INTRODUCTION
In 2001, guidance for prevention of hepatitis A changed in the United Kingdom when vaccine, rather than human normal immunoglobulin (HNIG), was recommended as post-exposure prophylaxis for contacts, on condition that it can be given within 1 week of onset of illness in the index case, where onset is defined as the start of jaundice [Reference Crowcroft1, Reference Markov, Ahmed and Crowcroft2]. The condition of use reflected the limited evidence base for using vaccine for post exposure prevention of hepatitis A [Reference Sagliocca3], which is why hepatitis A vaccines are not licensed for post-exposure prophylaxis. Despite this, vaccine is often given late without HNIG [Reference Markov, Ahmed and Crowcroft2]. Reasons given by public health specialists for avoiding use of HNIG include difficulties in obtaining it and concerns about using a human blood product. As these concerns do not appear to limit use of other similar products for exposure to infections such as hepatitis B, chicken pox and rabies, it may be that attitudes are influenced by a perception that hepatitis A is a mild disease, coupled with the apparent rarity of secondary cases. Hepatitis A can, however, have a severe outcome. During 2001–2004, 1–5 deaths per year were certified in England with hepatitis A as the underlying cause [4], and at least two patients with hepatitis A required liver transplantation (A. Mann, personal communication).
Hepatitis A is now infrequent in the United Kingdom, with fewer than 700 cases reported in England and Wales in 2004. In an audit in 2001, the median number of cases of hepatitis A that Consultants in Communicable Disease Control (CsCDC) reported managing was around one case per month [Reference Markov, Ahmed and Crowcroft2]. At such low rates of infection most physicians are unlikely to be able to rely on clinical judgement to evaluate the effectiveness of preventive interventions. In this context, we decided to estimate the number of cases a CCDC would manage before observing a single additional secondary case caused by a failure of vaccine or HNIG to protect a contact of a case of hepatitis A.
METHODS
The scenario of interest was a household setting. The average household size in the United Kingdom in 2004 was 2·3 members [5]. From seroprevalence studies we can assume that all these would be susceptible to hepatitis A [Reference Morris6]. Secondary attack ratios (AR) in susceptible household members were estimated from the literature at 10–25% [Reference Levy7–Reference Victor9]. Effectiveness of HNIG was estimated at 80% [Reference Crowcroft1]. Vaccine is unlikely to be as effective as HNIG in the second week following onset of disease in the index case because of the delay in developing an immune response [Reference Irwin and Millership10]. As post-exposure vaccine efficacy is unknown, we estimated it as between 0 and 80%.
If the average number of secondary cases occurring in each household following a case is r, for a single case occurring in a household of size n, with protective efficacy of intervention k and AR in susceptible contacts a, the following describes the average number of secondary cases per household:
The difference in number of secondary cases between vaccine and HNIG can be calculated for different values of vaccine effectiveness. The inverse of this difference is the average number of households that need to be treated with vaccine before one additional secondary case would occur, compared with HNIG.
RESULTS
The average number of secondary cases per household varies from 0 to 0·3 depending on the level of effectiveness of the intervention and the AR (Tables 1 and 2). In either the conservative or realistic scenarios, the number of secondary cases following use of HNIG is 0·03. The effectiveness of vaccine given late is unknown, but could be lower than 50%. If vaccine effectiveness in the second week was 50% then the average number of households which would need to be treated before one additional secondary case would be observed would be 26 in the conservative scenario (Table 1) or 7·7 in the realistic scenario.
Table 1. Number of cases needed to be managed before one additional case occurs for different levels of vaccine effectiveness and with 1·3 contacts per case [conservative scenario of human normal immunoglobulin (HNIG) efficacy 80% and secondary attack ratio 10%]
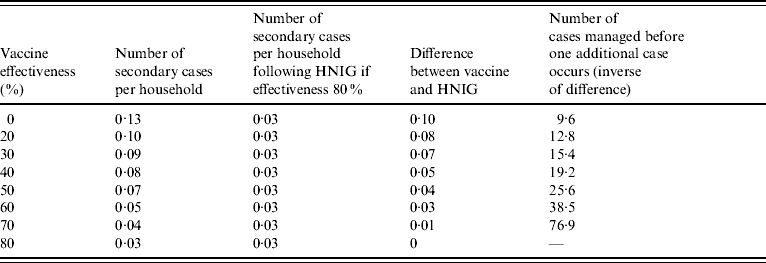
Table 2. Number of cases needed to be managed before one additional case occurs for different levels of vaccine effectiveness and with 1·3 contacts per case [realistic scenario of human normal immunoglobulin (HNIG) efficacy 90% and secondary attack ratio 25%]
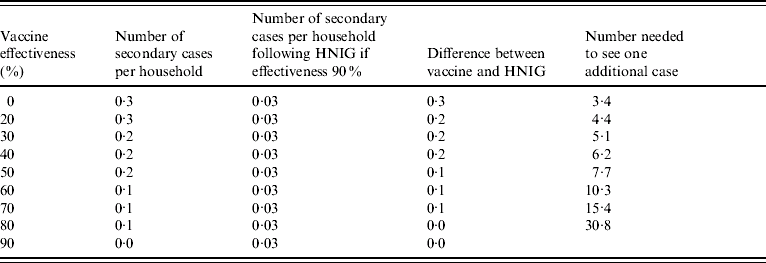
The number of households per secondary case observed depends upon the average size of household, the effectiveness of any intervention and the AR. Varying the average household size from 2 to 6 people changes the number of households per secondary case from 50 to 10 households. Varying the effectiveness of the intervention from 0% to 80% gives a range between 8 and 39 households. Varying the AR between 0% and 25% gives a range of 8–19 households. Household size has the greatest influence on the estimates.
DISCUSSION
Sixty per cent of public health professionals would recommend vaccine for contacts if the onset of illness in the index case was more than 1 week before [Reference Markov, Ahmed and Crowcroft2]. If vaccine efficacy in the second week after onset was 50%, in the conservative scenario 26 households and in the realistic scenario 8 households would need to be treated with vaccine after the first week before one additional case would be observed. Public health professionals in England manage around one case of hepatitis A per month, of which only a proportion are reported more than one week after onset. It would therefore take from 8 months to >2 years for an additional secondary case to occur amongst the households managed by an average CCDC if they used only vaccine, over that which would have been observed if they were also using HNIG. The time may be shorter if the size of households affected by hepatitis A is greater than average, which is plausible. Nevertheless, the difference in outcome is probably not noticeable, but most practitioners would probably agree that a 30% difference is a clinically important lower level of effectiveness.
No vaccine is currently licensed for post-exposure prophylaxis. As antibody has not been detected at fewer than 12 days following vaccination [Reference Irwin and Millership10] the effectiveness of vaccine given more than 1 week after onset in the index case may be less than 50%. In clinical trials, cases of hepatitis A were observed up to 17 days post-vaccination, indicating limited post-exposure efficacy [Reference Wertzberger11]. Vaccine may, like HNIG, have a beneficial impact on reducing clinical severity and infectivity of cases. For an individual household contact, the risk of acquiring hepatitis A is 10–30%, depending on the AR. HNIG, with efficacy of 80–90%, reduces this risk to 1–2%. Vaccine will reduce it to 5–15% if post-exposure vaccine efficacy (for vaccine given more than 1 week later) was 50%. The risk of that individual dying is the product of the risk of their acquiring the infection and the case fatality, and for someone aged >50 years the case fatality may be around 2% [Reference Fiore, Wasley and Bell12]. The risk of death for a household contact aged >50 years would therefore be between 1/200 and 1/500. This would fall to between 1 and 2/5000 after HNIG, compared to between 1 and 3/1000 after vaccine (if efficacy was 50%). The relative risk of death using vaccine would be 2·5–5 times that for HNIG. Patients need to make an informed choice, and will need full information about the possible lower efficacy of vaccine compared with HNIG, the low or theoretical risks of HNIG, and the real risks of acquiring hepatitis A infection. Vaccine has the advantage of providing longer duration of protection and should always be offered in addition to HNIG to individuals in risk groups and during outbreaks, to prevent tertiary cases.
Although policy and practice must be based upon the best available evidence, a shift in both has occurred in the United Kingdom without the evidence to support it. One published study supports using vaccine for post-exposure prophylaxis [Reference Sagliocca13]. Other countries including the United States and Canada have not changed their policy on use of HNIG on the basis of this evidence. In the United Kingdom, sensitivity about the use of blood products post-BSE has led to many professionals avoiding using HNIG. This is a reasonable concern, and the rational response is both to improve the speed of reporting so that contacts do not require HNIG, which appears feasible in other countries [Reference Sagliocca13], and to maximize uptake of pre-exposure vaccine in groups at greatest risk. The perception that hepatitis A is a mild disease is misplaced. Deaths and liver transplantation continue to occur in the United Kingdom as a result of hepatitis A infection. It will be a failure of public health should contacts of a case of hepatitis A develop fulminant hepatic failure or die because they were not offered HNIG, and were given vaccine too late.
ACKNOWLEDGEMENTS
Thanks to Anjna Mistry, Usha Gungabissoon and Amy Glasswell for extracting the hepatitis A mortality data, and Andrea Mann and Mary Ramsay for providing the UK Liver Transplant Registry data.
DECLARATION OF INTEREST
None.




