1. Introduction
The human cognitive system is constantly bombarded with environmental stimuli. This, combined with the fact that our cognitive resources are limited, underlines the need for attentional mechanisms that can pick out the environmental information one should attend to. An additional complexity is that this process has to support us in the modern world. Humans have to integrate goal-oriented behaviours with contextual information, rule following with environmental feedback and responding to new information, and memory and present informational processing or reasoning. To succeed, such cognition has to be adaptive and flexible, and to this end, the human brain has evolved an array of mechanisms termed ‘cognitive control’.
An example of a particularly challenging situation for cognitive control is switching between one task and another. A shift in the behavioural programme and information processing is needed, as well as a shift in attention, as new task demands make different information relevant. An often-neglected additional factor comes from the fact that everyday functioning is also shaped by the affective or emotional states that individuals experience. In this paper, we wanted to tackle the role of affect (defined as activation and valence) in cognitive control that is needed to switch from one task to another.
1.1. Cognitive control and switching between tasks
The modern world surrounds us with a huge amount of different stimuli and competing tasks that we are not capable of processing all at once (Tillman & Wiens, Reference Tillman and Wiens2011). Cognitive control is a mechanism that enables information selection, by which information irrelevant from a goal achievement perspective is ignored (Li et al., Reference Li, Hill and He2014); cognitive control, therefore, allows one to act in accordance with goals and intentions (Desimone & Duncan, Reference Desimone and Duncan2003; Miller & Cohen, Reference Miller and Cohen2003; Norman & Shallice, Reference Norman, Shallice, Davidson, Schwartz and Shapiro1986). To avoid a conflict induced by excess stimuli in combination with limited resources, cognitive control needs to be applied with such functions as working memory and response inhibition (Nigg, Reference Nigg2000). Additionally, the fact that the same stimuli result in different actions in different situations provides evidence of cognitive control, which questions the sole influence of the environment on action (Meiran, Reference Meiran2000). A complex mechanism of cognitive control consists of several subcomponents: updating, defined as the ability to actualise the information; shifting, which describes the changing of tasks or goals and inhibition, which requires holding the automatic reaction and implementing a reflexive response (Gratton et al., Reference Gratton, Cooper, Fabiani, Carter and Karayanidis2018; Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000; Miyake & Friedman, Reference Miyake and Friedman2012).
The mechanism of shifting between different tasks (and hence short-term goals) and coordinating several actions simultaneously is a daily aspect of human functioning that can be observed in the task-switching paradigm (Allport et al., Reference Allport, Styles, Hsieh, Umiltà and Moscovitch1994; Meiran, Reference Meiran1996; Rogers & Monsell, Reference Rogers and Monsell1995a). This type of task involves switching between cognitive tasks that are not complicated and require simple cognitive operations. A subject is asked to complete two or more uncomplicated tasks requiring attention to different elements, features or classifications of stimuli (Monsell, Reference Monsell2003). The task might also consist of recovery from the memory or computation of the attribute of the stimuli. The subject performs a cognitive operation on the stimulus presented in each of a series of trials of alternate tasks. As Monsell (Reference Monsell2003) indicated, there are different ways of instructing the subject about which task is to be performed at a given moment; however, the task might or might not change from one trial to the next.
This type of paradigm allows cognitive performance to be assessed during the trials of one task and when switching between tasks, which enables one to observe the increase of processing demand resulting from the need to reconstruct the task during changes. The general tendency observed in this paradigm is an increase of reaction time in the new task in comparison with repetition of the same task (Dreisbach & Haider, Reference Dreisbach and Haider2006). However, the result provoked by the task novelty can be diminished by prior preparation (Altmann, Reference Altmann2004; Rogers & Monsell, Reference Rogers and Monsell1995b). The measured performance is determined by the maintenance of access to the ultimately coded task in episodic memory; therefore, when performing the next task, a proactive interference can be observed (Altmann & Gray, Reference Altmann and Gray2008). This interference affects the latency of responses and rate of errors, which are the results of switching cognitive costs.
1.2. Emotions influencing cognitive control
One of the most important traits of processing emotions is their activating value (Kagan, Reference Kagan2007; Russell, Reference Russell1980). Emotions tend to activate central and autonomic nervous systems (Hagemann et al., Reference Hagemann, Waldstein and Thayer2003; Porges, Reference Porges1997; Shiota et al., Reference Shiota, Neufeld, Yeung, Moser and Perea2011); they also cause strictly cortical activation (Fossati, Reference Fossati2012; Freudenthaler et al., Reference Freudenthaler, Fink and Neubauer2006; Garg & Verma, Reference Garg and Verma2020; Kassam et al., Reference Kassam, Markey, Cherkassky, Loewenstein and Just2013). This activating charge brought by emotions is common for most feelings (Hung & Picard, Reference Hung and Picard2016; Reisenzein, Reference Reisenzein1994), independent of other factors that could describe emotions.
Historically, the activating factor in emotional processing was labelled as arousal (Russell, Reference Russell1980). The arousing value could be brought by emotions differing in other traits, but the activating value could be similar between them, which is supported by ratings of emotional stimuli (Imbir, Reference Imbir2016a; Marchewka et al., Reference Marchewka, Żurawski, Jednoróg and Grabowska2014) and physiological evidence (Hung & Picard, Reference Hung and Picard2016). Some researchers have argued that arousal is a core component of emotions (Reisenzein, Reference Reisenzein1994; Schachter & Singer, Reference Schachter and Singer1962), whereas others have proposed, in the theory of cognitive appraisal, that identifying other traits of emotions may be strictly tied to arousal, as we perceive the changes brought by arousal and interpret them, creating a mental representation of a certain emotion (Moors et al., Reference Moors, De Houwer, Hermans, Wanmaker, van Schie, Van Harmelen, De Schryver, De Winne and Brysbaert2013a; Russell, Reference Russell2009). Identifying the level of arousal seems to be easier for basic emotions such as fear and anger compared with complex emotions. (Imbir, Reference Imbir2016b; Johnston, Reference Johnston1999). Recently, a theory has been proposed that divides emotions into basic and more complex states based on their processing by the automatic/heuristic or reflective/systematic mind, respectively (Jarymowicz & Imbir, Reference Jarymowicz and Imbir2015). This theory is based on the dual-mind models of thinking proposed by many researchers, which divide our thought processes into two categories: automatic (heuristic and experiential) processes, which are fast and based on heuristics, one’s own experience and easily accessible information in general; and reflective (systematic) processes, which are slower and consume more energy but can process more complex problems by using the rules of logic and taking many aspects of a problem into account (Epstein, Reference Epstein2003; Kahneman, Reference Kahneman2011).
In the discussed two-system approach to emotions, it is postulated that arousal is an activating factor primarily for automatic emotions, whereas in the case of reflective emotions, it is assumed that there is a different activation mechanism associated with subjective significance (Imbir, Reference Imbir2016b; Jarymowicz & Imbir, Reference Jarymowicz and Imbir2015). This factor can be defined as an importance of an object to the perceiver. A subjective significance is a concept similar to impact of picture stimuli understood as a picture property to draw people’s attention and being memorised (Ewbank et al., Reference Ewbank, Barnard, Croucher, Ramponi and Calder2009). The processing of picture with high impact is more conscious and subjective-based process in comparison with arousal activation. Subjective significance can also be compared with salience, which demonstrates the prominence of obtained results (Kahnt & Tobler, Reference Kahnt and Tobler2013). A salience reflects that some outcomes are valued more than the others, as a result of a decision-making process that includes an assessment of risk, profit and loss. Finally, the concept of subjective significance can be at least partially related to the appraisal notion. Appraisal notion is understood as a mechanism of verification of the value of the stimulus in relation to its consistency with the goals of the individual and the ability to control these goals (see, e.g., Moors et al., Reference Moors, Ellsworth, Scherer and Frijda2013b); it also refers to individual’s standards (Clore & Ortony, Reference Clore, Ortony, Lewis, Haviland-Jones and Barrett2010).
High subjective significance should intensify the processing of emotional stimuli, especially when it comes to complex and abstract objects or ideas that would not carry the basic arousing charge (Imbir, Reference Imbir2016b). This is why subjective significance was proposed as the activating factor for the rational/systematic mind (Imbir, Reference Imbir2016a, Reference Imbir2016b), which functions on the basis of verbalisation (Rolls, Reference Rolls2000) and deliberation (Kahneman, Reference Kahneman2003, Reference Kahneman2011). Results of normative study exploring both of these activating factors show that ratings of emotional stimuli in terms of arousal are positively correlated with ratings in terms of subjective significance, which further supports the claim that both dimensions are related to activating value (Imbir, Reference Imbir2016b). However, this correlation (at 0.38) is small enough to claim that they are separate dimensions of evaluation.
Besides the activating factor, the most intuitive dimension for describing emotions is evaluating the feelings as positive or negative, which is defined as emotional valence (Imbir, Reference Imbir2019; Russell, Reference Russell1980). Next to activation, the concept of feeling good or bad is one of the most primal ways of characterising emotions (Kagan, Reference Kagan2007; Reisenzein, Reference Reisenzein1994). In the proposed series of experiments, we explore the influence of both activating factors on cognitive control and we have to acknowledge the non-linear way in which the activating factors correlate with emotional valence (Imbir, Reference Imbir2016a). Studies show that extremely negative or positive stimuli are perceived as more arousing (Bradley & Lang, Reference Bradley and Lang1999; Marchewka et al., Reference Marchewka, Żurawski, Jednoróg and Grabowska2014; Soares et al., Reference Soares, Comesaña, Pinheiro, Simões and Frade2012; Wierzba et al., Reference Wierzba, Riegel, Pucz, Leśniewska, Dragan, Gola, Jednoróg and Marchewka2015) and more significant (Imbir, Reference Imbir2016a) than neutral stimuli. It was also proved that the level of arousal as a function of valence has the form of a V-shaped relationship; however, this relationship is rather weak and the high individual variation is observed, and therefore this relationship might also be determined both by the person and situation (Kuppens et al., Reference Kuppens, Tuerlinckx, Russell and Barrett2013). The shape of the relationship between arousal and valence can also be influenced by culture, and it has been shown that the increase in the steepness of the function is determined by the level of extroversion of a given culture and the distinction between eastern and western cultures (Kuppens et al., Reference Kuppens, Tuerlinckx, Yik, Koval, Coosemans, Zeng and Russell2017). In fact, recent studies on Chinese words have shown that differences in the evaluation of valence between Chinese and English words may be determined by cultural and socio-political factors (Xu et al., Reference Xu, Li and Chen2022).
By incorporating stimuli differing in activating value in the experiments, we can expect the relationship with valence to play its role in the results (Demanet et al., Reference Demanet, Liefooghe and Verbruggen2011; Koole & Coenen, Reference Koole and Coenen2007; Riemann & McNally, Reference Riemann and McNally1995), which has to be factored into the model of the study.
In previous experiments using various cognitive tasks (Emotional Stroop Task and its modified version), we have found that a word’s high load of arousal slowed down the reaction times (Imbir et al., Reference Imbir, Spustek, Bernatowicz, Duda-Goławska and Żygierewicz2017, Reference Imbir, Jurkiewicz, Duda-Goławska, Pastwa and Żygierewicz2018, Reference Imbir, Duda-Goławska, Pastwa, Jankowska and Żygierewicz2021). Contrary to the influence of arousal, in a series of experiments using procedures requiring cognitive control, we have found that a high level of subjective significance shortens reaction times in comparison with lower subjective significance levels (Imbir et al., Reference Imbir, Spustek, Bernatowicz, Duda-Goławska and Żygierewicz2017, Reference Imbir, Duda-Goławska, Pastwa, Jankowska, Modzelewska, Sobieszek and Żygierewicz2020a). Both arousal and subjective significance tend to interact in tasks requiring cognitive control, with high subjective significance reducing the disruption brought by the high arousal and shortening the reaction time (Imbir, Reference Imbir2016b; Imbir et al., Reference Imbir, Spustek, Bernatowicz, Duda-Goławska and Żygierewicz2017). When it comes to emotional valence, both positive and negative words tend to speed up reaction times – importantly, this is independent of the arousal effect (Imbir et al., Reference Imbir, Pastwa, Jankowska, Kosman, Modzelewska and Wielgopolan2020b, Reference Imbir, Duda-Goławska, Pastwa, Jankowska and Żygierewicz2021); however, in some previous studies employing the Stroop paradigm, this effect was significantly faster processing for positive words (compared with negative and neutral words; Crossfield & Damian, Reference Crossfield and Damian2021; McKenna & Sharma, Reference McKenna and Sharma2004). In the group of neutral words, the effect of high arousal slowing down the reaction times was especially visible (Imbir et al., Reference Imbir, Duda-Goławska, Pastwa, Jankowska, Modzelewska, Sobieszek and Żygierewicz2020a, Reference Imbir, Duda-Goławska, Pastwa, Jankowska and Żygierewicz2021).
When it comes to the task-switching paradigm, the results of manipulating the affective states of participants seem to vary between experiments. A high load of arousal should disrupt cognitive control, and it has been shown that emotional stimuli of high arousal, both positive and negative, yielded longer reaction times than neutral words of low arousal during the switch between tasks in comparison with neutral stimuli (Demanet et al., Reference Demanet, Liefooghe and Verbruggen2011). These results are congruent with studies showing that a low heart rate of participants (which could be related to low arousal) is a significant predictor of faster performance in task switching (Grol & De Raedt, Reference Grol and De Raedt2020). Results of another study show that switching between the tasks in emotional conditions may be slower than in the neutral condition, but only when participants had a high tendency to ruminate (Koster et al., Reference Koster, De Lissnyder and De Raedt2013). In contrast, a number of studies show an increase of performance during the switch in emotional conditions. When emotional stimuli were presented as feedback for participants, the performance of participants was faster in the emotional conditions (both positive and negative) in comparison with neutral conditions (Ludwig et al., Reference Ludwig, Dignath and Lukas2021). This general effect of emotionality could be supported by a study showing that stress related to exams, which is inevitably related to high emotional arousal, promoted faster reactions in switching between tasks (Kofman et al., Reference Kofman, Meiran, Greenberg, Balas and Cohen2011).
A number of studies exploring the influence of emotions on cognitive control in task switching have been focusing on the specificity of the influence of positive and negative emotions independently. It has been shown that positive emotions may promote faster reactions during the switch between tasks when compared with neutral conditions (Hsieh & Lin, Reference Hsieh and Lin2019; Wenzel et al., Reference Wenzel, Conner and Kubiak2013); positive affect may also contribute to shortening the reaction time in comparison with negative affect (Tae et al., Reference Tae, Almasi, Weldon, Lee, An and Sohn2021). A proposed mechanism for this phenomenon has been that positive affect facilitates cognitive flexibility, whereas negative affect inhibits it (Kanske & Kotz, Reference Kanske and Kotz2011). When incentives were used in a task-switching procedure as a way to motivate participants but also to evoke positive emotions, responses during the switch were faster with the incentive than in the condition without the incentive (Savine et al., Reference Savine, Beck, Edwards, Chiew and Braver2010). Furthermore, it has been shown that increasing the reward in the task (causing a positive emotional reaction) resulted in reducing the time cost of the switch between the tasks, whereas reducing the reward (causing a negative emotional reaction) was tied to a larger time cost of the switch (Shen & Chun, Reference Shen and Chun2011). However, it has to be mentioned that a number of studies did not recognise the significant behavioural effects of different emotional conditions in task-switching procedures (Cudo et al., Reference Cudo, Francuz, Augustynowicz and Stróżak2018; González-García et al., Reference González-García, García-Carrión, López-Benítez, Sobrado, Acosta and Ruz2021; Nusbaum et al., Reference Nusbaum, Wilson, Stenson, Hinson and Whitney2018). Subjective significance has not been a frequently explored factor influencing cognitive control, and the present study is one of the first to verify the influence of this factor on task switching.
1.3. Aim and hypotheses
In a series of two experiments, we aimed to determine the role of activational factors of arousal and subjective significance (Experiment 1), as well as valence (Experiment 2), in the effectiveness of cognitive control needed to switch between two different tasks: a grammatical gender detection task and an emotional categorisation task. In general, we expected that arousal would lead to the impairment of cognitive control effectiveness and that subjective significance would enhance the effectiveness of cognitive control when switching attention. At first, we decided to investigate only activation load (with neutral valence), as our expectations were mostly focused on activation dimensions. The rationale for this was the results of studies showing that arousal level is an important factor for generating cognitive control difficulties in task switching (Demanet et al., Reference Demanet, Liefooghe and Verbruggen2011; Grol & De Raedt, Reference Grol and De Raedt2020). Subsequently, we were interested in whether the inclusion of valence (negative, neutral and positive) would modify the effects of activation obtained for neutral stimuli. As the emotionality of stimuli is intuitively associated with valence, we wanted to find a pure valence influence, with precise control of arousal and the subjective significance of stimuli.
We expected that reaction latencies would be shorter for the grammatical gender-marking task in comparison with the emotional categorisation task (H1), because the emotional categorisations of neutral valence stimuli should take more time. At the same time, we supposed that reaction time latencies would be lower for highly subjectively significant stimuli (H2). We also expected that reaction time latencies would be higher for highly arousing stimuli (H3), as well as the effects of arousal and subjective significance to be more pronounced, when the levels of valence were moderate (H4).
2. Method
2.1. Participants
2.1.1. Sample size estimation
In order to estimate the sample size needed to obtain reliable results in the experiments, we conducted a priori power analyses using G-Power 3.1 software (Erdfelder et al., Reference Erdfelder, Faul, Buchner and Lang2009). The estimation was based on partial eta squared sizes from previous studies exploring the relationship between emotions and performance in task switching (Demanet et al., Reference Demanet, Liefooghe and Verbruggen2011) and other tasks such as dual task (Tae et al., Reference Tae, Almasi, Weldon, Lee, An and Sohn2021), emotional Stroop task (Imbir, Reference Imbir2016a; Imbir et al., Reference Imbir, Duda-Goławska, Pastwa, Jankowska and Żygierewicz2021) and modified Stroop task (Imbir et al., Reference Imbir, Duda-Goławska, Pastwa, Jankowska, Modzelewska, Sobieszek and Żygierewicz2020a). Effect sizes in previous research varied from ηp 2 = 0.06 to ηp 2 = 0.15 for main effect of one factor, with accuracy as a dependent variable (Demanet et al., Reference Demanet, Liefooghe and Verbruggen2011; Tae et al., Reference Tae, Almasi, Weldon, Lee, An and Sohn2021). For reaction times, the effect sizes varied from ηp 2 = 0.06 to ηp 2 = 0.29 for main effect of one factor (Demanet et al., Reference Demanet, Liefooghe and Verbruggen2011; Imbir et al., Reference Imbir, Duda-Goławska, Pastwa, Jankowska, Modzelewska, Sobieszek and Żygierewicz2020a, Reference Imbir, Duda-Goławska, Pastwa, Jankowska and Żygierewicz2021; Tae et al., Reference Tae, Almasi, Weldon, Lee, An and Sohn2021) and from ηp 2 = 0.05 to ηp 2 = 0.28 for interaction of two factors (Imbir, Reference Imbir2016a; Imbir et al., Reference Imbir, Duda-Goławska, Pastwa, Jankowska, Modzelewska, Sobieszek and Żygierewicz2020a, Reference Imbir, Duda-Goławska, Pastwa, Jankowska and Żygierewicz2021; Tae et al., Reference Tae, Almasi, Weldon, Lee, An and Sohn2021). The smallest effect of interest we would expect in our experiments, based on presented studies, could be ηp 2 = 0.05 – this size of effect would be expected for interaction of two emotional factors, with reaction times as a dependent variable. Relying on the presented data, we estimated that in order to achieve high statistical power of 0.8, we would need at least 15 participants for the main effects of one emotional factor and 36 participants for the interaction of two factors (low number of participants needed to observe effects derives from the design of the study, including from 45 to 135 repeated measure for each level of one emotional factor). We decided to increase the number of participants from the initial estimation for two reasons: in Experiment 1, we were verifying the effects of subjective significance, which is a recently proposed dimension of emotional processing, and, thus, we could not reliably estimate the expected effect sizes; in Experiment 2, we manipulated three factors simultaneously – larger number of participants could have allowed us to observe the interaction of all three variables.
2.1.2. Experiment 1
A total of 64 participants took part in the experiment (32 women and 32 men); however, data verification procedures (described in Section 3) lead to reducing the final sample to 61 participants (32 women and 29 men). They were all aged 30–60 years (M = 39.97; SD = 7.28), were inhabitants of one of the Warsaw estates (in Wilanów district) and were members of a group community dedicated to discussions about this estate. They were invited to take part in the experiment via e-mail using the group community’s mailing list.
All participants were native speakers of the Polish language (and declared at least a basic understanding of the English language, necessary to answer a few questions at the beginning of our experiment, such as subject’s number and age), had normal or corrected-to-normal eyesight and declared their right hand as dominant. They were graduates from different colleges and faculties (e.g., pharmacy, law and management). For participation in our study, they received material remuneration worth about 20 PLN (about 5 USD).
2.1.3. Experiment 2
Our second experiment was conducted among students at the University of Warsaw. A total of 60 participants (30 women and 30 men) aged 19–27 years (M = 22.34; SD = 2.47) took part voluntarily. All participants were studying at the University of Warsaw in different faculties and colleges, such as law, journalism, internal security studies, musicology and others. A balanced proportion of participation of students from the various departments was maintained. Each participant received a remuneration of 20 PLN (about 5 USD).
All of the procedures involving human participants were conducted in accordance with the ethical standards of the institutional research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All of our participants gave their written informed consent for taking part in both experiments; they were ensured about the anonymous character of the study and the possibility of withdrawing the consent at any moment without providing any reason. They were also told that their anonymised data will be analysed only at a group level and used in a scientific purpose only.
2.2. Design
A full-factorial, within-subject design was used in the two experiments. For both, the behavioural response to determining the word’s grammatical gender (masculine vs. non-masculine) or emotionality (emotional vs. unemotional) was investigated. Both tasks were displayed alternately within subjects and were held in the task-switching paradigm. In the first experiment, the verbal stimuli used were selected for the three levels of arousal (low, moderate and high) and three levels of subjective significance (low, moderate and high). In the second experiment, verbal stimuli were matched based on the three levels of valence (positive, neutral and negative), three levels of arousal (low, moderate and high) and three levels of subjective significance (low, moderate and high).
2.3. Word materials
2.3.1. Experiment 1
A total of 135 target words were used in Experiment 1. They were all nouns derived from the Affective Norms for Polish Words Reload database (Imbir, Reference Imbir2016a). In creating this database, each word was evaluated by 50 participants on eight different dimensions: valence, arousal, dominance, origin, subjective significance, concreteness, imageability and subjective age of acquisition. Responses (indicated on Self-Assessment Manikin Scales consisting of nine points) were then calculated into means for each of the dimensions.
In Experiment 1, stimuli were divided into nine groups (15 words each) based on their level of arousal (low, moderate and high) and subjective significance (low, moderate and high), 135 words in total. We also controlled for other variables, namely, valence (neutral), concreteness, frequency of use in the Polish language [these data were transformed into natural logarithms (NLs) to meet the normal distribution assumption] and number of letters for this set of words. For the dimension of arousal, mean ratings were M = 3.22 (SD = 0.24) for low arousal, M = 3.85 (SD = 0.27) for moderate arousal and M = 4.87 (SD = 0.4) for high arousal. For subjective significance, mean ratings were M = 2.92 (SD = 0.4) for low significance, M = 3.67 (SD = 0.3) for moderate significance and M = 4.69 (SD = 0.4) for high subjective significance. All of the stimuli used in Experiment 1 can be found in Supplementary Appendix 1.
In order to verify our stimuli selection, we also conducted analysis of variance (ANOVA) using a 3 (levels of arousal) × 3 (levels of subjective significance) model. We were expecting statistically significant results for arousal and subjective significance levels in their respective ratings. None of the other effects should be significant, proving that the words were different only on the dimensions of arousal and subjective significance.
We found significant differences only between the groups of arousal divided by arousal ratings: F(2, 132) = 305.31, p < 0.001, ηp 2 = 0.822. There was no effect for subjective significance divided by arousal ratings: F(2, 132) = 0.61, p = 0.55, ηp 2 = 0.009; valence: F(2, 132) = 1.48, p = 0.23, ηp 2 = 0.022; concreteness: F(2, 132) = 0.03, p = 0.97, ηp 2 < 0.001; frequency of usage in the Polish language: F(2, 132) = 1.24, p = 0.29, ηp 2 = 0.018 or number of letters: F(2, 132) = 0.58, p = 0.56, ηp 2 = 0.009.
Similarly, we found the effect only for subjective significance divided by significance ratings: F(2, 132) = 257.27, p < 0.001, ηp 2 = 0.80. There was no significant effect for arousal: F(2, 132) = 0.16, p = 0.85, ηp 2 = 0.002; valence: F(2, 132) = 1.93, p = 0.15, ηp 2 = 0.028; concreteness: F(2, 132) = 2.86, p = 0.06, ηp 2 = 0.042; frequency: F(2, 132) = 3.06, p = 0.05, ηp 2 = 0.044 or number of letters: F(2, 132) = 1.33, p = 0.27, ηp 2 = 0.02. In Supplementary Appendix 1, we also provide further analyses verifying the stimuli selection and their exact mean ratings.
2.3.2. Experiment 2
In Experiment 2, there were 27 groups of words, with 15 words in each one, 405 words in total. However, the division was different, reflecting three levels of valence (negative, neutral and positive), three levels of arousal (low, moderate and high) and three levels of subjective significance (low, moderate and high).
The following mean and standard deviation values were obtained for valence: M = 3.80 (SD = 0.59) for negative, M = 5.10 (SD = 0.29) for neutral and M = 6.14 (SD = 0.46) for positive stimuli. For the dimension of arousal, mean ratings were M = 3.36 (SD = 0.31) for low, M = 3.96 (SD = 0.18) for moderate and M = 4.76 (SD = 0.42) for high arousal. For the dimension of subjective significance, mean ratings were M = 3.01 (SD = 0.28) for low, M = 3.62 (SD = 0.18) for moderate and M = 4.35 (SD = 0.41) for high subjective significance. We controlled factors such as word length (number of letters) and frequency of usage in the Polish language (Kazojć, Reference Kazojć2011) calculated as an NL. All the words used in the experiment can be found in Supplementary Appendix 1.
To verify the adequacy of stimuli selection, ANOVA was conducted using a 3 (levels of valence) × 3 (levels of arousal) × 3 (levels of subjective significance) model. We expected a significant main effect of valence on valence rating, arousal on arousal rating and subjective significance on subjective significance rating. We obtained a significant effect of valence rating on valence: F(2, 402) = 750.85, p < 0.001, ηp 2 = 0.78, but not on frequency of usage in the Polish language: F(2, 402) = 0.65, p = 0.53, ηp 2 < 0.01, number of letters: F(2, 402) = 1.09, p = 0.37, η2 < 0.01, arousal: F(2, 204) = 0.19, p = 0.83, ηp 2 < 0.01 or subjective significance: F(2, 204) = 0.40, p = 0.67, ηp 2 < 0.01.
Similarly, we found a significant effect of arousal rating on arousal: F(2, 402) = 656.11, p < 0.001, ηp 2 = 0.76, but not on frequency of usage in the Polish language: F(2, 402) = 0.68, p = 0.50, ηp 2 < 0.01, number of letters: F(2, 402) = 0.06, p = 0.94, ηp 2 < 0.01, valence: F(2, 204) = 1.32, p = 0.26, ηp 2 < 0.01 or subjective significance: F(2, 204) = 0.16, p = 0.86, ηp 2 < 0.01.
Furthermore, we found a significant effect of subjective significance rating on subjective significance: F(2, 402) = 663.04, p < 0.001, ηp 2 = 0.77, but not on the frequency of usage in the Polish language: F(2, 402) = 2.91, p = 0.6, ηp 2 = 0.01, number of letters: F(2, 402) = 2.67, p = 0.07, ηp 2 = 0.01, arousal: F(2, 402) = 0.73, p = 0.48, ηp 2 < 0.01 or valence F(2, 402) = 0.16, p = 0.86, ηp 2 < 0.01.
2.3.3. Control (non-target) words
Apart from experimental stimuli, treated as target words in our study, we also used some non-target neutral words, displayed to participants between target words and treated as control stimuli (excluded from analyses). Control stimuli were taken from a base of 849 nouns, neutral in valence (M = 5.33; SD = 0.6), arousal (M = 3.65; SD = 0.43) and significance (M = 3.4; SD = 0.47).
2.3.4. The competing tasks: Word gender-marking and emotional categorisations
In the Polish language, nouns have three main genders: masculine (usually, but not always, ending with a consonant), feminine (often ending with an ‘a’) and neutral (often ending with an ‘o’). In the grammatical sense, it is similar to Latin (a noun’s gender is set by rules, and it changes the forms of pronouns and adjectives in a sentence). It is possible – and natural for Polish native speakers – to automatically recognise the gender of a noun without a sentence (or an article).
The first task used in both of our experiments was a gender-marking task based on this ability to quickly recognise the gender of a noun. Participants were asked to recognise the gender of a noun from two options: male (masculine form; this option was named M) and non-male (feminine and neutral form; ~M). They gave their answers by pressing one of two keyboard keys; both of the choices (M and ~M) were displayed on the computer screen during the whole task. The selection of tasks with binary choices was motivated by their simplicity, which can aid investigation of the decision and switch processes. When facing binary choices, participants can easily answer to each task with only one hand (two keys per hand), minimising confounding factors on reaction times in the experimental procedure.
The second task in our experiments was the emotional-marking task. Participants were asked to evaluate the emotional load of a noun, choosing from two options: emotional (this option was named EMO) and unemotional (~EMO). They gave their answers by pressing one of two keyboard keys. Also, in this case, both of the choices (EMO and ~EMO) were shown on the screen during the task.
2.3.5. Task switching
For the task-switching procedure, we employed the above-described lists of target and control words and the two tasks mentioned above. The emotional and gender-marking tasks were presented in sequences (with one to five iterations of a task in one sequence), and the length of all the sequences was random. Target words were presented only after switching tasks: for example, if the sequence consisted of three trials with a gender-marking task, the next trial after this sequence was the emotional task and, in this exact trial, the target word was presented; if the next trial was the emotional task again, the control word was presented until the next switch to the gender-marking task, where the target word was presented. The control words were presented randomly, without repetition. The target words were presented in 15-word blocks, differing on the dimensions of arousal and subjective significance; the order of the blocks was random for each participant, and the order of words in each block was also random. We added one control word to each of the experimental groups (the words were excluded from the pool of control words used in the experiment) to make the number of words in each experimental group even; in this way, we can avoid a possible effect of one of the two tasks dominating, which could load the results in a specific way. The control words added to the experimental groups were excluded from the final analyses. As the length of the sequences between the switching of tasks was always random (from zero to four buffer trials), Experiment 1 could consist of 135–675 trials and Experiment 2 could consist of 405–2025 trials, but the extreme values never appeared. The actual distribution of buffer trials was: Experiment 1 – Min = 251; Max = 324; M = 289.3; SD = 17.9; Experiment 2 – Min = 801; Max = 944; M = 860.5; SD = 31.4. The task-switching procedure is presented in Fig. 1.
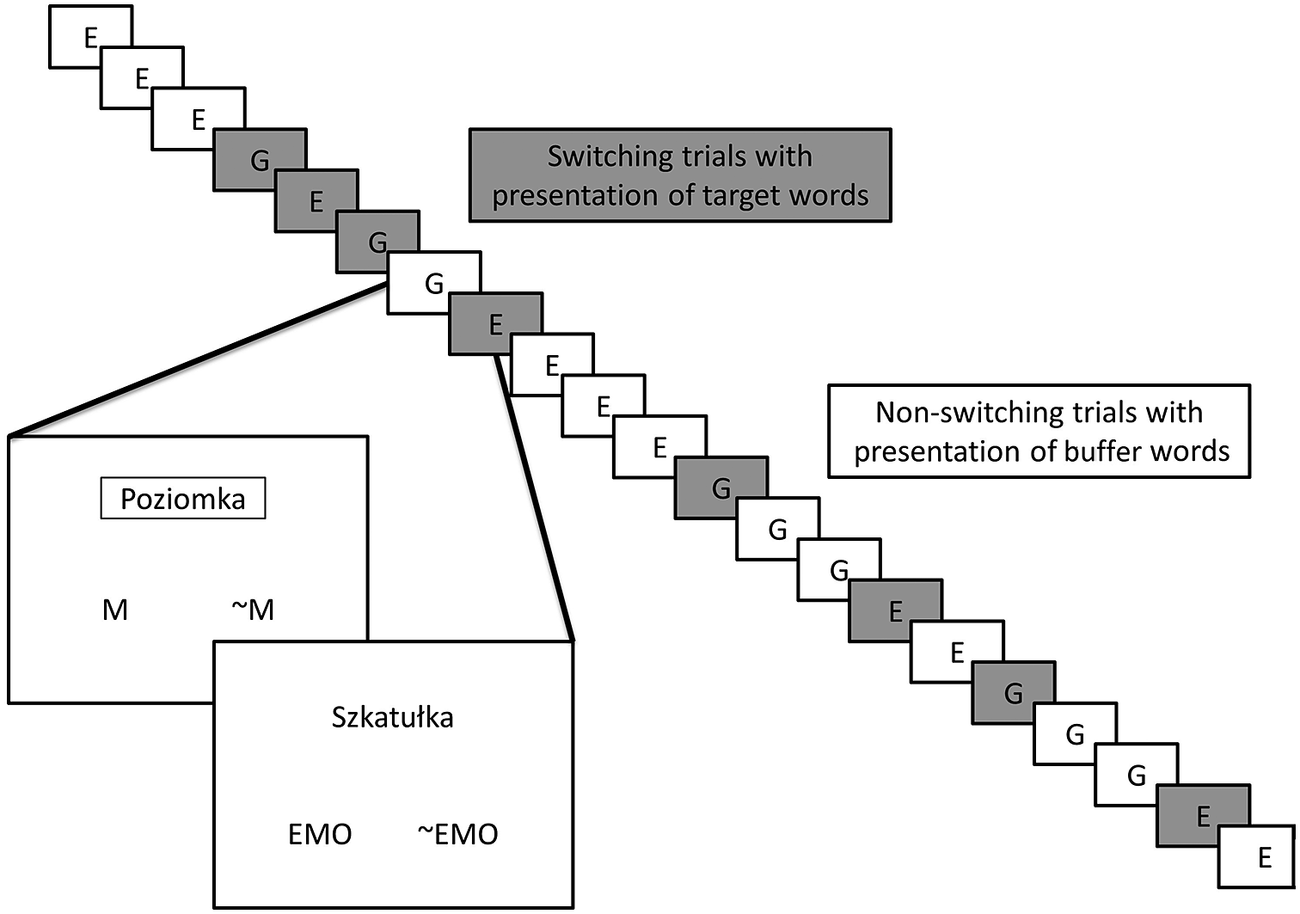
Fig. 1. Illustration of the experimental procedure. E, emotional task; G, gender-marking task; M, male; ~M, non-male; EMO, emotional; ~EMO, unemotional. The words showed at the trials are examples (‘poziomka’, meaning ‘wild strawberry’ in Polish, and ‘szkatułka’, meaning ‘small casket’). A rectangle was displayed in the gender-marking task to help participants notice a task switch.
2.4. Apparatus
Both of the experiments were conducted on a standard laptop with a 15-inch diagonal screen. We used E-Prime 2.0 software to prepare the display of experimental procedures as well as the reaction time recordings.
2.5. Procedure
The experimental procedure was identical in Experiments 1 and 2, the only difference being the various lists of words used as stimuli. Participants performed the tasks between 9 am and 9 pm in a properly lit room. The experimenter was present in the room throughout the whole procedure, sitting on the side at some distance from the participant. At the beginning, participants read a set of instructions explaining both tasks and how they would be switched during the procedure. The first part was a set of practice trials where participants had the chance to get to know the emotional and gender-marking tasks: five trials for the emotional task, followed by five trials for the gender-marking task. Participants then began the practice part of the task-switching procedure, where 18 trials (nine with the emotional task and nine with the gender-marking task) were presented in random order. This practice sequence allowed participants to get to know the tasks and the mechanism of task switching that would be presented during the whole experiment. In the practice set, we used only control words, which were not included in the further part of the experiment. Half of the participants had the keys ‘A’ and ‘S’ linked to the emotional task and ‘K’ and ‘L’ linked to the gender-marking task; for the other half of the participants, the keys were inverted between the tasks. Nevertheless, the participants did not see the mentioned letters, as stickers were placed on the keys with, respectively, EMO or ~EMO and M or ~M. The response keys were the same for a particular participant in both the practice and the actual experiment. After finishing the practice sequence, the participant started the main experiment. Mean time of completing the experimental session (excluding instructions and practice sequence) was M = 11.8 minutes (SD = 25 seconds) for Experiment 1 and M = 35.5 minutes (SD = 46 seconds) for Experiment 2. On completion of the task-switching procedure, the participants received the remuneration. The analysed data were the reaction latencies from the target trials only.
3. Results
3.1. Experiment 1
In Experiment 1, there were initially 8,640 trials, but the following were excluded: trials with answers taken with incorrect keys (298 trials), trials with incorrect answers in gender-marking task (318 trials), trials shorter than 300 ms (2 trials) or longer than 2 SD subjectwise (424 trials). These operations left three participants with some of the word groups empty, which lead to deleting their data from the final sample. After that, we transformed response latencies into NLs and analysed the data using a 2 (type of task: emotional or gender-marking) × 3 (levels of arousal: low, moderate and high) × 3 (levels of subjective significance: low, moderate and high) repeated-measures ANOVA. If the data did not meet the sphericity assumption, they were reported using the Greenhouse – Geisser correction; for further pairwise comparisons, we used t-tests with the Bonferroni correction.
Before the analysis, taking into consideration the fact that we provided our participants with two tasks, we investigated reaction times in both experiments to confirm that the procedure induced a cognitive cost associated with task switching. This allows for the interpretation of subsequent effects of emotional factors as modifying the ‘switching cost’ – the difference in reaction times after a task switch and when the task was repeated. We calculated the switching cost for each task separately, comparing reaction times in the first and second executions of each task. For the emotional task, responses were longer after a task switch (M = 2,137.43 ms; SEM = 15.79), than on the second trial (M = 1,382.06 ms; SEM = 25.23); t(2,861) = 9.66, p < 0.001, d = 0.18, with an average switching cost (the difference between those two means) of 255 ms with 95% CI: [232.27, 350.47]. For the gender-marking task, responses were longer after a task switch (M = 2,060.21 ms; SEM = 18.66), than on the second trial (M = 1,554.31 ms; SEM = 21.67); t(2,808) = 16.94, p < 0.001, d = 0.32, with an average switching cost (the difference between those two means) of 505.91 ms with 95% CI: [447.34, 564.47]. We present the example of task switch and first repeated trial, but those differences were identical between task switch and each of the subsequent repeated trials (which in our experiments were varying from one to four repetitions; see Section 2). Furthermore, none of the differences between repeated trials were statistically significant (Fig. 2).

Fig. 2. Mean reaction times as a function of the number of repetitions of a task for (A) emotional task and (B) gender-marking task. The error bars show standard deviations, and the black horizontal lines show statistically significant comparisons (***p < 0.001).
We obtained a significant main effect of the type of task (Fig. 3): F(1, 60) = 30.58, p < 0.001, ηp 2 = 0.34. Response latencies for the emotional task (M = 2,386.5 ms; SEM = 80.8; LNs: M = 7.67; SEM = 0.032) were significantly longer than latencies for the gender-marking task (M = 2,063 ms; SEM = 70.49; LNs: M = 7.53; SEM = 0.03); t(67) = 5.51, p < 0.001, d = 0.66.

Fig. 3. Bar graph showing the main effect for type of task. The error bars show standard deviations.
We also found a significant main effect of subjective significance (Fig. 4): F(1.68, 100.47) = 3.36, p = 0.04, ηp 2 = 0.054. Reaction times for moderate subjective significance (M = 2,253 ms; SEM = 68.71; LNs: M = 7.62; SEM = 0.029) were significantly longer than for high subjective significance (M = 2,192 ms; SEM = 72.7; LNs: M = 7.59; SEM = 0.03); t(67) = 3.35, p = 0.001, d = 0.41 (Fig. 4). The main effect for arousal was not significant: F(2, 120) = 0.116, p = 0.89, ηp 2 = 0.002.

Fig. 4. Bar graph showing the main effect for subjective significance. The error bars show standard deviations, and the black horizontal lines show statistically significant comparisons (*p < 0.05; **p < 0.01; ***p < 0.001).
There was also a significant interaction effect between type of task and level of arousal and subjective significance: F(4, 240) = 4.37, p = 0.002, ηp 2 = 0.07. Namely, we found significant differences for the emotional categorisation task in moderate arousal, whereby reaction times for stimuli of moderate subjective significance (M = 2,535 ms; SEM = 107.85; LNs: M = 7.73; SEM = 0.04) were significantly longer than for those of high subjective significance (M = 2,334 ms; SEM = 100.62; LNs: M = 7.64; SEM = 0.043); t(67) = 3.17, p = 0.002, d = 0.14. We also found differences for the emotional task in low subjective significance, whereby stimuli of low arousal elicited significantly longer (M = 2,485 ms; SEM = 97.12; LNs: M = 7.71; SEM = 0.04) responses than highly arousing words (M = 2,288 ms; SEM = 79.61; LNs: M = 7.65; SEM = 0.29); t(67) = 2.23, p = 0.03, d = 0.27. These differences are shown in Fig. 5.

Fig. 5. Bar graph showing the interaction effect between type of task, arousal and subjective significance for the emotional task. The error bars show standard deviations, and the black horizontal lines show statistically significant comparisons (*p < 0.05; **p < 0.01; ***p < 0.001).
For the gender-marking task, there were differences in reaction times for high arousal stimuli, whereby responses for moderate subjective significance (M = 2,117 ms; SEM = 72.9; LNs: M = 7.57; SEM = 0.031) were significantly longer than for stimuli of high subjective significance (M = 1,933 ms; SEM = 83.1; LNs: M = 7.47; SEM = 0.037); t(67) = 3.73, p < 0.001, d = 0.45. These differences are shown in Fig. 6.

Fig. 6. Bar graph showing the interaction effect between type of task, arousal and subjective significance for the gender-marking task. The error bars show standard deviations, and the black horizontal lines show statistically significant comparisons (*p < 0.05; **p < 0.01; ***p < 0.001).
3.2. Experiment 2
In Experiment 2, there were 24,300 trials in total; as in Experiment 1, we deleted trials with answers taken with incorrect keys and trials with incorrect answers in gender-marking task (815 trials), trials shorter than 300 ms (14 trials) or longer than 2.5 SD subjectwise (768 trials); additionally, we excluded 18 trials that were longer than 15 seconds.
In Experiment 2, again, we saw that reaction times were longer after a task switch. For the emotional task, the reaction time was slower (M = 1,995.90 ms; SEM = 13.53) than on the first repeat (M = 1,444.89 ms; SEM = 16.99); t(9,092) = 25.28, p < 0.001, d = 0.27 as well as in the gender task, where switching trials (M = 1,921.27 ms; SEM = 12.39) were significantly slower than on the repeated trial (M = 1,416.77 ms; SEM = 14.94); t(9,084) = 26.07, p < 0.001, d = 0.27. Switching costs for both tasks were, respectively, 551.01 ms with 95% CI [508.29, 593.74] for the emotional task and 504.49 ms with 95% CI [466.57, 542.42] for the gender-marking task (Fig. 7). Similarly to Experiment 1, we once again noticed significant differences between the switch trial and all repeated trials (1–4) and no differences between the repeated trials.

Fig. 7. Mean reaction times as a function of the number of repetitions of a task for (A) emotional task and (B) gender-marking task in Experiment 2. The error bars show standard deviations, and the black horizontal lines show statistically significant comparisons (***p < 0.001).
Response latencies were once again transformed into NLs and analysed using a 2 (type of task: emotional vs. gender-marking task) × 3 (levels of valence: positive, neutral and negative) × 3 (levels of arousal: low, moderate and high) × 3 (levels of subjective significance: low, moderate and high) repeated-measures ANOVA. In the general analysis, there was no main effect for type of task: F(1, 58) = 0.26, p = 0.61, ηp 2 = 0.005; valence: F(2, 101) = 1.05, p = 0.35, ηp 2 = 0.033; arousal: F(2, 102) = 0.76, p = 0.58, ηp 2 = 0.013 or subjective significance: F(2, 88) = 0.015, p = 0.96, ηp 2 = 0.001.
We found the interaction effect between three factors: task type, subjective significance and valence (F(4, 232) = 2.79, p = 0.027, ηp 2 = 0.046). The significant differences could be observed only within the group of neutrally valenced words. For words of medium significance, there was a difference between task types, with longer responses in the emotional task (M = 2,097 ms; SEM = 94; LNs: M = 7.48; SEM = 0.04) than in the gender-marking task (M = 1,871 ms; SEM = 71; LNs: M = 7.41; SEM = 0.03); t(179) = 2.43, p = 0.03, d = 0.36. In the emotional task, words with high subjective significance elicited faster reaction times (M = 1,904 ms; SEM = 76; LNs: M = 7.41; SEM = 0.04) compared with words of medium significance; t(179) = 2.59, p = 0.019, d = 0.39.
However, we found an interaction effect between type of task, valence, arousal and subjective significance: F(8, 464) = 3.45, p = 0.001, ηp 2 = 0.056. After that, further analyses were conducted within the valence factor with the assumption of each level (positive, neutral and negative) being a separate cluster of data, using the repeated-measures ANOVA and then paired sample t-tests with the Bonferroni correction for more detailed pairwise comparisons.
For words of a neutral valence, we found no statistically significant main effects for type of task: F(1, 58) = 0.40, p = 0.53, ηp 2 = 0.007; arousal: F(2, 57) = 0.30, p = 0.74, ηp 2 = 0.01 or subjective significance: F(2, 57) = 0.96, p = 0.39, ηp 2 = 0.03. There was, however, a significant effect of interaction between type of task and subjective significance: F(2, 57) = 5.35, p = 0.007, ηp 2 = 0.16. The shape of the interaction reflected the interaction between type of task and subjective significance for neutral words, that was found in the main analysis. For words of moderate subjective significance, response latency in the emotional task was significantly longer (M = 2,095 ms; SEM = 92.8; LNs: M = 7.48; SEM = 0.04) than in the gender-marking task (M = 1,868 ms; SEM = 69.8; LN: M = 7.40; SEM = 0.04); t(59) = 2.48, p = 0.02, d = 0.32. There was also a difference between moderate and high subjective significance for the emotional task, whereby reaction times were longer for moderate significance than for high significance (M = 1,899 ms; SEM = 75; LNs: M = 7.41; SEM = 0.04); t(59) = 2.76, p = 0.008, d = 0.36. These differences were presented in Fig. 8.

Fig. 8. Bar graph showing the interaction effect between type of task and subjective significance (for words of neutral valence only). The error bars show standard deviations, and the black horizontal lines show statistically significant comparisons (*p < 0.05; **p < 0.01; ***p < 0.001).
For words of a positive valence, we found no main effects for type of task: F(1, 59) = 0.58, p = 0.45, ηp 2 = 0.01; arousal: F(2, 58) = 1.04, p = 0.36, ηp 2 = 0.04 or subjective significance: F(2, 58) = 0.09, p = 0.92, ηp 2 = 0.03, but we did find a significant effect of interaction between type of task, arousal and subjective significance: F(4, 56) = 5.20, p = 0.001, ηp 2 = 0.27. In the case of moderate arousal and high subjective significance, the response time was significantly longer for the emotional task (M = 1,968 ms; SEM = 98.6; LNs: M = 7.43; SEM = 0.039) than for the gender-marking task (M = 1,727 ms; SEM = 64; LNs: M = 7.35; SEM = 0.033); t(59) = 2.40, p = 0.02, d = 0.31.
Similarly, there were differences between the emotional task (M = 2,112 ms; SEM = 101; LNs: M = 7.50; SEM = 0.40) and the gender-marking task (M = 1,868 ms; SEM = 87; LNs: M = 7.40; SEM = 0.40) for words of high arousal and low subjective significance; t(59) = 2.74, p = 0.008, d = 0.35.
For the gender-marking task, there were also differences in the case of high subjective significance between the response latency of words of low arousal (M = 2,017 ms; SEM = 118; LNs: M = 7.46; SEM = 0.04) and moderate arousal (M = 1,727 ms; SEM = 64; LNs: M = 7.34; SEM = 0.033); t(59) = 3.29, p = 0.002, d = 0.42; this was also the case for moderate arousal and high arousal (M = 1,993 ms; SEM = 84; LNs: M = 7.47; SEM = 0.039); t(59) = 3.75, p < 0.001, d = 0.49. Differences for positively valenced words are shown in Fig. 9.

Fig. 9. Bar graph showing the interaction effect between type of task, arousal and subjective significance (for words of positive valence only) for: (A) low arousal, (B) medium arousal and (C) high arousal. The error bars show standard deviations, and the black horizontal lines show statistically significant comparisons (*p < 0.05; **p < 0.01; ***p < 0.001).
For negative-valence stimuli, we did not find any significant main effect for type of task: F(1, 59) = 0.004, p = 0.95, ηp 2 < 0.001; arousal: F(2, 58) = 1.47, p = 0.24, ηp 2 = 0.048 or subjective significance: F(2, 58) = 0.66, p = 0.52, ηp 2 = 0.02. There was also no significant interaction effect.
4. Discussion
As expected, we observed an effect of the type of task, either as a main effect (Experiment 1) or an interaction effect (Experiment 2). This confirms the first of the proposed hypotheses. Reaction times among our participants were significantly longer for the emotional task than for the gender-marking task. Categorising the gender of a word seems to be easier for participants than assessing the emotionality of the word. This result underlines the difference between these two tasks: we can easily mark the gender of a word, but evaluating its emotionality is more subjective, ambiguous, demanding and, thus, takes longer. It is also important to mention that while the gender-marking task has an objectively good answer (supported by grammatical rules), assessing emotionality does not. Participants assessing emotionality might have deliberated longer over their decision because they were not totally confident and had to rely on their intuition instead.
We have also partially confirmed our hypothesis concerning subjective significance (H2): high subjective significance elicited shorter reaction times than moderate significance. Interestingly, we observed this effect for neutral words only – either as a main effect (Experiment 1) or as an interaction effect ( Experiment 2). In our second experiment, we could also see that the influence of subjective significance level is linked to the type of task: the differences are visible in the task requiring emotional assessment. This result is very much in line with previous studies and the theory concerning subjective significance (Imbir, Reference Imbir2016a, Reference Imbir2016b; Imbir et al., Reference Imbir, Spustek, Bernatowicz, Duda-Goławska and Żygierewicz2017); the subjective significance load of an object is important for an individual in relation to the context and some goals, rules and values. It is not relevant when marking the gender of the word, but it might provide an excellent clue to the emotionality of the word, thus shortening the reaction times for highly significant stimuli (i.e., those considered as important and requiring a fast answer).
The results for arousal did not confirm our hypothesis that high arousal will lead to slower task-switching reactions (H3). We did not observe a main effect of arousal or a two-way interaction between arousal and type of task; this may be due, in part, to the difference that controlling subjective significance has on the main effect of arousal compared with previous studies that did not manipulate this factor. Indeed, we saw an interaction between type of task, arousal and subjective significance. There was an effect of arousal for low significance in the emotional categorisation task, but its direction was opposite to what we expected: words with high arousal had shorter reaction times than words with low arousal. This can be explained by looking at the task demands. In Experiment 1, we asked participants to judge the emotionality of only neutral words. In such a context, the role of arousal and significance becomes more relevant to the answer. The effect may derive from the fact that it was harder to judge the emotionality of an arousing word than a word that is both significant and low in arousal, rather than from arousal’s positive effect on task switching. What corroborates this account is that the positive effect of high subjective significance could be seen in both tasks, while no significant post hoc difference in arousal has been observed for the gender-marking task. It is important to note, however, that the effect of shorter reaction times in the face of high emotional arousal, despite being observed in the present experiment only for words of low subjective significance and only in the emotional categorisation task, is in line with some of the results of previous studies that showed faster reaction times after presentation of arousing stimuli (Ludwig et al., Reference Ludwig, Dignath and Lukas2021) or during being exposed to real-life stress conditions evoking emotional arousal (Kofman et al., Reference Kofman, Meiran, Greenberg, Balas and Cohen2011). The change in the time of reaction dependent on arousal may be modified by the emotional source of the arousing factor, which is supported by the diversity of the procedures used in the previous studies reporting such influence, as well as the specificity of the interaction in which we identified this effect – only among the low significant stimuli in the emotional decision task (Kofman et al., Reference Kofman, Meiran, Greenberg, Balas and Cohen2011; Ludwig et al., Reference Ludwig, Dignath and Lukas2021).
Interpreting the two sets of results together, we see that feeling high subjective significance may, in fact, lead to better cognitive control, as indexed by the faster reaction times during a task-switching procedure. This may shed light on previous findings and perhaps explain some of the inconsistencies in their results. For example, our study showed how high subjective significance may diminish the adverse effects of arousal on cognitive control, in that in the medium arousal condition, reaction times were faster for high subjective significance. A real-world case where the existence of such a mechanism may prove useful is in situations that require strong performance but are also very arousing (high significance and arousal) – an example of such an event may be a student taking an important exam. Recall that this was the scenario investigated by Kofman et al. (Reference Kofman, Meiran, Greenberg, Balas and Cohen2011), where students before an exam showed faster task-switching performance. Although interpreting this finding purely in the context of arousal may seem contradictory to the results of other established studies, it can be resolved by describing how the students who felt high arousal may also have felt high subjective significance, which increased their performance and reduced the adverse effects of arousal. Although further confirmation of such results is needed, it shows the usefulness of subjective significance in describing real-world behaviour and the importance of studying it as an emotional dimension. It has to be remembered, that real-life emotional experiences are much more dynamic than long-known affective connotations of words from a first language; however, some authors attempt to overcome this issue, for example, in the context of learning a new language (Kanazawa, Reference Kanazawa2020).
With regard to the influence of valence, which we assessed in Experiment 2, we observed a complex interaction of all four factors: type of task, valence, arousal and subjective significance. Investigating this interaction on different levels of valence allowed us to show that the results of Experiment 1 replicate under neutral valence (H4). This is also confirmed by the interaction of three factors (type of task, valence and subjective significance) in the main analysis, that showed the same differences depending on subjective significance for the neutral words. This could mean these effects are at least fairly robust to the introduction of positive and negative stimuli, which are relevant to the emotional decision task. However, this approach can also allow us to compare whether these effects are influenced by the level of valence – for example, are they different when valence is positive instead of neutral? Indeed, we saw that the introduction of valence changes the observed effects.
First, we observed no main effects of arousal and subjective significance, or interactions between them, for negative words. It appears that negative valence eliminates the effects of arousal and subjective significance, inhibiting the influence of subjective significance on shortening the reaction times. This may be caused by intensive and unambiguous affective meaning of negative words. We did, however, observe effects for positive valence. These results can be interpreted in the context of the hypothesis that positive affect facilitates cognitive flexibility (Kanske & Kotz, Reference Kanske and Kotz2011). We observed a three-way interaction between task type, arousal and subjective significance, with the fastest task-switching reaction time for medium arousal and high subjective significance. These reaction latencies were significantly faster than for low or high arousal. One way of interpreting the shape of this interaction for positive words is that higher significance indeed helps to speed up processing but only to a certain level of emotionality. When words are both positive and arousing, subjective significance does little to speed up the processing. This supports the interpretation that valence masks the effects of subjective significance; however, significance matters only for positive, and not negative words. The difference in the shape of results, depending on the valence condition, is partly in line with previous studies exploring this dimension of emotional processing in task switching (Hsieh & Lin, Reference Hsieh and Lin2019; Tae et al., Reference Tae, Almasi, Weldon, Lee, An and Sohn2021; Wenzel et al., Reference Wenzel, Conner and Kubiak2013), despite not obtaining a main effect of valence. It has to be noted that the lack of main effects of the well-established dimensions of emotionality, namely arousal and valence, is in line with studies showing no influence of emotions on performance in task switching (Cudo et al., Reference Cudo, Francuz, Augustynowicz and Stróżak2018; González-García et al., Reference González-García, García-Carrión, López-Benítez, Sobrado, Acosta and Ruz2021; Nusbaum et al., Reference Nusbaum, Wilson, Stenson, Hinson and Whitney2018).
The differences between switch and repetition trials were expected, and confirm the validity of both the task and the manipulation. The switch cost, that was seen in both experiments, derives both from the habituation of the task in the repetition trials (Kleinsorge, Reference Kleinsorge1999; Kraut & Smothergill, Reference Kraut and Smothergill1978; Thompson & Spencer, Reference Thompson and Spencer1966) and only neutral words being presented in the repetition trials, while the cluster of words presented in the switch trials consisted mostly of emotional words.
Among the most important limitations of the current experiments are the orthogonal manipulations applied and the lengthy procedure involving a large number of trials. With regard to the orthogonal manipulations, they lead to the selection of stimuli that are aligned in important variables and, thus, the differentiation of effects due to each factor is possible; however, such an approach also leads to the selection of stimuli that are rather mild/moderate in emotional intensity, which can shape the results by lowering the strength of the expected effects and, thus, limits the generalisation of results for more intense emotional stimuli. Also, the differences between particular groups of stimuli (e.g., for the dimensions of arousal) are rather small in the absolute values from the normative study (Imbir, Reference Imbir2016b), so regardless the fact that the differences between groups are significant in ANOVA comparisons (cf. Linguistic Stimuli), they are not especially pronounced. Nevertheless, orthogonal manipulation is the only method that allows the separation of investigated effects from each dimension of affect. With regard to the lengthy procedure, multiple repetitions of stimuli presentation in two tasks, accompanied by a large number of buffering neutral stimuli, could lead to habituation to the repetitive exposure of stimuli, or simply fatigue. The length of the procedure was also increased by the orthogonal manipulations used, requiring several word items for each category of stimuli. The length of procedure may, to some extent, account for the more subtle and more challenging interpretation results recorded in Experiment 2. The last limitation of our design concerns the ability to choose truly neutral stimuli. Although stimuli in Experiment 1 were chosen to have neutral mean valence ratings in each group, this does not guarantee that stimuli will be neutral for all participants. As ratings of each stimuli have their own distribution among participants, subjective stimuli valence covaries with idiosyncratic characteristics and this variance could be larger for some of the words used (e.g., ‘authority’). This limitation always warrants caution as to the confounding effect of valence, which we explored in Experiment 2.
The dimension of subjective significance is recently proposed and not yet thoroughly explored (Jarymowicz & Imbir, Reference Jarymowicz and Imbir2015). We were particularly interested in exploring the influence of this dimension on cognitive processing; however, as we use the word stimuli rated on the scale of subjective significance in a normative study (Imbir, Reference Imbir2016b), it can be questioned whether the affective ratings from that study are relevant to the groups of participants recruited for the experiments. The normative ratings were collected on a group of students and young adults, which could be relatable to the group from Experiment 2; thus, the results could be interpreted reliably. The group taking part in Experiment 1 on the other hand was significantly older than the group from Experiment 2 (around 18 years of difference on average), which leads to limited interpretation of results regarding subjective significance. It also has to be mentioned, that both emotional processing (Kappes et al., Reference Kappes, Streubel, Droste and Folta-Schoofs2017; Laulan et al., Reference Laulan, Catheline, Mayo and Robert2022; Reed et al., Reference Reed, Chan and Mikels2014) and solving cognitive tasks (Allard & Isaacowitz, Reference Allard and Isaacowitz2008; Grady, Reference Grady2012) change with ageing, which means that also the results of both experiments could be compared only to a limited extent. However, as valence of the stimuli was not manipulated in Experiment 1, the results should be free from one of the most commonly reported changes in emotional processing of stimuli that develops with age, which is positivity effect (Kappes et al., Reference Kappes, Streubel, Droste and Folta-Schoofs2017; Laulan et al., Reference Laulan, Catheline, Mayo and Robert2022; Reed et al., Reference Reed, Chan and Mikels2014). Nevertheless, the group taking part in Experiment 2 (students and young adults) is more in line with the specificity of participants taking part in other studies (Demanet et al., Reference Demanet, Liefooghe and Verbruggen2011; Imbir, Reference Imbir2016a, Reference Imbir2016b; Tae et al., Reference Tae, Almasi, Weldon, Lee, An and Sohn2021); thus, the results of this experiment could be concerned as comparable with previous research.
In conclusion, the two experiments showed that subjective significance is a distinct form of activation from arousal perceived in emotional words. Increasing the subjective significance level reduces cognitive control costs among neutral stimuli in general (Experiment 1) or mainly for the emotional categorisation task ( Experiment 2); that is an effect which should be further verified in a procedure closer to everyday life, for example, studying some real subjective significant of an object, such as financial consequences of a decision (even in the laboratory conditions that could be measured by assigning different values to some stimuli and checking how well will they be remembered; Brainerd et al., Reference Brainerd, Chang and Bialer2021). Applying the manipulation which we showed is significant in words stimuli to other procedures could be also a way to overcome the limitations of the present study (such as orthogonal manipulations of words and long, repetitive procedure) and further confirm the status of the subjective significance dimension. Furthermore, we did not find a clear effect of arousal or valence. From the perspective of the dimensional approach to emotions, subjective significance is a relatively new dimension, but the current investigation is in line with previous findings showing a beneficial role of this factor for cognitive control in the Emotional Stroop Task (Imbir, Reference Imbir2016b; Imbir et al., Reference Imbir, Duda-Goławska, Pastwa, Jankowska, Modzelewska, Sobieszek and Żygierewicz2020a).
Supplementary materials
To view supplementary material for this article, please visit http://doi.org/10.1017/langcog.2023.6.
Data availability statement
All of the data obtained in the experiment are publicly available in the figshare repository: https://doi.org/10.6084/m9.figshare.19411169.v3.
Funding
The research, data analysis and manuscript preparation were funded by the National Science Center on the basis of decision 2017/25/B/HS6/00198.












