Premenstrual syndrome (PMS) is a cyclical late luteal phase disorder of the menstrual cycle whereby the daily functioning of women is affected by emotional and physical symptoms substantially interfering with her quality of life( Reference Rapkin and Winer 1 , Reference O’Brien, Rapkin and Dennerstein 2 ). It is estimated that approximately 8–20 % of reproductive-aged women meet clinical diagnostic criteria for PMS( Reference Johnson 3 , Reference Halbreich, Borenstein and Pearlstein 4 ). Several treatment options exist (e.g. oral contraceptives, gonadotropin-releasing hormone agonists, antidepressants); however, these may have side effects and efficacy is relatively low( Reference Chocano-Bedoya and Bertone-Johnson 5 ). Furthermore, emerging research suggests that PMS may be an early sentinel for risk of hypertension( Reference Bertone-Johnson, Whitcomb and Rich-Edwards 6 ). Thus, it is important to identify modifiable risk factors to prevent the initial development of PMS, particularly those that are feasible to implement, such as dietary changes.
The American Congress of Obstetricians and Gynecologists recommends reducing fat intake to treat PMS( 7 ). However, evidence supporting these recommendations is limited, and it is unclear whether they apply to the prevention of PMS development( Reference Houghton and Bertone-Johnson 8 ). A small number of retrospective studies have reported inconsistent relationships between premenstrual symptoms and consumption of fats( Reference Nagata, Hirokawa and Shimizu 9 , Reference Gold, Bair and Block 10 ). Among retrospective studies, because of issues related to establishing temporality, it is unknown whether increased fat and fatty acid intake precedes the development of PMS. In addition, little attention has been given to specific types of fat, with most studies evaluating either total fat( Reference Nagata, Hirokawa and Shimizu 9 , Reference Gold, Bair and Block 10 ) or supplementation with n-3( Reference Sohrabi, Kashanian and Ghafoori 11 – Reference Rocha Filho, Lima and Pinho Neto 13 ), γ-linolenic acid( Reference Watanabe, Sakurada and Tsuji 14 ) and evening primrose oil( Reference Budeiri, Li Wan and Dornan 15 ).
Although the aetiological cause of PMS has yet to be elucidated, suggestions include chronic inflammation and alterations in hormones. Dietary fats may affect a woman’s cytokine and hormone levels( Reference Houghton and Bertone-Johnson 8 ). Dietary fat and SFA have been shown to act as pro-inflammatory factors increasing C-reactive protein (CRP) concentrations( Reference Aeberli, Molinari and Spinas 16 , Reference Santos, Oliveira and Lopes 17 ), whereas the unsaturated n-3 fatty acids have been shown to act as anti-inflammatory factors decreasing CRP and IL-6 concentrations( Reference Turunen, Jula and Suominen 18 – Reference Calder 20 ). Higher CRP and other inflammatory cytokine levels have been linked to PMS( Reference Bertone-Johnson, Ronnenberg and Houghton 21 ) and premenstrual symptoms( Reference Gold, Wells and Rasor 22 – Reference Azizieh, Alyahya and Dingle 24 ). Second, higher intakes of SFA are associated with higher plasma total and free estradiol levels and lower concentrations of luteinizing hormone among premenopausal women( Reference Tsuji, Tamai and Wada 25 ). Hormone levels have been long implicated in the aetiology of PMS due to its cyclical nature of symptoms( Reference Bäckström, Andreen and Birzniece 26 ).
To our knowledge, no previous study has prospectively evaluated whether total fat, fat subtypes or individual fatty acid intake is associated with risk of developing PMS. Therefore based on prior epidemiological studies and suggested biological pathways, we evaluated the hypothesis that higher intakes of total, SFA and trans-fat and lower intakes of unsaturated fats and fatty acids were related to increased PMS risk, in the Nurses’ Health Study II (NHS2) PMS Sub-Study, a case–control study nested within the prospective NHS2.
Methods
Study population
The NHS2 is a large prospective cohort of 116 429 US female registered nurses, aged 25–42 years in 1989, that has assessed demographics, health-related behaviours, diet, and medical history biennially and diet quadrennially for over 25 years( Reference Bertone-Johnson, Hankinson and Bendich 27 ). Response rates have been ≥89 % for all questionnaire cycles. The original NHS2 study protocol was approved by the Institutional Review Board at Brigham and Women’s Hospital in Boston, Massachusetts.
Classification of premenstrual syndrome cases and controls
The NHS2 PMS Sub-Study has been described previously( Reference Bertone-Johnson, Hankinson and Bendich 27 , Reference Bertone-Johnson, Hankinson and Johnson 28 ). In brief, we identified all NHS2 members who had not reported a diagnosis of PMS by a clinician in either 1989 or 1991 and thus were at risk of developing PMS. Premenopausal women reporting a new clinician-made diagnosis of PMS during the follow-up period in 1991–2005 were selected as potential cases (n 4108) with the diagnosis year assigned as their reference year. Potential controls were randomly selected from women who did not report a diagnosis of PMS from 1991–2005 (n 3248). These women were randomly assigned a reference year (1991–2005) corresponding to the cases’ diagnosis years, and then were frequency matched to cases on age and reference years. Women who had reported a history of cancer (other than non-melanoma skin cancer), endometriosis, extremely irregular menstrual cycles, infertility, hysterectomy or menopause before their assigned reference year were excluded to reduce likelihood of inclusion of women with symptoms similar to PMS due to other conditions. In addition, because of our interest in diet, those with implausible energetic intakes (i.e. those below 2092 kJ/d (500 kcal/d) and above 14 644 kJ/d (3500 kcal/d)) were also excluded.
All potential cases and controls were sent a modified version of the Calendar of Premenstrual Experiences questionnaire( Reference Bertone-Johnson, Hankinson and Johnson 28 , Reference Mortola, Girton and Beck 29 ) in 2003 for the 1991–2001 follow-up cycles and then in 2007 for the 2003–2005 follow-up cycles, that assessed the occurrence of twenty-six physical and affective symptoms in the 2 years before their specific reference year, the timing of symptoms and the impact of symptoms on several domains of daily functioning. Of those that were sent the questionnaire, 87 % of the potential cases (n 3579) and 95 % of the potential controls (n 3087) returned a completed questionnaire. We used the responses to the premenstrual questionnaire to confirm potential cases as meeting clinical diagnostic guidelines and potential controls as having no premenstrual symptoms.
There were 1257 women who met clinical diagnostic guidelines for PMS. Specifically, the cases reported: (1) ≥1 physical and ≥1 affective menstrual symptoms; (2) overall symptom severity of ‘moderate’ or ‘severe’ or ‘moderate’ or ‘severe’ effect of symptoms on at least one life activity or relationship domain; (3) symptoms began ≤14 d before start of menses; (4) symptoms ended ≤4 d after start of menses; and (5) symptoms were not present in week after menses ended( Reference Bertone-Johnson, Hankinson and Bendich 27 ).
Women who had no or minimal symptoms that did not impact daily function domains were verified as controls (n 2463). Specifically they: (1) confirmed no PMS diagnosis; (2) reported either no menstrual symptoms, or an overall symptom severity of ‘minimal’ or ‘mild’; and (3) reported either ‘no effect’ or ‘mild’ effect of symptoms on all life activities and relationship domains( Reference Bertone-Johnson, Hankinson and Bendich 27 ).
Participants who did not meet these definitions were excluded from further analysis. This allowed for a comparison of women at the two extreme ends of the spectrum of premenstrual symptom experience and reduced the likelihood of misclassification of cases as controls and vice-versa. Our approach for identifying PMS cases and controls has been validated previously and found to be comparable with prospective charting of symptoms( Reference Bertone-Johnson, Hankinson and Bendich 27 ).
Assessment of fat intake
In the NHS2, a 131-item semi-quantitative FFQ was first given in 1991 and then repeated every four years thereafter( Reference Willett 30 ). We used food intake information reported on the FFQ to assess the intake of total fat, SFA, MUFA, PUFA, trans-fat, dairy fat, animal fat, vegetable fat, total n-3, total n-6, the ratio of n-6:n-3, and specific fatty acids hypothesised to play a role in PMS development including stearic acid (18 : 0), oleic acid (18 : 1n-9), arachidonic acid (20 : 4n-6), linoleic acid (18 : 2n-6), conjugated linoleic acid (cis-9, trans-11 18 : 2), linolenic acid (18 : 3), EPA (20 : 5n-3), and DHA (22 : 6n-3). The FFQ included food high in fat such as red meat, chicken with skin, bacon, processed meats, fish, eggs, butter, margarine, whole milk, cheese, ice cream, French fries, potato chips, peanut butter and nuts. In addition, participants were asked about the types of fat used for frying and baking and whether fish oil or cod liver oil supplements are used. Participants indicated the frequency with which they consumed a specific portion size of each food item, with a nine-option range from ‘never or less than once per month’ to ‘6 or more times per d’. To calculate each participant’s dietary fat intakes, the portion size of each food item was multiplied by the indicated frequency of consumption and fat content and then summed across all foods. The fat and fatty acid content of each food is based on the Harvard University Food Composition Table( Reference Harvard 31 ), which is updated every 4 years based on information from the US Department of Agriculture, food manufacturers, academic publications and direct analyses of fatty acids (e.g. trans-fat) in commonly used foods to reflect changes in manufacturing and food supply over time. Dairy fat is a subset of animal fat (i.e. animal fat also includes dairy fats), which includes all fat from the foods in the ‘Dairy Foods’ section on the FFQ except for margarine and non-dairy whitener and fat from the dairy products used as ingredients in other FFQ items. Nutrient intakes were adjusted for total energy using the residual method( Reference Willett 30 ).
The reproducibility and validity of similar FFQs have been evaluated previously in the NHS( Reference Willett 30 , Reference London, Sacks and Caesar 32 – Reference Sun, Ma and Campos 34 ). The energy adjusted correlation between intakes reported by FFQ and mean intake measured via two 1-week diet records in 1986 (n 191) was 0·51, 0·59, 0·41 and 0·51 for total fat, SFA, PUFA and MUFA, respectively( Reference Willett 30 ). Further, dietary intake correlated with subcutaneous adipose tissue aspirate levels of PUFA (r 0·37), n-3 marine fatty acids (r 0·48) and trans-fatty acids (r 0·51) but not serum SFA or MUFA levels (r 0·16 and 0·07 respectively) in a study of 115 postmenopausal US women using a similar FFQ( Reference London, Sacks and Caesar 32 ).
Primary analyses used the most recent FFQ that preceded the reference year (i.e. 2–4 years). For example, if a woman was diagnosed with PMS in 1999 then information from the 1995 FFQ was used. We additionally examined intakes from the baseline (1991) FFQ, a more distal exposure. Dietary information from 3638 Sub-Study participants was available for analyses of intake 2–4 years before a woman’s reference year and for 3660 women for analyses of intake at baseline in 1991.
Assessment of covariates
We considered as covariates variables associated with PMS in our population. Height and menstrual cycle characteristics were assessed on the 1989 questionnaire. History of depression and antidepressant use were assessed on the premenstrual questionnaire. Childhood trauma was assessed in 2001 on a separate questionnaire( Reference Bertone-Johnson, Whitcomb and Missmer 35 ). Other factors were collected on each biennial questionnaire and included age, smoking status, weight (used to calculate BMI in kg/m2), pregnancy history and oral contraceptive use. Lastly, other nutrients such as B-vitamins, Fe and Ca were assessed by FFQ and were calculated using similar methods as fat intake.
Statistical analysis
Age-adjusted baseline characteristics of PMS cases and controls were compared using generalised linear modelling. We used unconditional logistic regression models to estimate relative risks (RR) of PMS for women across quintiles of fat intake at 2–4 years before each woman’s reference year and calculated 95 % CI comparing the risk of PMS in each of the four highest quintiles compared with the lowest quintile adjusting for age. Multivariable logistic regression models additionally adjusted for reference year, age in 1991, age at menarche, calculated BMI (weight (kg)/height (m2)), physical activity, oral contraceptives, parity (pregnancies lasting ≥6 months), smoking (pack-years), previous use of antidepressants, significant childhood trauma, previous diagnosis of depression, total intake of vitamins B6, B1, Fe and Ca 2–4 years before the reference year. Secondary analyses were run using the intake at baseline to assess the possibility of latent effects adjusting for covariates measured at baseline in 1991. Additional multivariable models also adjusted subtypes of fat for the effect of other subtypes (e.g. SFA was adjusted for MUFA, PUFA and trans-fat) and each source of fat was adjusted for the other sources (e.g. dairy fat was also adjusted for vegetable and animal fat). The Mantel extension test for trend was used to examine the presence of linear trend across quintiles, modelling the median of each quintile as a continuous variable.
We further assessed whether the relationship of dietary fat and PMS varied by age at reference year (<40 v. ≥40 years) and smoking (past/never v. current) via stratified analyses. The multiplicative interaction terms were evaluated using likelihood ratio test, where the interaction terms were calculated as the products of a binary stratification factor and indicators of the macronutrient quintile. In a sub-analysis, we also restricted analyses to non-oral contraceptive users.
SAS 9.3 (SAS Institute Inc.) for UNIX was used for all analyses. Two-sided P<0·05 were considered statistically significant for all analyses.
Results
Baseline characteristics of PMS cases and controls are shown in Table 1. Cases were heavier at baseline and age 18 years, had a slightly earlier age at menarche, and had higher history of oral contraceptive use, smoking, significant childhood trauma, depression and antidepressant use. In addition, cases on average consumed less vitamin D, Ca and higher vitamins B6 and B12 than controls.
Table 1 Age-standardised characteristics of premenstrual syndrome (PMS) cases and controls at baseline (n 3660): Nurses’ Health Study II PMS sub-study, 1991–2005 (Mean values and standard deviations; percentages)
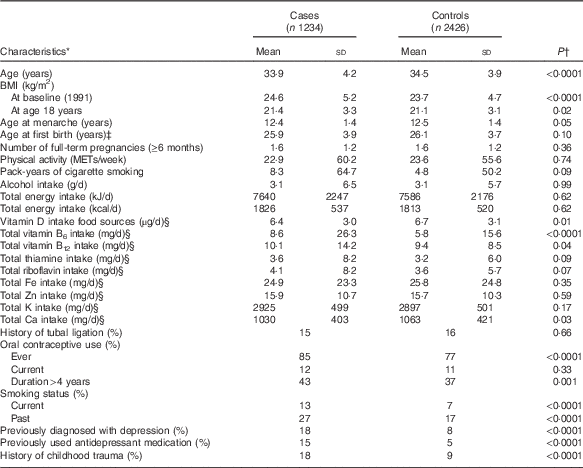
METs, metabolic equivalents of task.
* All characteristics, except age, standardised to the age distribution of participants in 1991.
† Calculated using the generalised linear model.
‡ Limited to parous women.
§ Energy adjusted values.
Table 2 presents age-adjusted and multivariate-adjusted RR and 95 % CI for types of fats consumed 2–4 years before the reference year and the risk of developing PMS. In analyses adjusted only for age, total fat, PUFA and MUFA were each positively associated with risk of developing PMS (P trend≤0·05 for all). However, after controlling for BMI, smoking and additional covariates, these positive associations were attenuated and no longer significant. The strongest confounders of the fat and PMS relation were BMI, smoking, and vitamin D. In multivariable adjusted models (model 1), high SFA intake was inversely associated with risk of PMS (RR quintile 5 v. quintile 1=0·75; 95 % CI 0·58, 0·98). When the models were additionally adjusted for other fats (model 2), SFA estimates were slightly stronger (RR quintile 5 v. quintile 1=0·63; 95 % CI 0·44, 0·92). The estimates for other subtypes of fats were largely unchanged and interpretations did not change.
Table 2 Quintiles of dietary fat subtypes 2–4 years before diagnosis and risk of premenstrual syndrome (PMS) (n 3638): Nurses’ Health Study II PMS Sub-Study, 1991–2005 (Relative risks (RR) and 95 % confidence intervals)
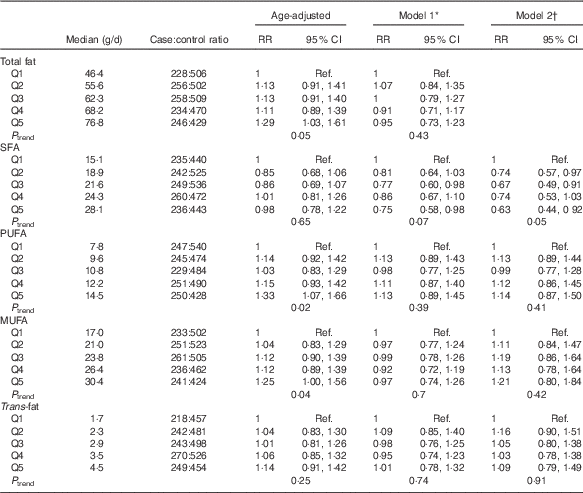
Ref., referent values.
* Model 1 adjusted for age in 1991 (continuous), reference year (1991–1992, 1993, 1994–1996, 1997–1998, 1999–2000, 2001–2002, 2003–2004), age at menarche (continuous), current BMI (≤19·9, 20·0–22·4, 22·5–24·9, 25·0–27·4, 27·5–29·9, ≥30 kg/m2), physical activity (<3, 3–8, 9–17, 18–26, 27–41, ≥42 metabolic equivalents), duration of oral contraceptive use (none, 1–23, 24–71, 72–119, ≥120 months), parity (nulliparous, 1–2, 3–4, ≥5 pregnancies>6 months), smoking (never, past 1–14, past 15–34, past 35+, current 1–14, current 15–34, current 35+ cigarettes/d), previous use of antidepressants (never, ever), childhood trauma score (5, 6–10, 11–15, 16–20, 21–25), previous diagnosis of depression (never, ever) and quintiles of total intake of vitamins B6, B1, Fe and Ca 2–4 years before reference year.
† Model 2 adjusted for factors included in model 1+mutually adjusted for other fat subtypes.
Table 3 shows results from models assessing risk of PMS related to sources of fats. Intakes of vegetable and dairy fats were unrelated to PMS risk, with estimates for all quintiles approximately equal to 1. High animal fat intake was related to a non-significant lower risk of PMS in age-adjusted (P trend=0·53) and multivariable adjusted (model 1) models (P trend=0·17). Additional adjustment for the other sources of fat (model 2) did not affect the interpretations of any of the estimates.
Table 3 Quintiles of dietary fat sources 2–4 years before reference year and risk of premenstrual syndrome (PMS) (n 3638): Nurses’ Health Study II PMS Sub-Study, 1991–2005 (Relative risks (RR) and 95 % confidence intervals)
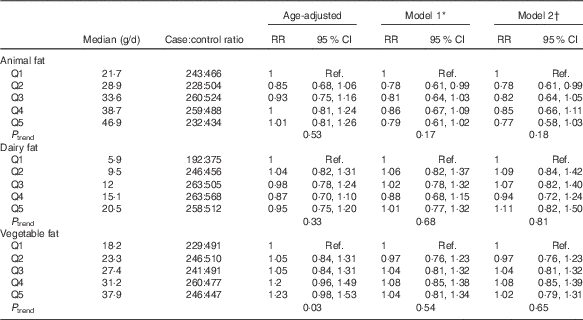
Ref., referent values.
* Model 1 adjusted for age in 1991 (continuous), reference year (1991–1992, 1993, 1994–1996, 1997–1998, 1999–2000, 2001–2002, 2003–2004), age at menarche (continuous), current BMI (≤19·9, 20·0–22·4, 22·5–24·9, 25·0–27·4, 27·5–29·9, ≥30 kg/m2), physical activity (<3, 3–8, 9–17, 18–26, 27–41, ≥42 metabolic equivalents), duration of oral contraceptive use (none, 1–23, 24–71, 72–119, ≥120 months), parity (nulliparous, 1–2, 3–4, ≥5 pregnancies >6 months), smoking (never, past 1–14, past 15–34, past 35+, current 1–14, current 15–34, current 35+ cigarettes/d), previous use of antidepressants (never, ever), childhood trauma score (5, 6–10, 11–15, 16–20, 21–25), previous diagnosis of depression (never, ever) and quintiles of total intake of vitamins B6, B1, Fe and Ca 2–4 years before reference year.
† Model 2 adjusted for factors included in model 1+mutually adjusted for other fat subtypes (quintiles).
Results for models of fatty acid intake and PMS risk are presented in Table 4. Stearic acid, a SFA, was inversely associated with PMS risk. Women with the highest intake of stearic acid (quintile median=7·4 g/d) had a significant 25 % lower risk of PMS than women with the lowest intake (quintile median=3·7 g/d) (95 % CI 0·57, 0·97; P trend=0·03). We did not find intake of other individual fatty acids to be associated with risk.
Table 4 Quintiles of fatty acids 2–4 years before reference year and risk of premenstrual syndrome (PMS) (n 3638): Nurses’ Health Study II PMS Sub-Study, 1991–2005 (Relative risks (RR) and 95 % confidence intervals)
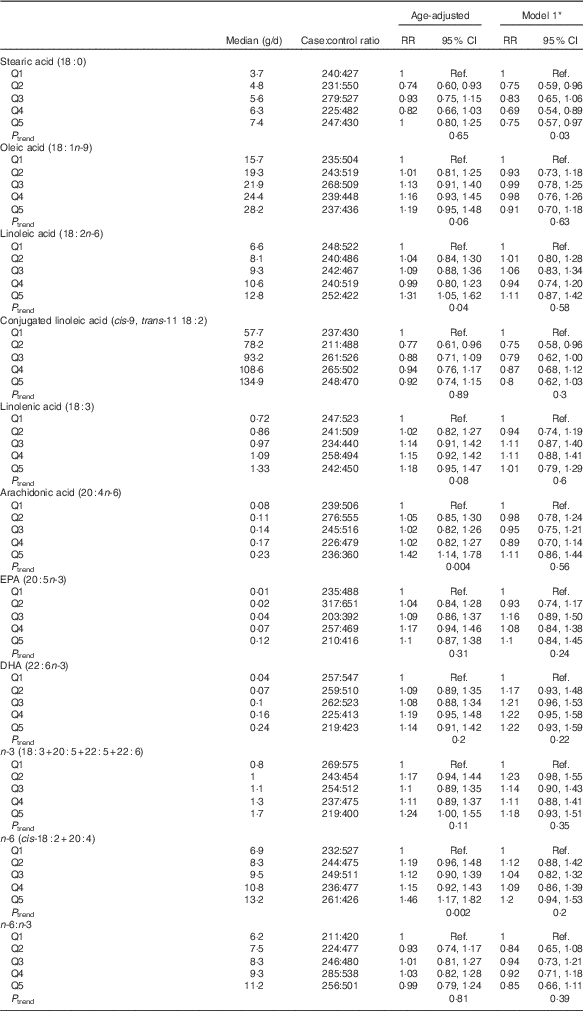
* Model 1 adjusted for age in 1991 (continuous), reference year (1991–1992, 1993, 1994–1996, 1997–1998, 1999–2000, 2001–2002, 2003–2004), age at menarche (continuous), current BMI (≤19·9, 20·0–22·4, 22·5–24·9, 25·0–27·4, 27·5–29·9, ≥30 kg/m2), physical activity (<3, 3–8, 9–17, 18–26, 27–41, ≥42 metabolic equivalents), duration of oral contraceptive use (none, 1–23, 24–71, 72–119, ≥120 months), parity (nulliparous, 1–2, 3–4, ≥5 pregnancies>6 months), smoking (never, past 1–14, past 15–34, past 35+, current 1–14, current 15–34, current 35+ cigarettes/d), previous use of antidepressants (never, ever), childhood trauma score (5, 6–10, 11–15, 16–20, 21–25), previous diagnosis of depression (never, ever) and quintiles of total intake of vitamins B6, B1, Fe and Ca 2–4 years before reference year.
Dietary intake of stearic acid was strongly correlated with SFA intake (r 0·93). The inverse association of stearic acid and PMS risk remained after adjustment for SFA; however, due to wider CI the association was no longer significant (RR quintile 5 v. quintile 1=0·67; 95 % CI 0·39, 1·16). Inclusion of stearic acid in the model for SFA attenuated the results, and the estimates for SFA became null (RR quintile 5 v. quintile 1=1·08; 95 % CI 0·63, 1·84).
The main contributors to stearic acid variation were beef from main dishes and hamburgers, cheese (e.g. Cheddar, American) and chocolate from chocolate bars or other candy bars. The associations between the individual foods and PMS were similar to that of stearic acid and PMS (results not shown).
Results evaluating fat and fatty acid intake at baseline (results not shown), to assess latent effects, were largely similar to those evaluating fat intake at reference year, including results for stearic acid. Women with high intake of stearic acid (quintile median=7·6 g/d) had 20 % lower risk than those with the lowest intake (quintile median=4·1 g/d; 95 % CI 0·61, 1·05; P trend=0·04). However, there were a few exceptions. The n-3s from marine sources, EPA and DHA, showed significant positive associations with PMS, with evidence of linear trend. In fully adjusted models, women with the highest EPA intake at baseline (quintile median=0·12 g/d) had 1·31 times the risk of PMS as those with the lowest (quintile median=0·01 g/d; 95 % CI 0·98, 1·75; P trend=0·05). Women with high DHA intake (quintile median=0·26 g/d) had 1·49 times the risk of PMS as those with the lowest intake (quintile median=0·05 g/d; 95 % CI 1·14, 1·95; P trend=0·01).
As BMI could potentially lie in the casual pathway of an association between fat intake and PMS risk and could mediate associations, multivariable analyses were repeated without adjusting for BMI in the model; however, estimates were unchanged. Analyses stratified by age and smoking status did not suggest effect modification by any of these factors and there were no significant interactions.
Discussion
We did not find intake of total fat to be associated with risk of developing PMS. High intake of SFA, specifically stearic acid, was significantly associated with lower risk. Other individual fatty acids, including n-3 fatty acids, were not consistently associated with risk.
Few studies have comprehensively assessed how fat and fatty acid intake may be related to PMS. In addition, to date no studies have prospectively assessed fat or fatty acid intake and risk of developing PMS. Prior research of the relation between fat intake and PMS has been limited to consideration of symptom presence and/or severity, rather than risk of developing PMS. Both Nagata et al. ( Reference Nagata, Hirokawa and Shimizu 9 ) and Gold et al. ( Reference Gold, Bair and Block 10 ) assessed total fat intake with premenstrual symptoms using cross-sectional study designs. Nagata et al. ( Reference Nagata, Hirokawa and Shimizu 9 ) examined whether total fat and fat subtypes were associated with the severity of premenstrual symptoms among 189 female Japanese students aged 19–34 years( Reference Nagata, Hirokawa and Shimizu 9 ). Total fat, SFA and MUFA were associated with higher overall symptom severity in the premenstrual phase as well as pain severity specifically. In contrast, in the Study of Women’s Health Across the Nation, Gold et al. ( Reference Gold, Bair and Block 10 ) found total fat intake to be associated with lower cravings/bloating symptoms in older premenopausal US women, but unrelated to the other symptoms assessed, including back pain/cramps, breast pain or headaches.
The inconsistencies in the findings among these studies may be due to several possible reasons: differences in population characteristics such as age and food sources in different geographical reasons, and study design characteristics (i.e. prevalent symptoms v. incident cases, cross-sectional v. prospective). In cross-sectional studies assessing PMS symptoms, temporality cannot be established. It is unclear whether symptomatic women consume foods with higher fat content due to cravings, in order to alleviate symptoms, or whether higher intakes of fat are physiologically involved in the development of premenstrual symptoms.
SFA was inversely associated with the risk of developing PMS in our study, and the association appeared to be largely attributable to stearic acid. The biological mechanism for this is unclear. Although the Dietary Guidelines for Americans suggests limited SFA to <10 % of total kcal/d due to adverse health effects( 36 ), laboratory and clinical evidence suggests that stearic acid may be physiologically different from other SFA( Reference Kris-Etherton and Mustad 37 – Reference Ding, Hutfless and Ding 40 ). For example, stearic acid appears to have a neutral effect or may even lower cholesterol levels as opposed to increasing levels( Reference Kris-Etherton and Mustad 37 , Reference Kelly, Sinclair and Mann 38 ), and may potentially lower risk of breast cancer( Reference Evans, Cowey and Siegal 39 ) and CVD( Reference Kelly, Sinclair and Mann 38 ). A common source of stearic acid is cocoa butter, which is a primary component of chocolate( Reference Ding, Hutfless and Ding 40 ). Many women report chocolate cravings premenstrually and may increase chocolate intake to improve symptoms( Reference Rossignol and Bonnlander 41 ). In post hoc analyses, we evaluated whether chocolate intake may explain the association between stearic acid and PMS risk. When we adjusted for chocolate intake the relationship was slightly attenuated (RR quintile 5 v. quintile 1=0·80; 95 % CI 0·60, 1·07), suggesting that chocolate explained some but not all of the observed association with stearic acid. As no other studies have evaluated the relationship between stearic acid and PMS, additional studies specifically evaluating whether stearic acid intake may reduce PMS risk or be beneficial in treating premenstrual symptoms are warranted along with examining physiological mechanisms.
Several clinical trials have tested the efficacy of treating premenstrual symptoms with fatty acid supplements. Clinical trials of supplementation with n-3s, a mixture of PUFA (γ-linolenic acid (18 : 3n-6), oleic, linoleic, other PUFA and vitamin E)( Reference Rocha Filho, Lima and Pinho Neto 13 ), and krill oil (high in n-3)( Reference Sampalis, Bunea and Pelland 12 ) have suggested that these supplements can alleviate premenstrual symptoms. Doses and durations of supplementation varied; Sohrabi used 2 g of n-3 for one full cycle and then only during the late luteal phase for two cycles( Reference Sohrabi, Kashanian and Ghafoori 11 ). Filho used 1–2 g of the PUFA for 6 months( Reference Rocha Filho, Lima and Pinho Neto 13 ), and Sampalis compared 2 g of krill oil with 2 g fish oil for one full cycle then during the late luteal phase for two more cycles( Reference Sampalis, Bunea and Pelland 12 ). These doses are comparable with our highest quintile of intake for n-3. However, our study did not find an association with risk of PMS for PUFA, oleic, linoleic or n-3 fatty acids. In addition, there was very little supplement use of fish oil or cod liver oil in our study.
We were unable to use prospective symptom charting to assess incident PMS in our large, ongoing prospective cohort. However, we used strict criteria to classify PMS cases and controls, excluding women in the middle of the symptom spectrum and thus reducing the likelihood of misclassification of cases as controls and vice-versa. Women who experience severe symptoms each month and women who experience no symptoms or very minimal symptoms are likely to accurately recall symptom experience and unlikely to be misclassified between the two groups( Reference Bertone-Johnson, Hankinson and Bendich 27 ). In addition, in a previous validation study our method of classifying PMS cases was found to be comparable with methods additionally using report of prospective symptom charting as part of clinical diagnosis( Reference Bertone-Johnson, Hankinson and Johnson 28 ).
Participants may either over-report or under-report their intake of foods containing fats or fatty acids either unintentionally or purposefully due to beliefs about what they should be eating, thus misclassification is likely. However, because dietary intake was collected prospectively, any misclassification of fat or fatty acid intake, therefore would not be related to PMS status and would likely bias result to the null. Moreover, previous validation studies have found fat intakes from FFQ data to be reasonably well correlated with diet record (r>0·41)( Reference Willett 30 ) and several previous studies within the NHS2 suggest that FFQ are sensitive enough to detect associations between fats and outcomes such as endometriosis or CVD at similar ranges of intake( Reference Missmer, Chavarro and Malspeis 42 , Reference Oh, Hu and Manson 43 ). Lastly, the range of intake for total fat and subtypes of fat (i.e. SFA, MUFA, PUFA and trans-fat) within our study was comparable with previous observational studies of PMS( Reference Nagata, Hirokawa and Shimizu 9 , Reference Gold, Bair and Block 10 ).
Overall, we did not find intake of dietary fats to be associated with higher risk of developing PMS, though high intake of stearic acid was associated with a statistically significant lower risk of PMS. As this is the first study to suggest this association and the fact that these findings may be by chance due to multiple testing, additional prospective studies are needed to further evaluate whether stearic acid may reduce risk of developing PMS or improve existing symptoms taking into account any potential adverse effects.
Acknowledgements
The authors thank the participants and staff of the NHS2 for their valuable contributions.
The NHS2 cohort is supported by the NIH grant CA176726. E. R. B.-J. was supported by National Institutes of Health, Department of Health and Human Services grant MH076274; a cy pres distribution from Rexall/Cellasene settlement litigation; and a grant from GlaxoSmithKline Consumer Healthcare.
J. E. M., S. E. H. and E. R. B.-J. designed the research; S. C. H. and E. R. B.-J. conducted the research; J. E. M. and S. E. H. provided essential materials; S. C. H. and E. R. B.-J. performed the statistical analysis; S. C. H. and E. R. B.-J. wrote the paper; B. W. W., L. M. T. and C. B. interpreted study results, reviewed manuscript for important intellectual content and contributed knowledge of underlying biologic mechanisms; S. C. H., J. E. M. and E. R. B-J. had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.






