control and Prevention, Gaobeidian.
RESULTS
Of 1032 students tested, the geometric mean of anti-PT IgG concentration (GMC) was 6·93 IU/ml (95% CI 6·42–7·48; Table 1); 101 (9787/100 000) students tested positive (anti-PT IgG levels ⩾40 IU/ml), of which 35 (3390/100 000) were found to have anti-PT IgG levels >100 IU/ml.
Table 1. Age-specific population seroprevalence and rates of recent infections, with 95% confidence intervals in parentheses
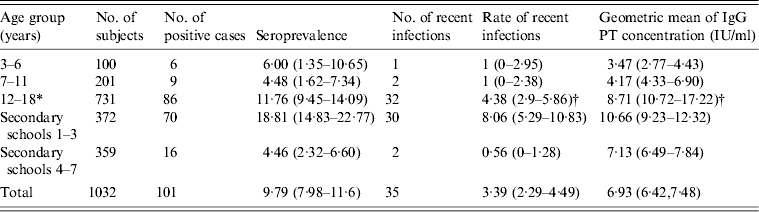
* 12–18 years age group, from secondary schools 1–7.
† Significantly higher than the other two age groups.
In this study, the students were separated into three age groups: 3–6 years (kindergarten), 7–11 years (primary school) and 12–18 years (secondary school). The distribution of anti-PT IgG levels and seroprevalence with the 95% CIs by age group and school are shown in Table 1 and Figure 1. Anti-PT IgG levels and seroprevalence between the 3–6 and 7–11 years age groups were very close, although seroprevalence in the 7–11 years age group was slightly lower. There were no significant differences between these two age groups with respect to anti-PT IgG levels (t = 1·213, P = 0·226) and seroprevalence (χ 2 = 0·084, P = 0·771). Anti-PT IgG levels in students aged 12–18 years were much higher than for those aged 3–6 and 7–11 years (P = 0·0001). The seroprevalence of pertussis in students aged 12–18 years was also higher than those in the other two groups (ages 7–11 years and 3–6 years), although it was not statistically significant (χ 2 = 2·97, P = 0·085).
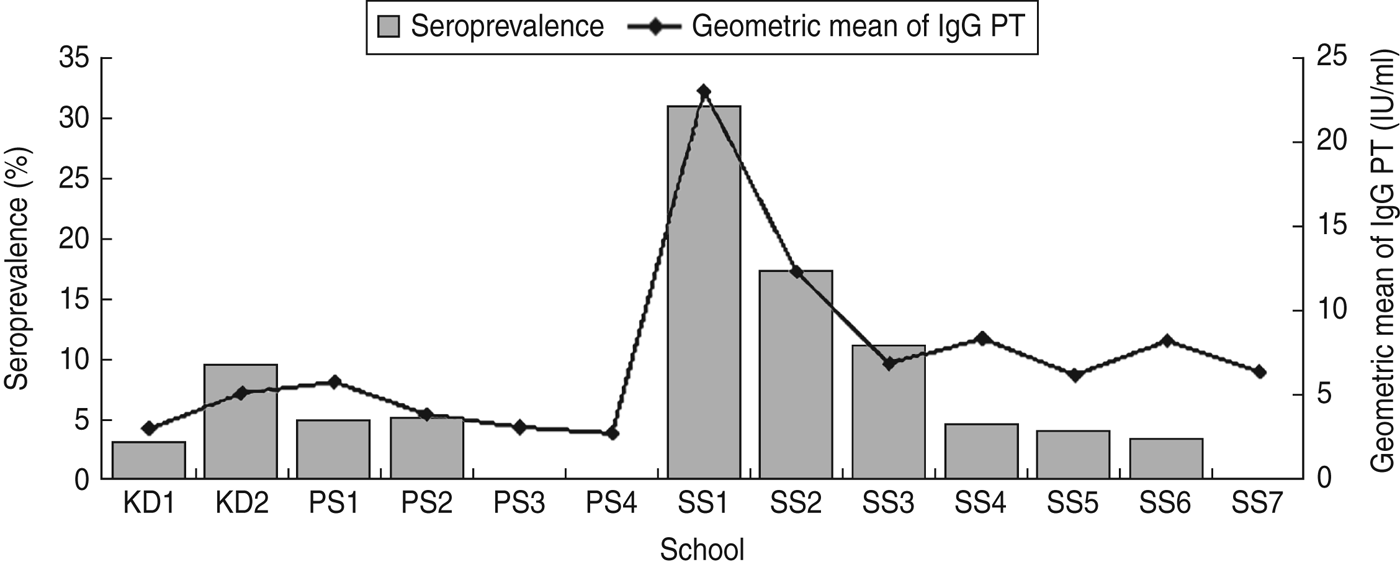
Fig. 1. Distribution of seroprevalence and pertussis toxin antibody (IgG PT) levels in kindergartens (KD), primary schools (PS) and secondary schools (SS).
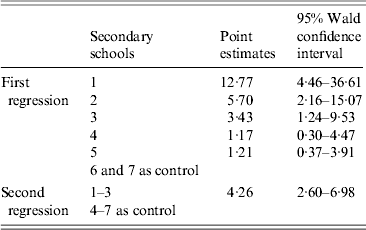
Students who tested positive for anti-PT IgG (⩾40 IU/ml) were assigned a value of ‘1’, and the rest were assigned a value of ‘0’. After regression analysis, we found that the OR in secondary schools 4 and 5 was not significantly different from that in secondary schools 6 and 7 (95% CI of OR including 1), so these four schools were merged into one group, with the remainde being treated as another group (Table 2). We repeated the regression analysis, and calculated an OR of 4·26 (95% CI 2·60–6·98), which suggests that secondary schools 1–3 were risk factors for pertussis infection compared to secondary schools 4–7.
Table 2. Odds ratio estimates of two logistic regressions against infection by pertussis (anti-PT IgG ⩾40 IU/ml) in secondary schools
In the 12–18 years age group, the anti-PT IgG levels, seroprevalence and rates of recent infections in secondary schools 1–3 were the highest of any group. In secondary schools 4–7, however, they were very similar in the 3–6 and 7–11 years age groups (Table 1). This suggested that the high prevalence and anti-PT IgG levels were probably caused by a clustering of cases in secondary schools 1–3.
DISCUSSION
Despite high vaccination rates, recent studies have reported cases of pertussis in schoolchildren and adolescents from some areas, due to a time-dependent decline in vaccine-induced immunity [8, Reference Versteegh18–Reference Horby20]. However, the case definition is based on clinical diagnosis and laboratory confirmation of B. pertussis infection is not routinely practised in China, therefore, many pertussis cases in vaccinated children and adolescents without classical paroxysmal cough symptoms are not reported. According to the present study, infections (anti-PT IgG levels ⩾40 IU/ml) were mainly distributed in adolescents. We therefore considered these cases to be histories of natural infections instead of vaccine-induced immunity. Anti-PT IgG levels >100 IU/ml (recent infection) is a valid marker of pertussis cases, although it is not the criteria of reported pertussis disease incidence in China [Reference Versteegh18, Reference Duranoglu21, Reference Melker22]. The high rate of recent infections (3390/100 000) suggests that pertussis was circulating in Gaobeidian, and the reported incidence of zero cases was inaccurate. In China, the reported annual incidence of pertussis has been about 0·2 infection/100 000 people since the 1990s, which appears to be an underestimation, according to the results presented in this study [Reference Ou14–Reference Yan16]. The rate of recent infection was also consistent with that of Hallander et al.'s study in Sweden, whose data were collected in 1997 at the end of a 17-year vaccine-free period and in 2007, 10 years after the introduction of TDaP [Reference Hallander23]. This phenomenon was considered to be caused by natural circulation and transmission of pertussis infection in the population.
The anti-PT IgG levels, seroprevalence and rates of recent infections in students aged 12–18 years were indeed higher than those in the other two age groups (3–6 years and 7–11 years), which was consistent with reports and studies from other countries [Reference Mossong9–Reference Greenberg11]. However, one notable finding was that cases clustered in three secondary schools (secondary schools 1–3). Students in the 12–18 years age group from the other secondary schools (4–7) shared nearly identical anti-PT IgG levels, prevalence and rates of recent infections with the other age groups (3–6 years and 7–11 years). This strongly suggests that the high seroprevalence was caused by clustered cases in certain schools. We have ever tested for the presence of antibodies against diphtheria in secondary schools 1 (the northeast), 2 (the northeast), and 6 (the west). Antibodies against diphtheria would be indicative of immune status because immunity against diphtheria is long lasting. Antibodies against diphtheria concentration >0·01 IU/ml was considered as seropositive according to the manufacturer's protocol. According to the above study, the seroprevalence of diphtheria in secondary schools 1, 2 and 6 was 65·22%, 78·26% and 78·26%, respectively. There was no statistical difference between secondary schools 1, 2 and 6 (χ 2 = 1·353, P = 0·508) with regard to diphtheria antibody levels, which suggested that immune coverage due to vaccination with DTaP was similar between schools with high and low anti-PT IgG levels. Therefore, the high levels of anti-PT IgG were not due to poor immune status. Secondary schools 1–3 were all private schools, and therefore, the students generally lived in the dormitories at the schools. School dormitories are often crowded in China, which could contribute to the transmission of infectious diseases [Reference Horby20]. According to our study, adolescents and children had the same susceptibility, but the crowded living conditions of adolescents could have contributed to their high risk of infection.
This serosurvey focusing on the prevalence of pertussis was performed only in children and adolescents in one city. The vaccination programme used in this city was nationally used in China. However, pertussis epidemiology may vary, particularly given the country's vast geographical and climatic ranges, and urban/rural settings. Thus, further studies should be advocated in populations in other cities of China, which will provide more detailed information for understanding the epidemiology of pertussis in China.
The passive surveillance system, based on notification, was affected by limitations such as underdiagnosis. The notified (reported) criterion was based on clinical definition, which was difficult due to the atypical clinical characteristics and the lack of laboratory confirmation. Seroepidemiology could be one possible improvement in pertussis surveillance activities, as it could discover outbreaks that might otherwise be missed. However, in order to better understand the epidemiology of pertussis in China, strengthening of surveillance system (e.g. case ascertainment and standard laboratory confirmation) would be beneficial [Reference Cherry24]. In conclusion, the present study suggests that pertussis is still circulating in adolescents in China and the reported annual incidence of pertussis might be underestimated.
ACKNOWLEDGEMENTS
This study was supported by the national 973 programme of China (2011CB504900) and the Priority Project on Infectious Disease Control and Prevention (grant no. 2013ZX10004221) from the Ministry of Health and the Ministry of Science and Technology, People's Republic of China.
DECLARATION OF INTEREST
None.





