INTRODUCTION
Brown bear Ursus arctos Linnaeus, 1758 is a Holarctic species distributed across boreal, montane, arctic, forest, and open environments in almost all over the Northern Hemisphere. Over this wide area, this carnivoran exhibits a huge variability in ecology, genetics, behavior, and morphology (e.g., Erdbrink Reference Erdbrink1953; Baryshnikov Reference Baryshnikov2007; Haroldson et al. Reference Haroldson, Clapham, Costello, Gunther, Kendall, Miller, Pigeon, Proctor, Rode, Servheen, Penteriani and Melletti2020 and references therein; Swenson et al. Reference Swenson, Ambarlı, Arnemo, Baskin, Ciucci, Danilov, Delibes, Elfström, Evans, Claudio Groff, Penteriani and Melletti2020). This species is well adapted to cold environments with dense pelage, large fat stores, adaptability to various food sources, and the ability to reduce energy expenditure during winter by decreasing metabolism because of hibernation. Survival skills of the brown bear are some of the highest among all the carnivores, and this gives it great adaptability, an enormous ecological tolerance, and the capability to occupy different habitats; thus, today, this species is widespread in the Holarctic. Due to such a wide distribution, the brown bear is highly polymorphic; therefore, it has been split into a number of subspecies based on phenotypic differences (see Erdbrink Reference Erdbrink1953 and Baryshnikov Reference Baryshnikov2007 for a review). The same results were obtained during the study of fossil materials. Because of the impossibility of collecting genetic information on older bones, morphological analyses are often the only approach that can be used to study the evolutionary lineage of the brown bear. However, this normally entails difficulties in defining and estimating intra- and interspecific variability, both chronologically and geographically. Recent studies have shown the continual presence of U. arctos in Europe, even during the coldest periods of the Late Pleistocene (Sommer and Benecke Reference Sommer and Benecke2005; Valdiosera et al. Reference Valdiosera, Garcia-Garitagoitia, Garcia, Doadrio, Thomas, Hänni, Arsuaga, Barnes, Hofreiter, Orlando and Götherström2008; Davison et al. Reference Davison, Ho, Bray, Korsten, Tammeleht, Hindrikson, Østbye, Østbye, Lauritzen and Austin2011; Edwards et al. Reference Edwards, Ho, Barnett, Coxon, Bradley, Lord and O’Connor2014; Ersmark Reference Ersmark2016; Ersmark et al. Reference Ersmark, Baryshnikov, Higham, Argant, Castaños, Döppes, Gasparik, Germonpré, Lidén and Lipecki2019; Marciszak et al. Reference Marciszak, Schouwenburg, Lipecki, Talamo, Shpansky, Malikov and Gornig2019). There were no sufficient natural barriers for brown bear dispersion, and its continuous gene flow across most of the European territory was observed during that time (Ersmark et al. Reference Ersmark, Baryshnikov, Higham, Argant, Castaños, Döppes, Gasparik, Germonpré, Lidén and Lipecki2019).
Despite that, in the last two decades, knowledge about the Pleistocene history of U. arctos in Europe has significantly increased, and there are still many countries where this topic is still relatively weakly known. Traditionally, the area of the Czech Republic is considered abundant in the ursid remains of the Late Pleistocene (Musil Reference Musil1980; Wagner Reference Wagner2001; Kyselý Reference Kyselý2005). Fossil lists are constantly supplemented and modified by new discoveries, both in the field and in collections, by rediscovering old fossils believed to be lost. Additionally, new dating and other analytic methods improve our knowledge about biochronology and provide new palaeobiological insights into the studied species and populations. Here, we present a new series of AMS dates obtained for the Late Pleistocene remains of U. arctos from the Czech Republic. The obtained dates are discussed in a wide biochronological and palaeoecological context.
SAMPLES AND METHODOLOGY
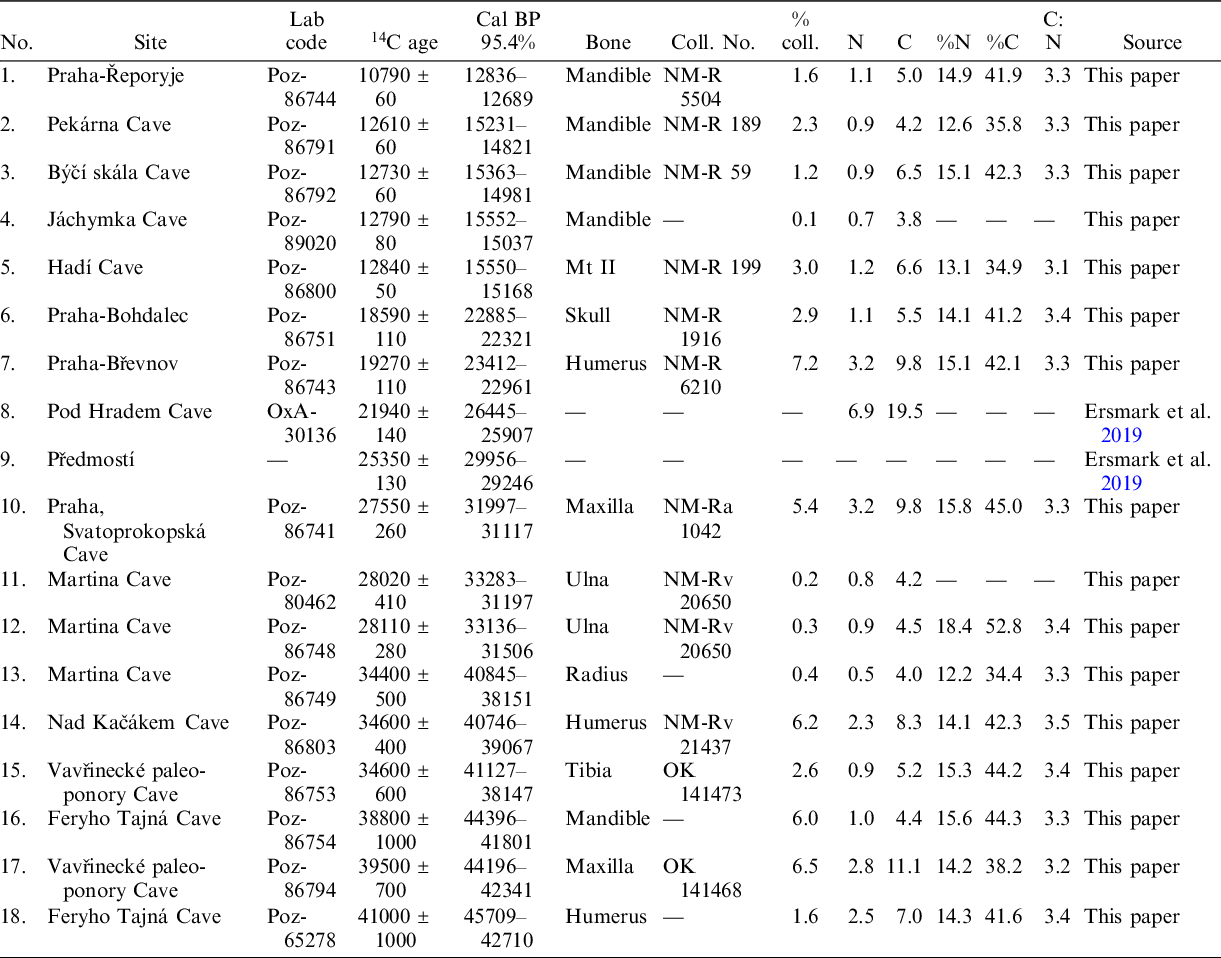
For the new dating, selected bones of U. arctos were pre-screened for their whole nitrogen content (Table 1). All 16 new radiocarbon dates (AMS) presented in this paper (see Table 1 for specimen information) were made in the Poznań Radiocarbon Laboratory (Poland), following their pre-treatment protocol for the extraction of collagen (Longin Reference Longin1971; Piotrowska and Goslar Reference Piotrowska and Goslar2002). Before extraction, the degree of collagen degradation was checked by measuring the nitrogen and carbon content in the bone using the analyser Flash EA 1112 Series (Thermo Scientific). The samples were regarded to be suitable for collagen dating if the nitrogen content was not lower than 0.6%, and C/N ratio was not higher than 5. Suitable bone fragments were crushed mechanically to granulation < 0.3 mm. The bone powder was then treated with 2M HCl (room temp., 20 min), and 0.1M NaOH (room temp., 1 hr). The sample was centrifuged, and the residuum was collected after each step of treatment. The extraction of collagen was processed in HCl (pH = 3, 80°C, 10 hr), and the residuum was removed after centrifugation. The extracted collagen was then ultra-filtered using pre-cleaned Vivaspin 15 MWCO 30 kD filters. The quality of collagen is ultimately assessed based on the C/N atomic ratio (interval of acceptance: 2.7–3.5) and collagen extraction yield (acceptance threshold: 0.5%). The carbon and nitrogen stable isotopic composition of collagen can be determined on demand (Bronk Ramsey et al. Reference Bronk Ramsey, Higham, Bowles and Hedges2004). All new dates were calibrated using the program IntCal 20 according to Reimer et al. (Reference Reimer, Austin, Bard, Bayliss, Blackwell, Bronk Ramsey, Butzin, Cheng, Edwards and Friedrich2020). Within the text, only calibrated data were used.
Table 1 Radiocarbon dates of the Late Pleistocene Ursus arctos remains from Czechia.
Specimens with inventory numbers starting with NM are housed in the National Museum, Prague. The infraspecific nomenclature and taxonomy of Late Pleistocene brown bears (U. arctos) is rather confusing, as is also the case for their recent counterparts. Traditionally, but not unreservedly, the last glacial brown bears from Europe were assigned to a separate subspecies, U. a. priscus Goldfuss, 1818. Its phenotypic peculiarities were mostly based on specimens from periglacial areas (e.g., Thenius Reference Thenius1956; Musil Reference Musil1964). The situation becomes confusing when Pacher (Reference Pacher2007) revised the holotype of U. a. priscus and found that this skull looks very recent and does not bear morphological characteristics traditionally associated with U. a. priscus. Moreover, some other subspecies were described for European brown bears from the Eemian, Weichselian or early Holocene, making the situation still more unclear, but it is above the scope of this paper to discuss this history in detail.
However, the specific cranial and dental characteristics of several Late Pleistocene brown bears, especially those from periglacial areas, cannot be disputed. There is good evidence from Central and East Europe to north Siberia (e.g., Baryshnikov and Boeskorov Reference Baryshnikov and Boeskorov2005; Marciszak et al. Reference Marciszak, Schouwenburg, Lipecki, Talamo, Shpansky, Malikov and Gornig2019 and references therein) that bears from the last glacial period bear a unique combination of features, unknown in recent representatives of the species. The problem is that, with the exception of some Eemian sites (e.g., Taubach, Germany), the record of Late Pleistocene brown bears is rather fragmentary, not allowing the sufficient characterisation of population variability. Therefore, we decided to leave the question of the taxonomic status of Late Pleistocene bears unanswered for the moment and used only informal taxa to describe the morphological characteristics of the studied specimens. We use the term priscus ecomorph for a large form, corresponding to the traditional concept of “U. a. priscus” (leaving aside the fact that these phenotypic characteristics do not fit the holotype of U. a. priscus itself). The term arctos ecomorph was restricted to the European Late Pleistocene brown bears, phenotypically more or less identical to recent, nominotypical subspecies U. arctos arctos. At this time, we were unable to resolve the relationship between these two ecomorphs and their taxonomic status. They can represent just two extremities of the population variability or, e.g., the arctos ecomorph can represent new immigrants from the south or east. Moreover, sometimes the material is so fragmentary that ecomorph determination is not possible. The assignation to the particular ecomorph was made by AM on the basis of a personal study of the material. The present study provides important new information about the temporal distribution of these ecomorphs in the territory of the Czech Republic.
RESULTS
As a result of this research, 18 radiocarbon dates, 16 of which were new, allowed us to specify the Late Pleistocene history of U. arctos in the Czech Republic. The dates obtained for brown bears from the Martina Cave (Bohemian Karst) agree with previously published data (Wagner et al. Reference Wagner, Zelinka, Frdlík and Kahlert2016). In most cases, cultural archaeological levels containing brown bear remains are well dated, and ages are consistent, as in the case of Gravettian sites, such as Dolní Věstonice 1 and 2, Pavlov, and Předmostí (Musil Reference Musil1959, Reference Musil1967, Reference Musil and Svoboda1994b, Reference Musil and Svoboda1997a, Reference Musil, van Andel and Davies2003a, Reference Musil, Orschiedt and Weniger2003b, Reference Musil2018; Wojtal et al. Reference Wojtal, Wilczyński, Bocheński and Svoboda2012). Some dates obtained for calcined bones, such as those from the Jáchymka Cave (Poz-89020) and Martina Cave (Poz-80462, Poz-86748, Poz-86749), yielded collagen lower than 1%, and the validity of these dates was restricted. All three dates from the Martina Cave cluster are tightly between 40.9–31.2 kyr, called BP, indicating good preservation of the bone, in keeping with a lower collagen amount. As standards, collagen yields above 1% and C/N ratios between 2.9 and 3.6 (DeNiro Reference DeNiro1985; Ambrose Reference Ambrose1990; van Klinken Reference Klinken1999; Brock et al. Reference Brock, Wood, Higham, Ditchfield, Bayliss and Bronk Ramsey2012) make the dates reliable. However, more recent analysis showed that we should be even stricter in this matter and that collagen with C:N ratios higher than 3.3 and quantity lower than 5% are potentially contaminated and will give dates that are too young (Zazzo et al. Reference Zazzo, Lepetz, Magail and Gantulga2019). Consequently, some cave dates do not fit well with the proposed age of layers in the analysed material. These dates were probably obtained from remains deposited after the formation of the layer or might have been re-deposited from the younger uppermost horizons. Other dated remains are isolated finds recovered from caves, loess, gravel pits or riverbanks where they occurred in fluvial sediments. In these cases, it should be considered that the material shifted from the place of its original deposition. In addition, it is always important to consider the possibility that humans could have transported some bone parts, such as canines, in the form of precious items over significant distances. Some of the bone collagen samples might have also been contaminated by a young, radiocarbon-enriched source of carbon, probably originating from the soil (Zazzo et al. Reference Zazzo, Lepetz, Magail and Gantulga2019).
These direct dates showed species’ range dynamics during that period. Predicting the response of U. arctos to climate allows us to better understand the mechanisms by which species and ecosystems can be affected by climate change (Bellard et al. Reference Bellard, Bertelsmeier, Leadley, Thuille and Carchamp2012). The mechanisms of the response of animal populations to climate fluctuations and environmental shifts can also be aided by the study of extinct analogues (Musil Reference Musil1994a; Hofreiter and Stewart Reference Hofreiter and Stewart2009). In these analyses, the transition from the last glaciation to the Holocene (MIS 2) is especially important. This was a time interval when major reorganisation of palaeocommunities and shifts occurred in species distributions as a response to abrupt climate fluctuations (Stewart et al. Reference Stewart, Lister, Barnes and Dalén2010; Palkoupoulou et al. Reference Palkopoulou, Dalén, Lister, Vartanyan, Sablin, Sher, Nyström Edmark, Brandström, Germonpré, Barnes and Thomas2013; Cooper et al. Reference Cooper, Turney, Hughen, Barry, Mcdonald and Bradshaw2015; Nadachowski et al. Reference Nadachowski, Lipecki, Baca, Żmihorski and Wilczyński2018; Niedziałkowska et al. Reference Niedziałkowska, Doan, Sykut, Górny, Stefaniak, Piotrowska, Jędrzejewska, Ridush, Pawełczyk and Mackiewicz2021).
The obtained data showed the continuous presence of U. arctos in the Czech Republic in the interval between 46 and 12.6 kyr. Most of the newly obtained dates are concentrated in the range of 45.7–29.3 kyr and covered the second half of MIS 3 (Table 2). The next three dates are especially important because they document the presence of U. arctos just before and during the LGM (Last Glacial Maximum, time frames according to Maier et al. Reference Maier, Mayr and Peresani2021). The dates from the sites Pod Hradem (26.5–25.9 kyr), Praha Břevnov (23.4–22.9 kyr) and Praha Bohdalec (22.9–22.3 kyr) imply that this species was present during cold phases of the LGM (Ersmark et al. Reference Ersmark, Baryshnikov, Higham, Argant, Castaños, Döppes, Gasparik, Germonpré, Lidén and Lipecki2019; Marciszak et al. Reference Marciszak, Schouwenburg, Lipecki, Talamo, Shpansky, Malikov and Gornig2019). The date fits well with the first part of the longest stadial during the LGM (GS-3 and GS-2.1c interval), dated 22.9–20.9 ka (Waelbroeck et al. Reference Waelbroeck, Paul, Kucera, Rosell-Melé, Weinelt, Schneider, Mix, Abelmann, Armand and Bard2009; Gavashelishvili and Tarkhnishvili Reference Gavashelishvili and Tarkhnishvili2016). During the coldest (∼20.9–19.0 kyr) part of the GS-2.1b interval (20.9–17.5 kyr), the maximum extent of the Scandinavian Ice Sheet had a profound impact on U. arctos populations in Europe, including the territory of the Czech Republic. There are indications of gaps in the pattern of dates in certain areas. Available records between 22 and 18 kyr came mostly from southern and southeastern parts of Europe in the alleged refugia (Ersmark et al. Reference Ersmark, Baryshnikov, Higham, Argant, Castaños, Döppes, Gasparik, Germonpré, Lidén and Lipecki2019). Since 19–18 kyr, European finds of U. arctos have considerably increased, and the number of dates was the largest during MIS 2, around the end of GS-2.1b (∼17.5 kyr) (Ersmark et al. Reference Ersmark, Baryshnikov, Higham, Argant, Castaños, Döppes, Gasparik, Germonpré, Lidén and Lipecki2019). The post-LGM time (GS-2.1a interval, 17.5–14.7 kyr) was characterised by intensive climate change processes and a growing number of brown bear dates from the Czech Republic (Table 1). Four radiocarbon dates documented the presence of this species during that time. Finally, the date from Praha-Řeporyje and the Kalvárie Cave (12.8–12.6 kyr) recorded U. arctos during the beginning of the LGM, i.e., during abrupt GI-1e (14.7–14.1 kyr) warming (Steffensen et al. Reference Steffensen, Andersen, Bigler, Clausen, Dahl-Jensen, Fischer, Goto-Azuma, Hansson, Johnsen and Jouzel2008).
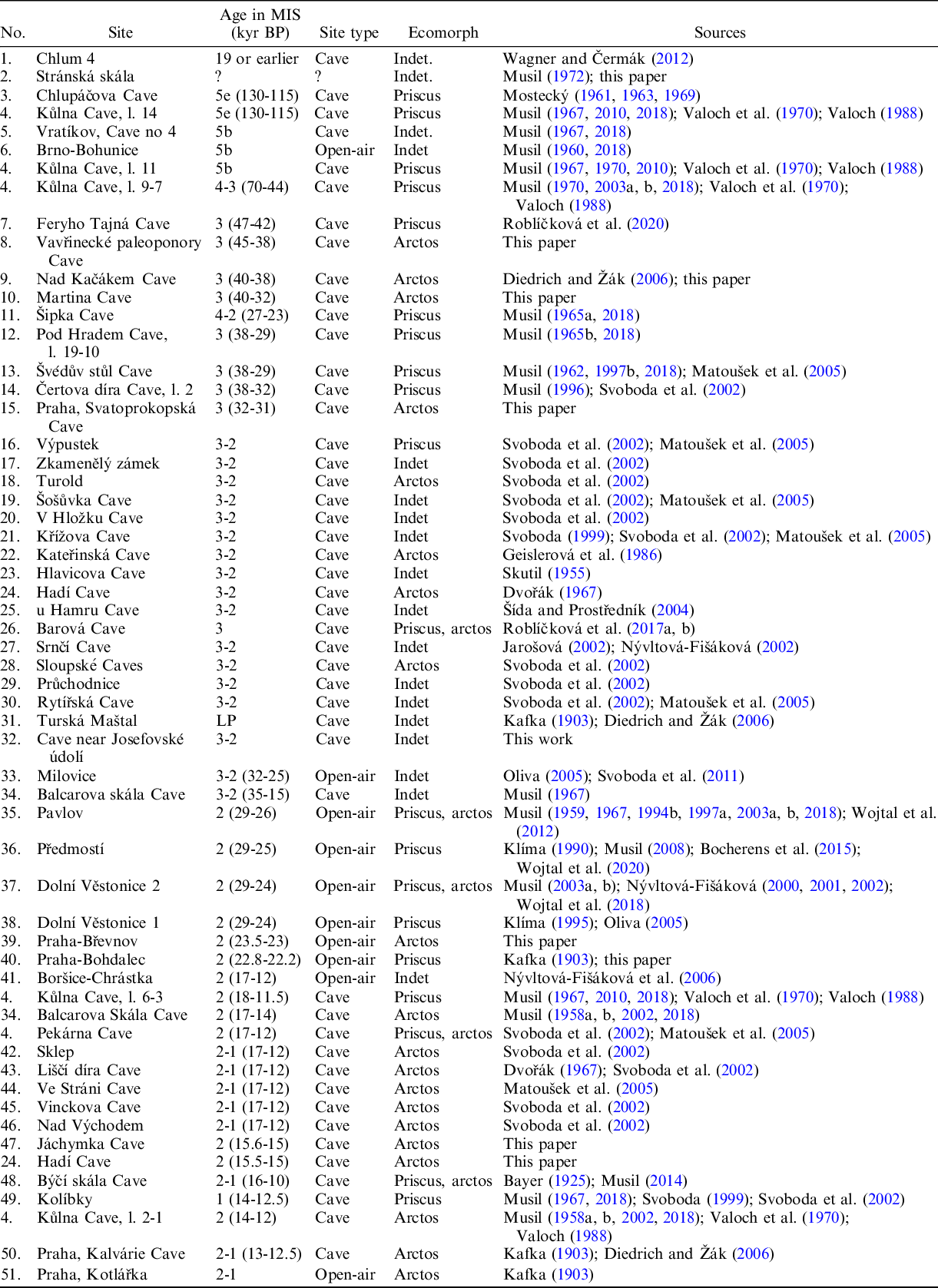
Table 2 Occurrence of Ursus arctos in the Middle and Late Pleistocene sites of the Czech Republic.
This was also a period of progressive growth in afforestation and the final disappearance of the steppe tundra in Central Europe. This warming caused a gradual transformation of Europe from semiarid, grassy steppes into boreal forests. Enormous herds of ungulates adapted to live in open grasslands disappeared as the forest took over their grassland habitats. The priscus ecomorph could not scavenge enough food to support its massive body, even if it could supplement its diet with plants. The process of disappearance and its mechanisms are still not resolved. The compact geographical range was split into isolated populations that survived across Europe. The decreasing size process took place, and U. a. priscus evolved into a more herbivorous form because of environmental changes. Leftovers blend into an abundant nominative form, which is much more herbivorous and omnivorous; they are also well adapted to forest conditions. Survival of the priscus ecomorph until the early part of MIS 1 has been confirmed by numerous finding of the great brown bear with particularly large and broad teeth (Marciszak et al. Reference Marciszak, Schouwenburg, Lipecki, Talamo, Shpansky, Malikov and Gornig2019 and references therein). From the Czech Republic, the late occurrence of the priscus ecomorph was documented by a robust mandible from the Býčí skála Cave, dated to 15.4–14.9 kyr.
DISCUSSION
The earliest possible record of U. arctos or at least its form from the arctoid lineage in the Czech Republic originates from the locality Chlum 4S, which is around the Early/Middle Pleistocene boundary in age, but the exact stratigraphical position is unknown at present (Wagner and Čermák Reference Wagner and Čermák2012; Horáček et al. Reference Horáček, Bláha, Wagner, Čermák, Žák and Ryšánek2016). New revisions, e.g., Lewis et al. (Reference Lewis, Pacher and Turner2010) and Rabeder et al. (Reference Rabeder, Pacher and Withalm2010), showed a higher variability among Middle Pleistocene arctoid bears than previously thought. However, according to Wagner and Čermák (Reference Wagner and Čermák2012), most of these Middle Pleistocene specimens belong to U. deningeri. Therefore, a new revision of the members of this lineage seems necessary for a more precise taxonomic determination. Therefore, a new revision of the members of this lineage seems necessary for a more precise taxonomical determination. Even if the presence of the species in the Middle Pleistocene is still not completely resolved, there is no doubt that the majority of the records belong to the Late Pleistocene. From the old collections, Musil (Reference Musil1972: 109) reported two maxillary fragments of U. arctos from Stránská Skála, which are in the same collection but differ in fossilisation. However, this material came from old excavations; therefore, its detailed location and exact stratigraphical position are strongly uncertain. Stránská Skála is a complex of large and small cavities and fissures filled with sediments of different ages. For this reason Stránská Skála cannot be regarded as a Middle Pleistocene record of U. arctos.
The Eemian (MIS 5e) is the time when true brown bears undoubtedly appeared in Czech territory in a form possibly identical with Ursus arctos “priscus” sensu lato (but see above regarding the nomenclatural confusion of this subspecies), a characteristic faunal element of European open land assemblages (Kurtén Reference Kurtén1968). This large, broad-toothed bear migrated from the East (Figure 1; Musil Reference Musil1996; Sabol Reference Sabol2001a, b) and appeared first in the late Middle Pleistocene (Kurtén Reference Kurtén1959; Baryshnikov Reference Baryshnikov2007; Marciszak et al. Reference Marciszak, Schouwenburg, Lipecki, Talamo, Shpansky, Malikov and Gornig2019; Marciszak and Lipecki Reference Marciszak and Lipecki2020). Earlier authors described the Eemian populations as independent subspecies called Ursus arctos taubachensis Rode, 1935 (Kurtén Reference Kurtén1956, Reference Kurtén1959, Reference Kurtén1968; Mostecký Reference Mostecký1961, Reference Mostecký1963, Reference Mostecký1969; Musil Reference Musil1996; Sabol Reference Sabol2001a, b; Wagner Reference Wagner2001). However, its taxonomic position was later revised, and it is now considered to be a synonym for U. a. priscus s. l. (Baryshnikov Reference Baryshnikov2007; Marciszak et al. Reference Marciszak, Schouwenburg, Lipecki, Talamo, Shpansky, Malikov and Gornig2019; Marciszak and Lipecki Reference Marciszak and Lipecki2020; Stefaniak et al. Reference Stefaniak, Kovalchuk, Marciszak, Stepanchuk, Rekovets, van der Made, Yanenko, Tsvelykh, Ratajczak-Skrzatek and Kotowski2021). The presence of this form during MIS 5e is rarely documented in the Czech territory, e.g., from layers 13–9 of the Kůlna Cave (Musil Reference Musil2010, Reference Musil2018), as well as from the Chlupáčova Cave (Mostecký Reference Mostecký1961, Reference Mostecký1963, Reference Mostecký1969; Wagner Reference Wagner2001).
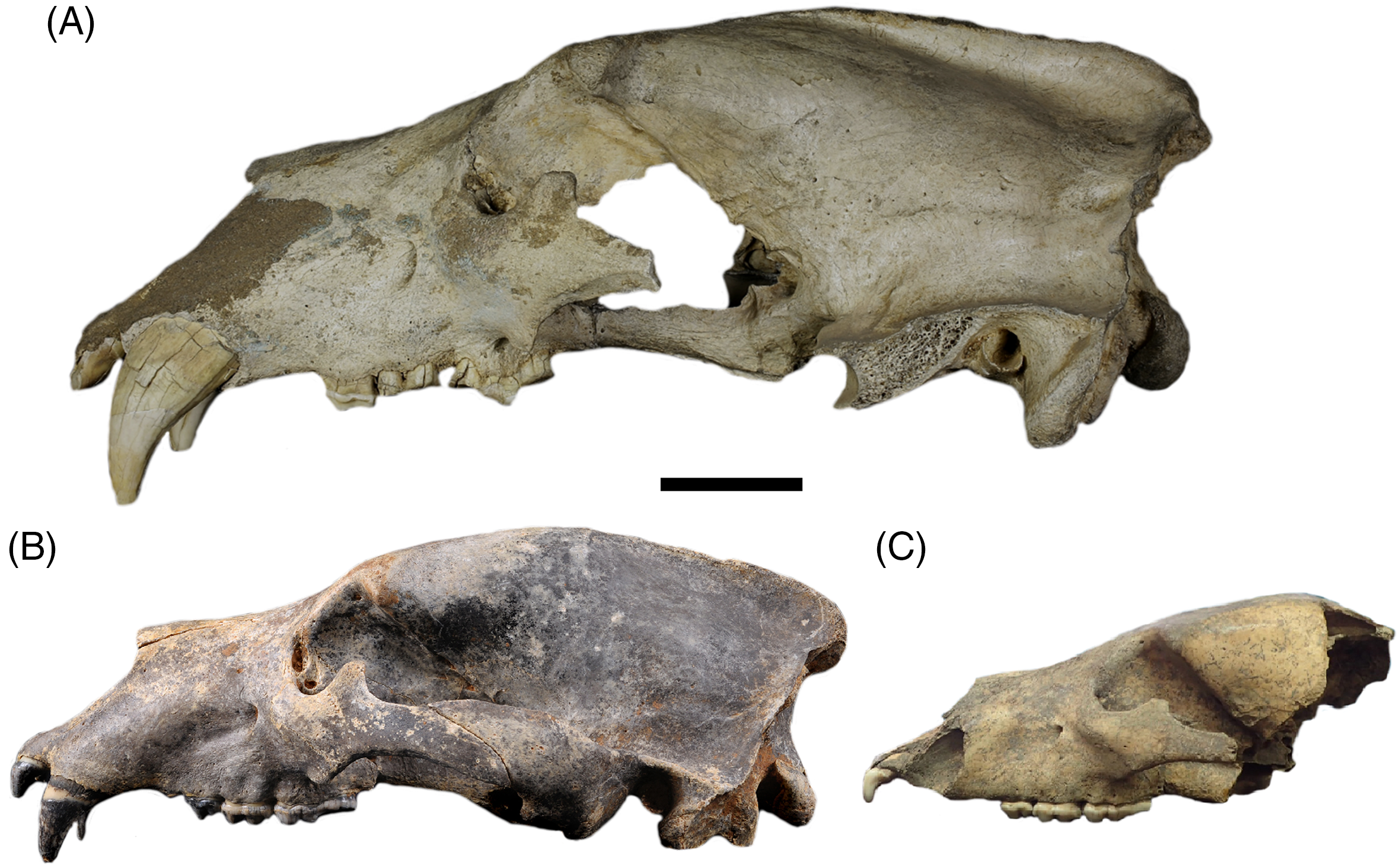
Figure 1 Skulls of the brown bear Ursus arctos from the Czech Republic. Ursus arctos, priscus ecomorph: A. male from Praha-Bohdalec (NM-R 1916), B. female from Feryho Tajná Cave. Ursus arctos, arctos ecomorph: C. female (P 135) from Klučov (P 135; Kudrnáč Reference Kudrnáč1970). Skulls showed in lateral view, scale bar 50 mm. Photos L. Váchová (A), A. Plichta (B), and A. Marciszak (C).

Figure 2 Distribution of the Middle and Late Pleistocene sites with Ursus arctos within the Czech Republic. See Table 2 for site numbers.
The occurrence of U. arctos was documented in 51 Late Pleistocene Czech localities (Figure 2, Table 2). After the Eemian, the number of records considerably increased, with 49 palaeontological records documenting the presence of this species within the Czech territory (Kořenský Reference Kořenský1884; Kafka Reference Kafka1903; Skutil Reference Skutil1955; Dvořák Reference Dvořák1967; Musil Reference Musil1962, Reference Musil1965a, Reference Musil1967; Mostecký Reference Mostecký1963, Reference Mostecký1969; Geislerová et al. Reference Geislerová, Seitl, Svoboda and Svobodová1986; Wagner Reference Wagner2001; Nývltová-Fišáková Reference Nývltová-Fišáková2002; Matoušek et al. Reference Matoušek, Jenč and Peša2005; Oliva Reference Oliva2005; Svoboda et al. Reference Svoboda, Bocheński, Čulíková, Dohnalová, Hladilová, Hložek, Horáček, Ivanov, Králík and Novák2011; Wojtal et al. Reference Wojtal, Wilczyński, Bocheński and Svoboda2012; Havlová Reference Havlová2014). Among them, there are 10 open-air and 41 cave localities. Plentiful remains have been considered either as a highly divergent form (e.g., Musil Reference Musil1962, Reference Musil1964, Reference Musil1965b, Mostecký Reference Mostecký1963), a subspecies of U. arctos or as a separate species (Wagner Reference Wagner2001). This mostly depends on which classification system has been used to recognise these forms. For carnivores, several systems have been proposed for different purposes, such as behaviour, ecology or morphological differences. However, the ecotype approach, when a population or a group of populations is associated with a particular set of environmental conditions, is adopted in conservation ecology. Ecotypes classify U. arctos according to different life history strategies and ecological conditions. Since the holotype of U. a. priscus does not contain the diagnostic characteristics traditionally used for defining this subspecies (Pacher Reference Pacher2007; Marciszak et al. Reference Marciszak, Schouwenburg, Lipecki, Talamo, Shpansky, Malikov and Gornig2019) and so far no formal re-description and/or neotype designation has been made, there is currently no consensus on the taxonomic status of this subspecies. In this paper, we recognise this bear as a distinct ecomorph distinguishing it from the nominotypical Ursus arctos arctos Linnaeus, 1758, whose occurrence in Czech territory is mostly related to MIS 1. However, the presence of individuals morphometrically indistinguishable from recent U. a. arctos was also sporadically distinguished from the Late Pleistocene (Table 2).
The immense priscus ecomorph roamed on Eurasian Late Pleistocene open grasslands (Baryshnikov and Boeskorov Reference Baryshnikov and Boeskorov2005), chasing other carnivores away from their carcasses and gorging themselves on scavenged meat (Marciszak et al. Reference Marciszak, Schouwenburg, Lipecki, Talamo, Shpansky, Malikov and Gornig2019). The significantly higher degree of carnivory in European pre-LGM brown bears, compared with recent U. arctos, has been confirmed by stable isotope analyses (Münzel et al. Reference Münzel, Stiller, Hofreiter, Mittnik, Conard and Bocherens2011; Bocherens Reference Bocherens2015; Bocherens et al. Reference Bocherens, Drucker, Germonpré, Lázničková-Galetová, Naito, Wissing, Brůžek and Oliva2015; Ersmark et al. Reference Ersmark, Baryshnikov, Higham, Argant, Castaños, Döppes, Gasparik, Germonpré, Lidén and Lipecki2019). Some of these authors also suggest that the shift to lower δ15N-values started after the LGM was synchronous with the extinction of speleoid bears. It has been proposed as the cause of this dietary shift, as it opened up a more herbivorous niche for brown bears (Münzel et al. Reference Münzel, Stiller, Hofreiter, Mittnik, Conard and Bocherens2011; Bocherens Reference Bocherens2015; Mackiewicz et al. Reference Mackiewicz, Baca, Popović, Socha, Stefaniak, Marciszak and Nadachowski2017). However, there is another possible scenario. Higher δ15N values commonly documented in the Late Pleistocene brown bears from MIS 3 and MIS 2 could have been an adaptation to colder and more barren habitats. Recent U. arctos are generally more carnivorous in open landscapes (Bojarska and Selva Reference Bojarska and Selva2012). Thenius (Reference Thenius1956) and Musil (Reference Musil1964) pointed out that U. a. priscus occurs in open grasslands. An abrupt warming at 14.7 kyr (onset of GI-1e), a reduction and fragmentation of the priscus-ecomorph range are suggested for a wide area of Europe, except in more oceanic north-western parts, such as the modern Baltic and North Sea coasts and perhaps the central and northern East European Plain. A possible explanation for the longer survival of this large bear is that the steppe-tundra ecosystem in these regions lasted longer. In addition, some areas were much larger because of the presence of a recently inundated huge landmass referred to as Doggerland in the modern North Sea (Coles Reference Coles, Pye and Allen2000). During the GI-1 interval (14.7–14.1 kyr), present day Baltic and North Sea coasts, as well as the north-western part of Europe, were still covered by open grassland vegetation suitable for the priscus ecomorph, in contrast to Central Europe, which was already covered by pine forests (Brewer et al. Reference Brewer, Giesecke, Davis, Finsinger, Wolters, Binney, de Beaulieu, Fyfe, Gil-Romera and Kühl2017).
CONCLUSIONS
The continuous occurrence of U. arctos during the Late Pleistocene (MIS 5e-2) in the territory of the Czech Republic was documented in 51 localities. Among them, 10 were open air sites, while 41 others were cave sites. A total of 18 radiocarbon dates showed the presence of the brown bear between 46 and 12.6 kyr ago during the Late Pleistocene. Most of the dates were concentrated in the range of 45.7–29.3 kyr and covered the second half of MIS 3. Later, its occurrence continued into the Holocene. Three dates documented the presence of U. arctos just before and during the LGM. The new dates imply that the species was present during some cold phases of the LGM. During the coldest (∼20.9–19.0 kyr) part of the GS-2.1b interval (20.9–17.5 kyr), U. arctos was probably absent in the Czech Republic. Similar to other parts of Central Europe, the maximum extent of the Scandinavian Ice Sheet had a profound impact on the Czech brown bears. During the Late Pleistocene, a large, broad-toothed and highly carnivorous priscus ecomorph occurred in the territory of the Czech Republic. It was adapted to live in open grasslands. The post-LGM time (GS-2.1a interval, 17.5–14.7 kyr) was characterised by intense climate changes and a growing number of brown bear finds from the Czech Republic. It was also a period of progressive growth in afforestation and the final disappearance of the steppe-tundra biome in Central Europe. It was also the time when the priscus ecomorph evolved into a more herbivorous form because of environmental changes. Leftovers blend into an abundant nominotypical subspecies, which is much more herbivorous and omnivorous, and are also well adapted to forest conditions. A robust mandible from the Býčí Skála Cave, dated at 15.4–14.9 kyr, is so far the latest known occurrence of priscus-ecomorph from the Czech Republic.
ACKNOWLEDGMENTS
This research was financed by subsidies for the activities of the University of Wrocław, no. 2021 (501) and by an internal grant no. BPIDUB.4610.6.2021.KP.A. “The Middle Pleistocene Revolution—How the modern theriofauna of Eurasia was developed” as part of the program “Inicjatywa Doskonałości-Uczelnia Badawcza (IDUB)” from the Faculty of Biological Sciences, University of Wrocław for Adrian Marciszak. Jan Wagner’s research was supported by the Ministry of Culture of the Czech Republic (DKRVO 2019-2023/2.I.d, National Museum, 00023272. Martina Roblíčková’s research was supported through the institutional support of long-term conceptual development of research institutions provided by the Ministry of Culture of the Czech Republic (ref. MK000094862).