Introduction
Phytate is widely distributed in plants for which it is the main storage form of phosphorus. This component forms insoluble complexes with zinc and limits its availability to non-ruminant species (O’Dell and Savage, Reference O’Dell and Savage1960). Consequently, besides the excessive excretion of phosphorus, the presence of high amounts of phytates in animal diets may cause environmental pollution due to zinc accumulation in soils (Mohanna and Nys, Reference Mohanna and Nys1999a; Burrell et al., Reference Burrell, Dozier, Davis, Compton, Freeman, Vendrell and Ward2004). Microbial phytase, which hydrolyses phytate, is an important means for environmental protection with regard to phosphorus excretion by both pigs and poultry (Kornegay, Reference Kornegay, Bedford and Partridge2001). Jondreville et al. (Reference Jondreville, Hayler and Feuerstein2005) reported that this enzyme efficiently improves zinc availability in pigs and estimated that 500 FTU is equivalent to 30 mg of zinc as sulphate. Moreover, in accordance with the response of phosphorus availability to pigs and broilers (Kornegay, Reference Kornegay, Bedford and Partridge2001), Jondreville et al. (Reference Jondreville, Hayler and Feuerstein2005) observed a greater magnitude of the response of zinc availability to dietary phytase per unit of phytase at lowest levels of supplementation. In chicks, lower improvements in zinc availability could be achieved by incorporating microbial phytase in maize–soya-bean meal diets without mineral zinc (Biehl et al., Reference Biehl, Baker and DeLuca1995; Yi et al., Reference Yi, Kornegay and Denbow1996; Mohanna and Nys, Reference Mohanna and Nys1999b). Yi et al. (Reference Yi, Kornegay and Denbow1996) reported a linear response of zinc utilisation to microbial phytase up to 600 FTU per kg diet, while the results by Biehl et al. (Reference Biehl, Baker and DeLuca1995) suggest a non-linear response up to 1200 FTU per kg diet.
European regulations recently moved to a drastic reduction of maximal zinc concentration authorised in animals diets from 250 to 150 mg/kg (European Commity, 2003). Such a reduction of safety margins requires improvements in dietary zinc utilisation by animals. Because microbial phytase is widely used in broilers’ diets, the sparing effect of this enzyme on the need for zinc supplementation is worth of being accurately established. Therefore, the present study was carried out to investigate the interest of adding graded levels of microbial phytase to a maize–soya-bean meal diet on zinc utilisation by chickens and to calculate zinc equivalency values of phytase up to 1000 FTU per kg diet. In addition, because of the negative effect of zinc on copper availability (Cousins, Reference Cousins1985), the effect of microbial phytase on the utilisation of dietary copper was assessed.
Material and methods
Experimental diets
A basal maize–soya-bean meal diet, containing 33 mg of zinc per kg, was formulated to meet all nutrient requirements of chickens from hatching to 3 weeks of age (Institut National de la Recherche Agronomique, 1989) (Table 1). In addition to the basal diet, 10 other experimental diets were obtained by supplementing the basal diet with 6, 12, 18, 24, 30 or 60 mg of zinc as sulphate per kg (feed grade, ZnSO4·7H2O, 321 mg zinc per g) or with 250, 500, 750 or 1000 FTU per kg (Natuphos®, produced by recombinant Aspergillus niger, BASF AG, Ludwigshafen, Germany, 6450 FTU per g). Zinc sulphate has been used as a reference in many studies dealing with the evaluation of zinc availability in different organic and inorganic sources of zinc for pigs and poultry (Jongbloed et al., Reference Jongbloed, Kemme, De Groote, Lippens and Meschy2002). When phytase was added to the diet, the levels of incorporation of monodicalcium phosphate and calcium carbonate were adjusted accounting to 0.16 g available phosphorus and 0.20 g total calcium per 100 FTU for levels of incorporation below 500 FTU per kg, and 0.08 g available phosphorus and 0.10 g total calcium per 100 additional FTU thereafter (Kornegay, Reference Kornegay, Bedford and Partridge2001). Zinc sulphate, microbial phytase and calcium carbonate were incorporated at the expense of monodicalcium phosphate and maize starch. Feedstuffs were ground in a hammer mill fitted with a 2.5-mm screen prior to incorporation in the diets. Diets were presented as pellets. During feed processing, temperature was not allowed to exceed 50°C in order to avoid any damage to dietary phytase.
Table 1 Composition and chemical composition of the basal diet (as-fed basis)

†The levels of incorporation of calcium carbonate in diets supplemented with 250, 500, 750 and 1000 FTU per kg were 12.98, 12.87, 12.81 and 12.75 g/kg, respectively; the levels of incorporation of monodicalcium phosphate (181 g Ca, 196 g P per kg) were 20.95, 18.40, 17.12 and 15.85 g/kg, respectively.
‡Zn-free vitamin–trace mineral mix that provided the following per kilogram of diet: vitamin A, 10 000 IU; vitamin D3, 2000 IU; vitamin E, 30 mg; vitamin K3 (menadione), 2 mg; vitamin B1 (thiamin), 1.5 mg; vitamin B2 (riboflavin), 4 mg; vitamin B3 (PP, niacin), 30 mg; vitamin B5 (Ca pantothenate), 10 mg; vitamin B6 (pyridoxine), 2.5 mg; vitamin B8 (biotin, H), 0.2 mg; vitamin B9 (folic acid), 0.4 mg; vitamin B12 (cyanocobalamin), 0.015 mg; choline, 500 mg; Fe (FeSO4), 50 mg; Cu (CuSO4), 10 mg; Mn (MnO), 85 mg; Co (CoSO4), 0.6 mg; I (Ca(IO3)2), 1 mg; Se (Na2SeO3), 0.25 mg.
§Analysed as described in the Material and methods section. In diets supplemented with 250, 500, 750 and 1000 FTU per kg, Ca concentration was 9.5, 7.9, 7.8 and 7.4 g/kg, respectively; P concentration was 7.6, 6.7, 6.5 and 6.3 g/kg, respectively; phytase activity was 320, 430, 700 and 390 FTU per kg. In diets supplemented with 6, 12, 18, 24, 30 and 60 mg zinc as sulphate zinc concentration was 39, 45, 50, 55, 63 and 94 mg/kg, respectively.
¶Calculated from Institut National de la Recherche Agronomique – Association Française de Zootechnie (Reference Sauvant, Perez and Tran2004).
Abbreviations are: CP = crude protein; DM = dry matter.
Animals, experimental procedures and analyses
The experiment was conducted under the guidelines of the French Ministry of Agriculture for Animal Research. From hatching till 2 days of age, 240 male Ross white chicks were fed a standard diet covering all nutrient requirements, including zinc. On day 2, chicks were individually weighed and the 192 chicks closest to the mean weight of 59.0 ± 3.57 g were blocked according to weight (16 blocks with 12 chicks). They were raised in individual plastic-coated cages and given the experimental diets for the subsequent 20-day period. In each block, each diet was randomly assigned to one chick, except the basal diet which was given to two birds. Twice as many chickens (n = 32) were thus assigned to the basal diet in order to provide a good baseline. The initial room temperature of 33°C was gradually decreased down to 26°C. During the first 2 days, birds were kept under 24 h light the first day and 23 h light the day after. Birds had free access to water analysed to contain less than 0.7 mg of zinc per l throughout the experiment. Individual feed consumption was recorded for the 20-day experimental period.
At the end of the experimental period, after an overnight fast, each chick was bled by means of heparinised tubes, weighed and then slaughtered by nembutal injection. Right tibiotarsi and liver were collected. Blood was centrifuged (3000 × g, 10 min, 4°C) and plasma was stored at −20°C. Liver was weighed, coarsely cut, freeze-dried, ground in a blender and stored at 4°C. Right tibiotarsi was autoclaved at 120°C for 20 min, cleaned of soft tissue and frozen at −20°C.
All the analyses were performed in duplicate. Dry matter (DM) was determined by drying to constant weight at 103°C. One ml of plasma, mixed with 0.5 ml of HCl 3 mol/l and 0.5 ml of 40% trichloracetic acid, was centrifuged at 3000 × g for 15 min. The supernatant was collected and diluted in 3 ml of deionised water. The bone was longitudinally sectioned, dried at 103°C overnight and weighed. The whole bone was ashed at 550°C for 12 h in a muffle furnace and the obtained ash was finely ground. Samples of diets and lyophilised liver were ashed at 550°C for 8 h in a muffle furnace. Bone, diet and liver ashes were solubilised with 16 mol/l HNO3 and 30% H2O2 on a digestion block until dry and diluted in 0.4 mol/l HNO3.
Analyses of minerals except phosphorus were performed by flame atomic absorption spectrophotometry (SpectrAA 220 FS, Varian, Springvale, Australia). Phosphorus was analysed by means of the Vanadate colorimetric method using a Cobas Mira apparatus (Hoffman-LaRoche, Nutley, NJ, USA).
Phytase activity in the basal diet and in the four diets supplemented with phytase was measured colorimetrically after incubation in a sodium phytate solution (Engelen et al., Reference Engelen, van der Heeft, Randsdorp and Smit1994). One phytase unit is the amount of enzyme that liberates 1 μmol of inorganic phosphorus from 5.1 mmol/l solution of sodium phytate per minute, at pH 5.5 and 37°C.
Statistical analysis
Statistical analysis of data was performed by means of the GLM procedure of the Statistical Analysis Systems Institute (SAS, 2000) as a complete-block design and using the individual chicken as experimental unit. A one-way analysis of covariance was performed on the indicators of growth performance (feed intake, weight gain, feed conversion ratio (FCR)) and of zinc status (plasma, bone and liver zinc) according to the following model:
where Yij = response measurement for block i and chick j (j = 1, 2, …, 12), Bi = block effect (i = 1, 2, … 16), Znij = zinc added as sulphate in diet given to chick j in block i (mg/kg diet); Phyt = microbial phytase added in diet given to chick j in block i (FTU per kg diet) and εij = residual error. This analysis was used to detect linear and quadratic effects of zinc and of phytase added to the diets. In this analysis of covariance, zinc and microbial phytase added were calculated from analysed and not scheduled dietary zinc concentrations and phytase activities. Differences were considered significant when P < 0.05 and trends were noted when P < 0.10.
Linear plateau models were fitted to the response of plasma and bone zinc concentrations to dietary supplemental zinc, either added as sulphate or released by phytase, by means of the non-linear (NLIN) procedure of SAS (2000). Linear plateau models were previously used to assess dietary zinc required for maximum plasma and bone zinc concentration in piglets (Jondreville et al., Reference Jondreville, Hayler and Feuerstein2005; Revy et al., Reference Revy, Jondreville, Dourmad and Nys2006) and in chicks (Wedekind et al., Reference Wedekind, Hortin and Baker1992). The models were of the following form:
If supplemental zinc <a, Y = c+b (supplemental zinc − a); if supplemental zinc ⩾ a, Y = c, with Y = response measurement, supplemental zinc = zinc added as sulphate or released by microbial phytase (mg/kg diet), a = breakpoint, b = slope of the response when supplemental zinc <a, c = maximum value of Y.
Supplemental zinc was written as the sum of zinc added as sulphate and as a function of phytase added. This function is the equation used to estimate equivalency values of phytase for zinc. Its form (linear or curvilinear) was chosen according to the results of the analysis of covariance previously performed. If the response of indicators of zinc status to phytase was linear, with no quadratic effect, then the equivalency of phytase for zinc as sulphate was considered as directly proportional to phytase. Thus, the model was as follows:
If Zn+d Phyt < a, Y = c+b (Zn+d Phyt − a); if Zn+d Phyt ⩾ a, Y = c, with Y = response measurement, Zn = zinc added as sulphate (mg/kg diet); Phyt = microbial phytase (FTU per kg diet), a = breakpoint, b = slope of the response, c = maximum value of Y, d = equivalency of one unit of phytase for zinc (mg zinc per FTU).
Linear plateau models were adjusted using treatment means. Zinc and microbial phytase were calculated from analysed dietary zinc concentrations and phytase activities. The coefficient of determination (R 2) of each generated equation was calculated as the square of the correlation coefficient between predicted and observed individual values. The root mean square error (r.m.s.e.) is the root square of the sum of squares of differences between predicted and observed individual values divided by the number of observations.
Meta-analyses of literature data were performed in order to (1) evaluate dietary zinc required for maximum plasma and bone zinc concentrations in chicks and (2) estimate equivalencies of microbial phytase for zinc as sulphate. In the reported experiments, plasma and bone zinc concentrations were recorded in chicks given plant feedstuffs-based diets added with graded levels of zinc as sulphate or microbial phytase. For the first meta-analysis, only dietary treatments without phytase were kept, whereas for the second, experiments in which diets without and with phytase were compared were considered. Experiments started and ended 0 to 5 and 16 to 35 days after hatching, respectively. Linear plateau models similar to those previously described were adjusted using treatment means. For the second meta-analysis, the equivalency of phytase for zinc as sulphate was considered as directly proportional to phytase activity, as previously described. To account for the variability between experiments, the parameter c (maximum value of the response parameter) was adjusted within experiments. The breakpoint a, the slope b and the equivalency of one unit of phytase for zinc d were adjusted between experiments. Since the number of replicates per treatment was very similar between experiments, this parameter was not introduced in the model. R 2 and r.m.s.e were calculated as described above.
Results
Dietary zinc analyses show that 6, 12, 17, 22, 30 and 61 mg zinc were added per kg diet, in accordance with the scheduled 6, 12, 18, 24, 30 and 60 mg zinc per kg, respectively (Table 2). As expected, the basal diet was devoid of significant phytase activity (40 FTU per kg). However, the range of microbial phytase addition to the basal diet was narrower than expected with 280, 390, 660 and 850 FTU per kg instead of 250, 500, 750 and 1000 FTU per kg.
Table 2 Growth performance and plasma, bone and liver characteristics of chickens given the basal diet supplemented with zinc as sulphate or microbial phytase

†r.m.s.e. = root mean square error; linear (L) and quadratic (Q) effects of zinc added as sulphate and of microbial phytase; *** P < 0.001; ** P < 0.01; * P < 0.05; NS P > 0.10.
‡Calculated from analysed zinc concentration and phytase activity in diets presented in Table 1.
§Estimates of the parameters were as follows: final weight (g) = 941+2.36 Zn – 0.0321 Zn2, R 2 = 0,10; weight gain (g) = 880+2.37 Zn–0.0322 Zn2, R 2 = 0,10; plasma Zn (mg/l) = 1.27+0.0398 Zn – 0.000406 Zn2+0.000481 Phyt, R 2 = 0.62; bone Zn (mg/kg ash) = 221+8.34 Zn – 0.0855 Zn2+0.121 Phyt, R 2 = 0.80; bone Zn (mg/kg DM) = 107+4.19 Zn – 0.0438 Zn2+0.0539 Phyt, R 2 = 0.79; Liver Zn (mg/kg DM): 89.3+0.418 Zn – 0.00379 Zn2, R 2 = 0.37; Liver Cu (mg/kg DM) = 21.1 – 0.337 Zn+0.00381 Zn2, R 2 = 0,15, with Zn = zinc added as sulphate (mg/kg diet); Phyt = microbial phytase (FTU per kg diet).
Abbreviation is: DM = dry matter.
Six birds died before the end of the experiment or displayed abnormal legs at slaughter and were removed from the data set. At the end, at least 14 replicates were available for each experimental treatment.
Growth performance and plasma, bone and liver characteristics
Results are presented in Table 2. Feed intake, weight gain and FCR were independent of the presence of phytase in the diet (P > 0.1). Compared with chickens fed the unsupplemented basal diet, chickens fed the diets with 12 and 30 p.p.m. of zinc added as sulphate displayed a higher weight gain. These differences led to a linear (P < 0.05) and quadratic (P < 0.05) response of weight gain to zinc added. Feed intake and FCR were independent of dietary zinc (P > 0.1).
Plasma zinc concentration increased linearly and quadratically (P < 0.001) with zinc added and tended to increase linearly (P = 0.07) with phytase. Bone weight and bone ash were independent of the dietary levels of zinc and of microbial phytase (P > 0.1). Bone zinc expressed as mg/kg ash or as mg/kg DM increased linearly and quadratically (P < 0.001) with zinc and increased linearly (P < 0.001; P < 0.01, respectively) with phytase. Birds given the diet supplemented with the highest level of phytase displayed lower plasma and bone zinc concentrations than birds on the diet supplemented with 60 mg zinc per kg (−21% for plasma zinc and −26% for bone zinc). Liver weight and DM concentration were independent of dietary zinc and phytase (P > 0.1). Liver zinc concentration increased linearly (P < 0.001) and quadratically (P < 0.05) with zinc added. On the contrary, liver copper concentration decreased linearly (P < 0.001) and quadratically (P < 0.05) with dietary zinc. Liver content of these two elements was independent of microbial phytase (P > 0.1).
Models of the response of indicators of zinc status to graded levels of zinc and phytase and equivalency values of zinc added as sulphate for microbial phytase
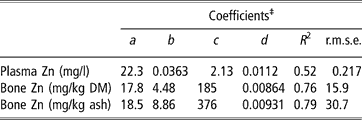
Parameters of linear plateau models generated for the response of plasma and bone zinc to supplemental zinc either added as sulphate or released by microbial phytase are presented in Table 3. Because the previous covariance analysis revealed that plasma and bone zinc increased linearly and not quadratically when phytase was added, the equivalency of phytase for zinc as sulphate was considered as directly proportional to phytase. Coefficients of determination were 0.52 for plasma zinc concentration and 0.76 to 0.79 for bone zinc concentration. These criteria linearly increased until supplemental zinc reached 22.3, 17.8 and 18.5 mg/kg diet, respectively, and reached a plateau at 2.13 mg zinc per l plasma, 185 mg zinc per kg bone DM and 376 mg zinc per kg bone ash.
Table 3 Adjustment of plasma, bone and liver zinc concentrations to zinc added as sulphate and to microbial phytase†
†Models were generated using treatment means and assayed dietary zinc concentrations and phytase activities.
‡If Zn+d Phyt < a, Y = c+b (Zn+d Phyt −a); if Zn+d Phyt ⩾ a, Y = c, with Y = response measurement, Zn = zinc added as sulphate (mg/kg diet); Phyt = microbial phytase (FTU per kg diet), a = breakpoint, b = slope of the response, c = maximum value of Y, d = equivalency of one unit of phytase for zinc (mg zinc per FTU).
R 2, coefficient of determination calculated as the square of the correlation coefficient between predicted and observed individual values; r.m.s.e., root mean square error calculated as the root square of the sum of squares of differences between predicted and observed individual values divided by the number of observations.
Abbreviation is: DM = dry matter.
Equivalency values of zinc as sulphate for microbial phytase are presented in Table 4. The response of the indicators of zinc status to phytase added being linear, these equivalencies also increased linearly between 280 and 850 FTU, by 1.1 and 0.9 mg zinc per 100 FTU for plasma and bone zinc, respectively.
Table 4 Equivalency values of zinc added as sulphate (mg) for microbial phytase (FTU) generated from the response of plasma and bone zinc concentrations to zinc added as sulphate and to microbial phytase†
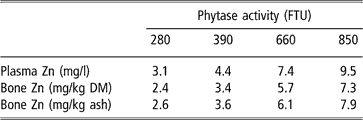
†Zn = d Phyt, with Zn = zinc added as sulphate (mg), Phyt = microbial phytase (FTU), d = 0.0112, 0.00864 and 0.00931 mg zinc per FTU for plasma zinc, bone zinc relative to bone dry matter and bone zinc relative to bone ash, respectively.
Abbreviation is: DM = dry matter.
Discussion
In the current study, growth performance was not affected by microbial phytase. Chicks given the basal diet supplemented with 12 mg zinc per kg displayed higher weight gain than those given the unsupplemented basal diet, but additional supply of zinc had no further effect in contrast to what was observed for blood or bone zinc concentrations. National Research Council (1994) states that the provision of diets containing 40 mg of zinc per kg is optimal for chick growth. However, in previous studies, no improvement in growth performance of chicks up to 21 days of age was observed by adding zinc (Mohanna and Nys, Reference Mohanna and Nys1999b; Burrell et al., Reference Burrell, Dozier, Davis, Compton, Freeman, Vendrell and Ward2004; Jondreville et al., 2007) or microbial phytase (Mohanna and Nys, Reference Mohanna and Nys1999b; Jondreville et al., 2007) to maize–soya-bean meal diets not supplemented with zinc, containing at least 28 mg of zinc per kg. In contrast, improvements in weight gain (Yi et al., Reference Yi, Kornegay and Denbow1996; Mohanna and Nys, Reference Mohanna and Nys1999a) and in FCR (Mohanna and Nys, Reference Mohanna and Nys1999a) were achieved when the unsupplemented maize–soya-bean meal basal diet contained around 20 mg of zinc per kg. According to Burrell et al. (Reference Burrell, Dozier, Davis, Compton, Freeman, Vendrell and Ward2004), improvements in growth rate could not be observed by adding zinc to a diet containing around 30 mg zinc per kg because zinc provision by the basal diet was too close to the recommended allowance.
The indicators of zinc status were plasma zinc concentration, which is an indicator of functional zinc and bone and liver zinc concentrations, which are indicators of body stores of zinc. Plasma and bone zinc were previously used for assessing zinc requirements in chicks by adjusting broken-line models to their response to dietary zinc (Wedekind et al., Reference Wedekind, Hortin and Baker1992; Mohanna and Nys, Reference Mohanna and Nys1999a). Liver zinc was shown to increase with dietary zinc in chicks (Yi et al., Reference Yi, Kornegay and Denbow1996) and in piglets (Jondreville et al., Reference Jondreville, Hayler and Feuerstein2005).
In diets without phytase, plasma and bone zinc concentrations linearly increased until dietary zinc reached 55 and 51 mg/kg diet, respectively, and plateaued thereafter. The slope of the response was 0.0363 mg plasma zinc per l and 4.48 mg zinc per kg bone DM for one additional mg of dietary zinc. In accordance with Mohanna and Nys (Reference Mohanna and Nys1999a) but at variance with Wedekind et al. (Reference Wedekind, Hortin and Baker1992), we did not observe any further increase in bone zinc beyond the breakpoint. Based on literature data, we adjusted linear plateau models to plasma and bone zinc concentrations in chicks given diets without added phytase supplemented with variable amounts of zinc as sulphate (Figure 1). Results of the current experiment are in accordance with the estimates for the slopes (0.0435 mg zinc per l plasma and 3.17 mg zinc per kg bone DM for one additional mg of dietary zinc) and for zinc required for maximum plasma and bone zinc concentrations (50 and 59 mg zinc per kg diet, respectively) derived from this analysis of the literature.

Figure 1 Response of plasma and bone zinc concentrations in chicks on diets without microbial phytase supplemented with variable amounts of zinc as sulphate – metaanalysis of literature data†. The model is if Zn < a, Y = c+b (Zn – a); if Zn ⩾ a, Y = c, with Zn = dietary zinc (mg/kg diet), Y = response measurement, a = breakpoint, b = slope of the response, c = maximum value of Y.

Between 0 and 24 mg zinc per kg diet, liver zinc concentration increased by 0.52 mg/mg dietary zinc, which is similar to the linear increase of 0.51 mg liver zinc per mg dietary zinc reported by Yi et al. (Reference Yi, Kornegay and Denbow1996).
The absence of any effect of experimental diets on bone ash concentration despite the decreased dietary P and Ca concentrations concomitant to microbial phytase addition suggests that this enzyme was effective in hydrolysing phytates and improving P and Ca availability. Improvements in zinc availability by microbial phytase added to low zinc diets fed to chicks were previously reported (e.g. Thiel et al., 1993; Mohanna and Nys, Reference Mohanna and Nys1999b; Yi et al., Reference Yi, Kornegay and Denbow1996). In some instances, no effect of microbial phytase on zinc availability could be detected because of the high zinc concentration in the experimental diets (Thiel et al., 1993; Sebastian et al., Reference Sebastian, Touchburn, Chavez and Lague1996; Mohanna and Nys, Reference Mohanna and Nys1999b). In chicks, Yi et al. (Reference Yi, Kornegay and Denbow1996) observed a linear response of bone and liver zinc to graded levels of phytase up to 600 FTU per kg introduced in a maize–soya isolate diet. The equivalencies of 3.8 and 5.5 mg zinc as sulphate for 600 and 1200 FTU estimated by Biehl et al. (Reference Biehl, Baker and DeLuca1995) suggest a decreasing efficacy of microbial phytase per unit when the level of incorporation of phytase increases. This is in accordance with the response of P availability to phytase supplementation (Kornegay, Reference Kornegay, Bedford and Partridge2001). Unfortunately, in the current experiment, the maximum level of phytase supplementation reached only 850 FTU per kg instead of 1000 FTU per kg expected. Within this range of phytase supplementation, the response of plasma and bone zinc remained linear. One mg of zinc as sulphate could be replaced per 100 FTU over the range of 280 to 850 FTU. This equivalency is in agreement with the estimate of 0.9 mg zinc as sulphate for 100 FTU up to 600 FTU per kg diet by Yi et al. (Reference Yi, Kornegay and Denbow1996). Based on literature data, we adjusted linear plateau models to the response of plasma and bone zinc to dietary zinc in diets containing different levels of microbial phytase (Figure 2). From this data set, no curvilinear response to phytase added could be detected; therefore, the release of zinc was considered as proportional to microbial phytase up to 1200 FTU. This proportionality may explain the slightly lower equivalency of 0.7 mg zinc as sulphate for 100 FTU derived from this literature review.

Figure 2 Response of bone zinc in chickens to graded levels of zinc as sulphate and of microbial phytase – metanalysis of literature data†. The model is: if Zn + d Phyt < a, Y = c+b (Zn + d Phyt – a); if Zn + d Phyt ⩾ a, Y = c, with Zn = dietary zinc (mg/kg diet), Phyt = dietary phytase (FTU per kg diet), Y = response measurement, a = breakpoint, b = slope of the response, c = maximum value of Y, d = equivalency of one unit of phytase for zinc (mg zinc per FTU).

These equivalencies in chicks are far below the estimates of 30 mg zinc as sulphate for 500 FTU in piglets (Jondreville et al., Reference Jondreville, Hayler and Feuerstein2005; Revy et al., Reference Revy, Jondreville, Dourmad and Nys2006). Moreover, over a range of 150 to 850 FTU introduced per kg of a maize–soya-bean meal diet, Jondreville et al. (Reference Jondreville, Hayler and Feuerstein2005) calculated that the release of zinc from phytates by phytase was not linear but proportional to the one of phosphorus. The results of a recent study conducted in our laboratory (Jondreville et al., 2007) suggest that the low pH in gizzard allows zinc–phytates complex to dissociate, even in the absence of phytase, whereas, in stomach of piglets, where the pH is higher, phytates must be hydrolysed by phytase before zinc can be released as soluble zinc. This phenomenon would result in a physiologically higher availability of zinc in chickens than in piglets, explaining the lower dietary requirements of birds than piglets for this element (50 to 60 mg zinc per kg of a maize–soya-bean meal based diet for a maximum plasma and bone zinc concentrations in chicks according to the current study v. 85 to 90 mg zinc per kg diet in piglets according to Jondreville et al. (Reference Jondreville, Hayler and Feuerstein2005) and Revy et al. (Reference Revy, Jondreville, Dourmad and Nys2006)). It would also explain the differential efficacy of phytase for improved zinc availability in chickens and in piglets.
It was calculated that zinc excretion by piglets could be reduced by 30% by replacing 30 mg of zinc as sulphate by 500 FTU microbial phytase in a maize–soya-bean meal diet formulated to contain 100 mg zinc per kg (Jondreville et al., Reference Jondreville, Hayler and Feuerstein2005). According to the current results, a chicken diet without microbial phytase containing 60 mg zinc per kg would result in similar performance and zinc retention than a diet containing 500 FTU microbial phytase and 55 mg zinc per kg. Considering a FCR of 1.25, a body-weight gain of 1000 g per bird and a zinc retention of 20 mg/kg body weight (Mohanna and Nys, Reference Mohanna and Nys1999a), this reduction of zinc ingested from 75 to 69 mg/bird, would result in a similar zinc retention of 20 mg per bird and consequently to a reduction of zinc excreted by 11% (55 v. 49 mg per bird).
Liver copper concentration decreased by 30% when 12 mg of zinc were added to the basal diet and remained steady thereafter. Although not significant because of a high variability within treatments, a negative effect of microbial phytase on liver copper concentration was also recorded. Decreased liver copper accompanying increased dietary zinc was previously reported in piglets (Zacharias et al., Reference Zacharias, Ott and Drochner2003; Revy et al., Reference Revy, Jondreville, Dourmad and Nys2004) and was interpreted as a result of the negative effect of zinc on copper availability (Cousins, Reference Cousins1985). In piglets, Zacharias et al. (Reference Zacharias, Ott and Drochner2003) reported an indirect impairment of copper status by phytase and suggested it was due to the release of zinc by phytase. However, the studies by Jondreville et al. (Reference Jondreville, Hayler and Feuerstein2005) and Revy et al. (Reference Revy, Jondreville, Dourmad and Nys2006) did not corroborate this hypothesis. On the contrary, a positive effect of phytase on copper balance was reported in piglets (Adeola et al., Reference Adeola, Lawrence, Sutton and Cline1995; Revy et al., Reference Revy, Jondreville, Dourmad and Nys2004) and in chickens (Sebastian et al., Reference Sebastian, Touchburn, Chavez and Lague1996) and was interpreted as an overall positive effect of this enzyme on mineral balance. Ultimately, the possible effect of phytase on copper availability remains debatable.
The current study confirms that, in diets without microbial phytase, 55 to 60 mg of zinc per kg diet are required to maximise bone and plasma zinc concentrations in chicks. Up to 850 FTU, the supplementation of 1 mg of zinc as sulphate can be spared per 100 FTU. In a chicken diet formulated to contain 60 mg zinc per kg, the replacement of 5 mg zinc per kg by 500 FTU as Natuphos® would allow a reduction of zinc excreted by chickens by around 10%.
Acknowledgements
The authors are grateful to Sandrine Hillion and to Anne-Marie Chagneau for their technical assistance. They also acknowledge Maryse Mills, Kléber Gérard, Jean-Marc Meslier, Frédéric Mercerand, Michel Derouet and Thierry Bordeau of the UR Recherches Avicoles of INRA at Nouzilly and Georges Guillemois, Raymond Vilboux and Patrick Touanel of the UMR Systèmes d’Elevage Nutrition et Humaine of INRA at Saint-Gilles.







