Introduction
Bloodstream infection (BSI) is associated with high morbidity and mortality rates. According to recent data from the China Antimicrobial Surveillance Network (CHINET), Klebsiella pneumoniae accounted for 15.4% of BSIs, and a mortality rate of 54.3% [Reference Xu, Sun and Ma1, 2]. The virulence of K. pneumoniae in the bloodstream is enhanced by its pks gene cluster, which is a hybrid non-ribosomal peptide synthetase-polyketide synthase assembly line that represents 19 genes (clbA to clbS) [Reference Fais3]. This gene cluster is responsible for colibactin synthesis and was first discovered in the extraintestinal pathogenic Escherichia coli strain IHE3034 [Reference Nougayrede4]. The cluster is associated with DNA double-strand breaking and chromosome aberrations, which leads to senescence of epithelial cells and apoptosis of immune cells [Reference Nougayrede4]. The presence of the pks gene cluster in E. coli has also been linked to bacteraemia, meningitis, and in patients with colorectal cancer [Reference McCarthy5, Reference Shimpoh6].
The pks genes, clbB and clbN, are significantly associated with BSIs and were found to be present in 58% of E. coli group B2 related bacteraemia isolates [Reference Johnson7]. Moreover, bacterial loads of K. pneumoniae in the bloodstream in an experimental meningitis model in mice were shown to decline in those infected with pks gene (clbA) knockout isolates [Reference Lu8]. The pks gene cluster is globally diverse, but was found to be relatively infrequent (3.5%) among K. pneumoniae clinical isolates in Europe [Reference Putze9]. However, the gene cluster was recently detected in 17% of bloodstream-sourced K. pneumoniae isolates from the Taiwan region and also related to serotype K1, the most common serotype of hypervirulent K. pneumoniae (hvKP) in Asia [Reference Chen10, Reference Siu11].
Data on the prevalence of the pks gene cluster in mainland China are limited. The current study therefore aimed to investigate its prevalence in bloodstream-sourced K. pneumoniae clinical isolates from a national multicentre longitudinal program in China. Microbiological, molecular and clonality characterisation of the pks gene cluster in a large collection of isolates was undertaken.
Materials and methods
Bacterial isolates collection
Five hundred and seventy-one K. pneumoniae isolates were selected from our previous 15 monthsstudy of extended-spectrum beta-lactamase (ESBL) production of E. coli and K. pneumoniae BSIs in mainland China [Reference Quan12]. Samples were included from 28 tertiary hospitals in 22 provinces and municipalities, which covered about one billion people. Community-onset BSIs were defined as infections which occurred in non-hospitalised patients, or less than 48 h after admission to hospital [Reference Rodriguez-Bano13]; 279 isolates were defined as community-onset K. pneumoniae (COK), and 292 as hospital-acquired K. pneumoniae (HAK). Isolates were identified by matrix-assisted laser desorption ionisation-time of flight mass spectrometry (Bruker Daltonics, Bremen, Germany). The study was approved by our hospital ethics committee (20130910-13) with a waiver of informed consent.
Detection of the pks gene cluster in K. pneumoniae isolates
The presence of pks genes (clbA, clbB, clbN and clbQ) among all isolates was screened for by the conventional polymerase chain reaction (Takara, Dalian, China) [Reference Nougayrede4] using specific primers and parameters as listed in Table S1.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing of 12 antimicrobial agents against all isolates was performed using an agar microdilution method and results were interpreted according to the Clinical & Laboratory Standards Institute [14]. Susceptibility to tigecycline, however, was determined using broth microdilution with cation-adjusted Mueller-Hinton broth, and interpreted according to the European Committee on Antimicrobial Susceptibility Testing breakpoint [15].
Whole-genome DNA sequencing and analysis
Total DNA was extracted from 117 pks-positive K. pneumoniae isolates (QIAGEN, Hilden, Germany) and subjected to whole genome sequencing with 2 × 150 bp paired-end reads (Illumina HiSeq X Ten, San Diego, California, USA). The derived short reads were assembled into contigs in a CLC Genomics Workbench 9.5.1 (QIAGEN, Germany) by automatic word size and bubble size, with a minimum contig length of 200 base pair. The genome sequence was submitted to the European Nucleotide Archive (accession number PRJEB32094). Multilocus sequence types (MLST) were identified by mapping the assembled contigs against the K. pneumoniae MLST database on the Center for Genomic Epidemiology (CGE) server [Reference Larsen16]. The assembled contigs were also used to screen for acquired antimicrobial resistance genes by ResFinder 2.1 on the CGE server [Reference Zankari17], with 90% identity and 60% minimum length. Virulence genes and wzi alleles were identified with reference to the Pasteur Institute website (https://bigsdb.pasteur.fr/klebsiella/klebsiella.html).
Clonality analysis by core-genome multilocus sequence typing (cgMLST)
FASTA files of each isolate were imported into SeqSphere + 4.1.9 (Ridom GmbH, Münster, Germany) for stable cgMLST analysis to identify cluster types (CT) with default parameters. K. pneumoniae NTUH-K2044 (GenBank accession no. NC_012731.1) was used as a reference with a standard set of 2358 genes for gene-by-gene comparison, and the cgMLST comparison table was established. SeqSphere + 4.1.9 was also utilised to obtain neighbour-joining (N-J) and minimum span trees. The genome sequence used for the construction of phylogenetic trees was searched on the National Center for Biotechnology Information (NCBI) database; the isolate identifier and location origin are listed in Table S2.
Statistical analysis
For categorical variables and continuous variables, comparisons were performed by the Fisher's exact test and t-test. A P-value < 0.05 was considered statistically significant. The statistical software used was Prism5 (Graph Pad Software, California, USA).
Results
In total, 117 pks gene cluster positive representatives were identified among 571 K. pneumoniae isolates; 79 were classified as COK (28.3%) and 38 (13.0%) as HAK isolates (P < 0.0001).
Antimicrobial susceptibility of isolates
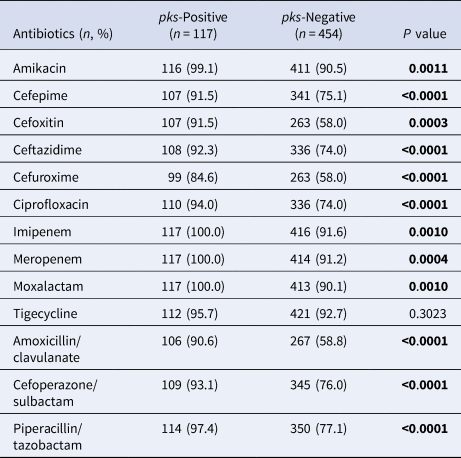
As compared with pks-negative K. pneumoniae, pks-positive isolates were significantly more susceptible to 12 antimicrobial agents (Table 1), including, β-lactams, β-lactam/β-lactamase inhibitors, fluoroquinolones, cephamycin, aminoglycosides and oxacephem, except for tigecycline. For instance, the susceptibility rate of cefepime, ceftazidime, ciprofloxacin and amoxicillin/clavulanate was 91.5%, 92.3%, 94.0% and 90.6% in pks-positive isolates, respectively, compared with pks-negative isolates, where the respective rate for these antibiotics was 75.1%, 74.0%, 74.0% and 58.8%. All pks-positive isolates were susceptible to carbapenem agents while over 90% of their pks-counterparts showed susceptibility. Multidrug resistant (MDR) was defined as acquired resistance to at least one agent in three or more antimicrobial classes, and 5.1% (6/117) of pks-positive isolates and 28.6% (130/454) of pks-negative isolates were MDR.
Table 1. Susceptibility of pks-positive and pks-negative K. pneumoniae isolates to antimicrobials
Clinical characteristics of community-onset infection patients
Clinical outcome data were available for 249 of the 279 patients classified as community-onset BSI. Table 2 shows that patients infected with pks-positive K. pneumoniae were significantly younger than those with pks-negative isolates (P < 0.05). Likewise, a much higher proportion of pks-positive patients presented with a liver abscess (P < 0.05), but were less likely to have biliary obstruction (P < 0.05). There was no significant difference in clinical prognosis and outcomes between patients harbouring pks-positive or pks-negative isolates (P > 0.05).
Table 2. Demographic and clinical data of patients with community-onset BSI according to isolation of pks-positive and pks-negative K. pneumoniae

Molecular characteristics of pks-positive K. pneumoniae
ST23 (78/117) and ST65 (20/177) were the dominant sequence types identified in pks-positive isolates and were similarly distributed in COK and HAK patients (P > 0.05); eight other STs (ST380 (6), ST268 (3), ST133 (3), ST1660 (2), ST2058 (1), ST2846 (1), ST1265 (1) and ST3 (1)) accounted for 15.4% of isolates, and one isolate had a novel ST (Table 3). Genome sequencing showed that K1 (81/117) and K2 (25/117) were the predominant serotypes among pks-positive isolates. Six isolates fell into other K serotypes (K3 (1), K34 (2) and K20 (3)), and five were serologically un-typeable. Virulence genes were ubiquitous in pks-positive isolates, particularly the siderophore encoding genes iroN (salmochelin), iucA (aerobactin) and ybtA (yersiniabactin) which were found in frequencies of 88.0%, 90.6% and 100% respectively. Moreover, 72.6% of all isolates were rmpA positive, the positive regulator of the mucoid phenotype [Reference Zhang18]. There was no significant difference in the distribution rate of K serotype and virulence genes between pks-positive COK and HAK (Table 3). As for drug resistance determinants, no isolate proved positive for carbapenemase genes, and only seven isolates representative of the CTX-M-1 group and six of the CTX-M-9 group were identified (Table 3). Likewise, we selected 23 pks-negative K. pneumoniae from this project, which fell into 19 different STs and 20 different K serotypes (wzi alleles), that indicated the diversity of pks-negative isolates from BSI. The screen of virulence genes showed the frequencies of rmpA, iroN, iucA and ybtA to be 21.7%, 39.1%, 34.8% and 39.1%, respectively. There were 56.5% (13/23) of isolates producing ESBLs (CTX-M-1 group (4), CTX-M-9 group (6) and SHV group (3)) and three isolates were bla KPC positive.
Table 3. MLST, K serotype, virulence genes and drug resistance genes, of pks-positive isolates of K. pneumoniae.

∆ ST of COK15 phoE: 28 (A53G)
Clonality of pks-positive isolates
To investigate clonal linkage of the pks-positive isolates, an N-J tree was constructed based on the cgMLST allelic profiles with default parameters. Except for two unidentified isolates, 115 isolates and 27 genome sequences from the NCBI database were divided into nine clades (allelic differences >1000). The two major clades (Clade 1 and Clade 2) mainly consisted of ST23 and ST65 clone groups, respectively (Fig. 1). The results suggested that either the source, or the geographical origin of pks-positive isolates was mixed in the phylogenetic tree, and indicated that community and hospital isolates were phylogenetically related, and no specific epidemic clone existed in mainland China. Moreover, by comparison with genomes from the worldwide database, ST23 isolates from Russia, Japan, USA, Austria, Singapore and Italy were all related to pks-positive ST23 isolates in Clade 1. Similar results were found for ST65 isolates, with those from Singapore, Malaysia, Japan, Ireland and Spain being closely similar to representatives of this ST from China, and demonstrate the sporadic presence of pks-positive K. pneumoniae worldwide.

Fig. 1. The N-J tree of pks-positive isolates (isolates in the current study and the genome from the NCBI database) was constructed. Sequence types, source and geographic location were shown.
To further explore the differences of the isolates from this study, the allelic distance of Clade 1 and Clade 2 was calculated. In Clade 1, after excluding HAK226 with an average distance of 318.4.4 alleles, there were 80 isolates with an average distance of 65.8 alleles (Fig. S1A), and 95% of them were ST23; the remainder included ST1265 (1), ST65 (1), ST1660 (2) and one unknown ST. Two paired isolates that showed no allelic difference were COK301/HAK290 (CT1895, Jiaxing) and COK247/COK248 (CT1876, Hohhot). COK255/COK256 (CT1869, Guiyang) and HAK253/HAK254 (CT1907, Hohhot) differed by one allele while COK107/HAK120 (CT1847, Guangzhou) had five allelic differences. The average distance was 47.0 alleles in Clade 2, and all of 19 isolates were ST65 with different CT, except that HAK280/HAK281 (CT1915, Fuzhou) had no allelic difference from isolates in the same hospital (Fig. S1B). Six paired pks-positive isolates from the same city showed less than 10 allelic differences.
Discussion
BSI caused by K. pneumoniae is associated with high morbidity and mortality, where the pks gene cluster plays a major role in the occurrence and prevalence of BSI [Reference Lu8]. Previous studies from the region have reported the prevalence of the pks gene cluster in K. pneumoniae to be 17% in Taiwan [Reference Chen10] and 26.8% in Changsha, China [Reference Lan19]. The current study extends these data to encompass a large number of BSI K. pneumoniae isolates from 28 hospitals in 22 provinces and municipalities in mainland China and reports an overall prevalence of 20.5%.
Bloodstream-sourced pks-positive isolates share many microbiological and clinical characteristics associated with the hvKP variant which first emerged in Taiwan in the mid 1980s [Reference Siu11]. Infection with such strains was most often characterised by community-onset, and relatively infrequent resistance to antibiotics [Reference Paczosa and Mecsas20]. In our study, 67.5% of pks-positive isolates were community-onset, which was twice that of the hospital-acquired infections, and were also more susceptible to antimicrobials, as reported by Chen et al. [Reference Chen10]. Moreover, previous studies have found that hvKP strains isolated from four different continents were almost exclusively of serotype K1 (93%), or K2 [Reference Struve21], and ST23 and ST65 were predominant in such strains [Reference Shi22]. The great majority (90.6%) of our pks-positive isolates were K1 or K2 serotype, and ST23 and ST65 accounted for 82% of the study sample. The pks gene cluster was shown to co-exist with the ybt locus and T4SS-mobBC on integrative conjugational elements Kp10 (ICEKp10) in the vast majority of the ST23 clonal group [Reference Lam23], which explains the presence of ybtA in all our pks-positive isolates.
There is no universal clinical definition of hvKP infections but it is generally agreed that they are most commonly isolated from patients with community-associated liver abscesses, metastatic meningitis and endophthalmitis, and that such cases usually have normal biliary and hepatic function [Reference Russo and Marr24]. In this study, patients infected with pks-positive K. pneumoniae had a higher proportion of liver abscesses and a lower rate of biliary disorders, which is consistent with the view that pks-positive isolates may play an important role in hvKP infections, as suggested by Lan et al. [Reference Shi22].
A recent study from South Korea reported that the pks gene cluster is a risk factor for 30-day mortality of K. pneumoniae BSI patients when accompanied MDR, but the relative MDR rates for pks-negative isolates is unclear [Reference Kim25]. In our study, 5.1% of pks-positive isolates were multidrug-resistant compared with 28.6% of pks-negatives. Although the MDR rate appears to be currently low in pks-positive isolates, active surveillance of these properties is warranted.
The threshold for the definition of strain relatedness within an outbreak cluster was set as a difference of <10 alleles, which would be considered a close relationship between strains [Reference Kleta26]. Genome sequencing showed that the pks-positive isolates were mostly sporadic in the phylogenetic tree, with a 65.8 and 47.0 average allele difference between Clade 1 and Clade 2, respectively; these values indicate that there does not appear to be a widespread epidemic lineage of pks-positive K. pneumoniae nationwide in China. Indeed, only isolates from the same hospital showed a difference of <10 alleles, and these strains were found in both COK and HAK patients. However, two exceptions were noted for COK301/HAK290 and COK107/HAK120, where close clonality was evident among the community-onset and hospital-acquired isolates, which is suggestive of transmission of such lineages between these presentations. Apparent clonality of E. coli bacteraemia strains from community-acquired and healthcare-associated cases has been previously observed [Reference Kang27] and it is therefore possible that our general classification of some infections as COK or HAK, might have masked subtle differences in acquisition of the organism and mistakenly interpreted as close clonality of strains.
In conclusion, our study of 571 K. pneumoniae BSI isolates from patients in 28 tertiary hospitals in 22 provinces in mainland China, classified according to the presence or absence of the pks gene cluster, showed that the genetic lineages ST23 and ST65 predominated among pks-positive isolates. There was no evidence of a widespread epidemic clone and infections were mostly sporadic. Although pks-positive isolates were generally susceptible to antimicrobials, their higher prevalence in the community and the genotoxicity, represents a potential risk for treatment of BSI due to K. pneumoniae.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268820000655
Author contributions
Qiucheng Shi participated in the study design, collected the specimens, carried out laboratory work, analysed the data and drafted the manuscript. Jingjing Quan participated in the study design, carried out laboratory work and analysed the data. Peng Lan analysed the data. Danyan Huang carried out laboratory work. Jiancang Zhou and Yan Jiang conceived the study, and edited the manuscript. Yunsong Yu conceived the study, participated in its design and coordination, edited the manuscript, and received the majority of funding needed to complete the research.
Financial support
This work was supported by the National Natural Science Foundation of China (81672067), the Natural Science Foundation of Zhejiang province, China (LY17H190004) and the Key Research and Development Programme of Zhejiang (2015C03046). These agencies had no role in the design of the study, data collection, analysis, interpretation of data or writing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.






