CASE REPORT
We present the case of a previously well 41-year-old male who presented to our emergency department (ED) after a witnessed syncopal episode. No further history was immediately available. A pre-hospital echocardiogram (ECG) showed ST-segment elevation in leads V1–V3 (Figure 1). Consequently, the patient was accepted to the ST-elevation myocardial infarction (STEMI) ED bypass protocol by our hospital and was being transported for immediate coronary angiography.
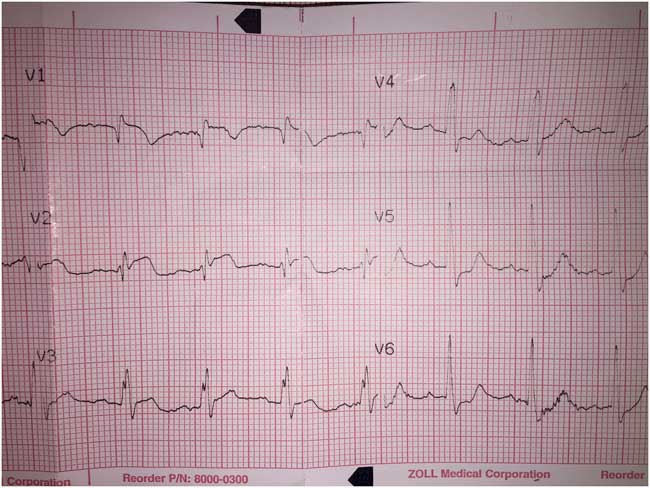
Figure 1 Pre-hospital ECG strip, initially interpreted as anterior ST-elevation MI by our hospital’s STEMI bypass program.
The patient’s clinical condition deteriorated en route, and attending paramedics elected to deliver the patient to the ED instead. Upon arrival at 16:40, the patient was cyanotic, dyspneic, and somnolent. Central pulses were palpable, although non-invasive blood pressure and oxygen saturation were undetectable. The skin of his lower extremities was mottled. Engorged superficial veins were noted across the patient’s abdomen and right flank; examination of the lower extremities revealed clusters of engorged varicose and superficial veins over the medial right thigh. Supplemental oxygen, intravenous (IV) normal saline, and norepinephrine infusion were initiated. His ED ECG showed lateral ST depression, ST elevation in aVR and V1, and an incomplete right bundle branch block pattern (Figure 2). Bedside point-of-care ultrasound (PoCUS), performed in the resuscitation room, revealed a hyperdynamic and partially collapsed left ventricle, dilated right ventricle, and global right ventricular systolic dysfunction (Video 1). A pulmonary embolus (PE) was strongly suspected.
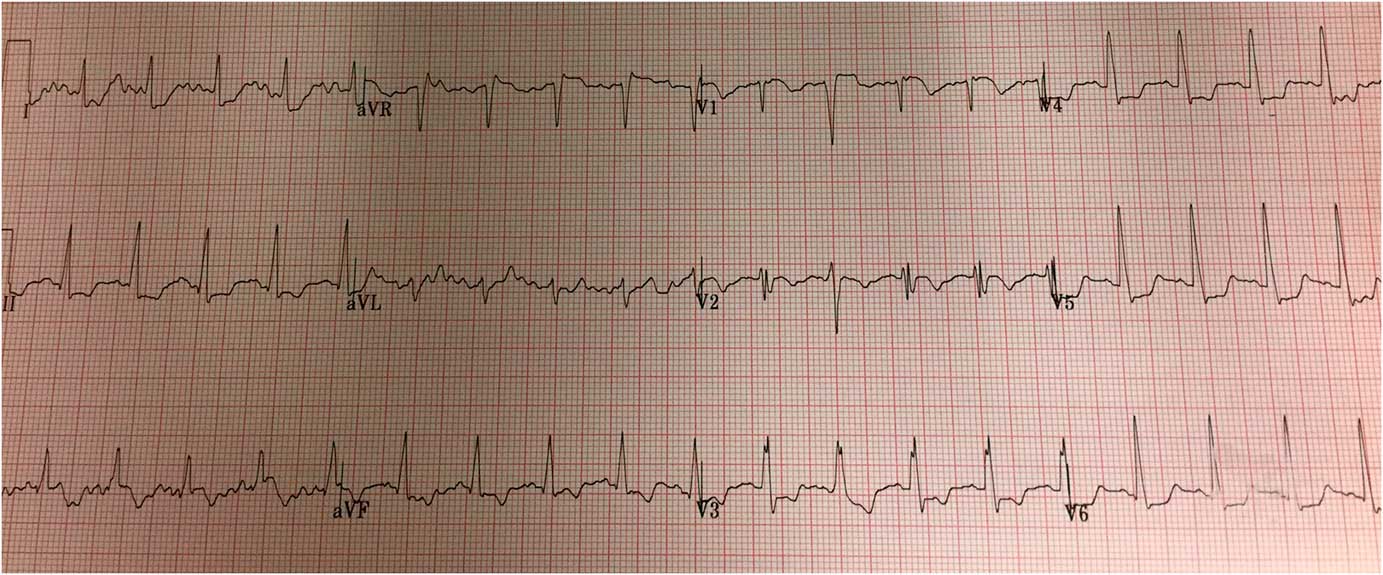
Figure 2 Emergency department 12-lead ECG. Findings include sinus tachycardia, lateral ST depression, ST elevation in aVR and V1, and incomplete RBBB. The QRS duration is 100ms.
The patient’s level of consciousness continued to deteriorate during his initial assessment and resuscitation. At 16:47, uncomplicated rapid sequence intubation was performed using 1 mg/kg of IV ketamine and 1 mg/kg of IV rocuronium. One hundred milligrams of IV alteplase (tPA) was initiated at 16:53; the first 10% was ordered as a bolus, with the remainder infused over one hour. However, the patient experienced a witnessed pulseless electrical activity (PEA) arrest at 16:56. Cardiopulmonary resuscitation (CPR) and standard advanced cardiac life support (ACLS) were initiated immediately, and a total 100 mg of tPA was deliberately infused as a rapid bolus over the next two minutes. After four minutes of CPR, the return of spontaneous circulation (ROSC) was achieved with a blood pressure of 138/90 and heart rate of 109. At 17:23, venous blood gas results were as follows: pH 6.97, pCO2 84, pO2 28, HCO3 12, and lactate 10.2. A portable chest X-ray confirmed clear lung fields bilaterally. An IV heparin bolus was initiated, and the patient was admitted to the intensive care unit (ICU).
After his resuscitation and admission to the hospital, additional medical history became available. Prior magnetic resonance imaging (MRI) demonstrated abnormally large and tortuous saphenous and perforator veins extending the length of the right thigh. The patient had experienced several episodes of right-sided superficial thrombophlebitis, but no prior deep venous thromboembolic (DVT) disease. He had been horseback riding a few days before his presentation, with subsequent shortness of breath and new right thigh discomfort.
Pressor support was successfully weaned overnight, and he was extubated without incident the following morning. That afternoon, the signs and symptoms of hemorrhagic shock were noted, and a large hemoperitoneum, likely from a splenic artery, was detected on abdominal computed tomography (CT). Parenteral anticoagulation was stopped, and five units of packed red cells were transfused. The patient was transferred to the interventional radiology suite, where he underwent successful endovascular coiling of the bleeding splenic vessels and insertion of an inferior vena cava (IVC) filter. On admission day six, the diagnosis of bilateral PE was confirmed by CT pulmonary angiography, which showed clots in the left main pulmonary artery, right upper segmental pulmonary artery, and multiple other smaller vessels. He was discharged from the hospital with no noted neurologic or functional deficits.
Two years after his presentation, our patient has no functional limitations or symptoms of post-thrombotic syndrome and has returned to full-time work in his highly active profession. His IVC filter has been removed, and anticoagulation maintained with rivaroxaban.
DISCUSSION
Diagnosis of high-risk PE
PE is the third most common cause of cardiovascular death and carries a high mortality rate when presenting with hemodynamic instability or cardiac arrest (52%–65%).Reference Er, Nia and Gassanov 1 - Reference Goldhaber, Visani and De Rosa 4 It may account for up to 15% of in-hospital deaths and 2% of out-of-hospital cardiac arrests.Reference Er, Nia and Gassanov 1
The definition of high-risk PE proposed by the European Society of Cardiology and American Heart Association is widely accepted: PE with hemodynamic instability, i.e., signs of shock requiring inotropic support, documented systolic blood pressure <90 mm Hg for >15 minutes, or both.Reference Konstantinides, Torbicki and Agnelli 5 - Reference Condliffe, Elliot and Hughes 8
Definitive diagnosis of PE in critically ill and peri-arrest ED patients remains a significant challenge. In many centres, D-dimer results may not be available within a clinically appropriate time frame. In patients who arrested, elevated D-dimer levels are also associated with other critical illnesses and systemic endothelial injury.Reference Deng, He, Yang and Wang 9 Most are too unwell for transfer to the radiology suite for imaging. Furthermore, clinicians may not always consider the diagnosis of PE if confronted with a peri-arrest patient, particularly if the history and collateral information are limited. Therefore, the clinician must rely on select historical points, physical exam findings, and bedside diagnostic tests:
1. History: prior DVT or PE; clotting disorder; active cancer; and recent immobilization, airline travel, or orthopedic surgery.Reference Logan, Pantle, Huiras, Bessman and Bright 10
2. Physical exam: unilateral leg swelling; oxygen saturation <95%; witnessed cardiac arrest; and initial arrest rhythm PEA (particularly with narrow QRS).Reference Logan, Pantle, Huiras, Bessman and Bright 10
3. ECG: precordial T-wave inversion; precordial ST depression; precordial Qr pattern; S1Q3T3; new right bundle branch block; right or indeterminate axis deviation; and ST elevation in aVR.Reference Livaditis, Paraschos and Dimopoulos 11 , Reference Ferrari, Imbert and Chevalier 12
4. PoCUS: right ventricular dilation, hypokinesis, or both; and non-compressible popliteal, femoral veins (suggesting concomitant DVT), or both.Reference Squizzato, Galli and Gerdes 13 , Reference Come 14
Anterior ST elevation, noted in our patient’s pre-hospital ECG, is uncommon in acute PE, though it has been described.Reference Livaditis, Paraschos and Dimopoulos 11 , Reference Lin, Lin and Yang 15 , Reference Falterman, Martinez, Daberkow and Weiss 16 Proposed mechanisms include right ventricular strain and injury because of increased afterload; rarely, this finding can reflect coronary occlusion due to a clot through a patent foramen ovale.Reference Lin, Lin and Yang 15 , Reference Cheng 17 It is often visually indistinguishable from the ST elevation seen in an acute myocardial infarction; however, the simultaneous (or subsequent) presence of more specific ECG findings may raise the index of suspicion for PE at a patient’s bedside.Reference Lin, Lin and Yang 15 In patients with cardiogenic shock, anterior T-wave inversions and ST elevation in leads aVR and V1 are associated with PE.Reference Ferrari, Imbert and Chevalier 12 , Reference Kukla, McIntyre and Fijorek 18
In addition to the previously listed PoCUS findings, bedside diagnosis of PE is based on the visualization of a thrombus in the right heart as has been described.Reference Sharifi, Berger and Beeston 19 While this particular finding was not noted during resuscitation in our case, a subsequent review of our patient’s bedside ultrasound images did reveal echogenic material in the right pulmonary artery (Figure 3). Clinically, the presence of a grossly abnormal right ventricular shape and function on PoCUS contributed significantly to our immediate treatment decisions. It is important to note that bedside echocardiography alone cannot safely rule out PE.Reference Konstantinides, Torbicki and Agnelli 5 , Reference Squizzato, Galli and Gerdes 13 Nevertheless, the presence of particular findings on PoCUS in the context of a suspicious clinical presentation may strongly suggest the diagnosis.Reference Squizzato, Galli and Gerdes 13 Other than a visible clot, specific echocardiographic findings reported in patients with PE are all consistent with right ventricular (RV) strain or overload: increased RV size, decreased RV function (both noted in our case), and tricuspid regurgitation.Reference Come 14
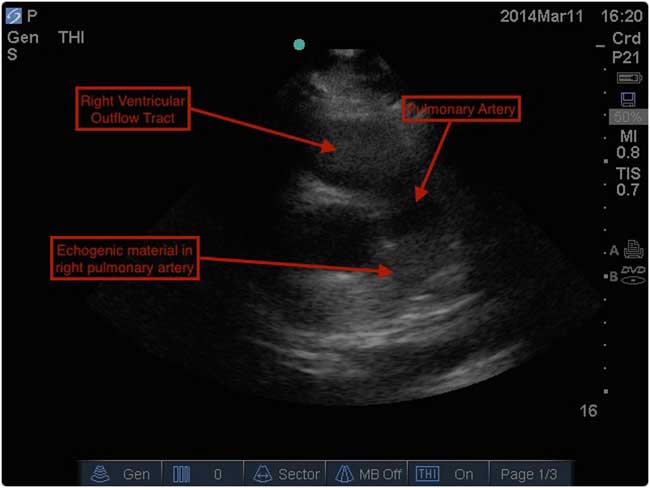
Figure 3 PoCUS still image showing echogenic material, suspicious for clot, in the patient’s right pulmonary artery.
Treatment of high-risk PE
Systemic thrombolysis is the standard of care in patients who present with high-risk PE, barring any contraindications (Figure 4).Reference Konstantinides, Torbicki and Agnelli 5 , Reference Jaff, McMurtry and Archer 6 , Reference Moorjani and Price 20 - Reference Kearon, Akl and Comerota 22 Existing literature has repeatedly confirmed a mortality benefit in patients with a high risk of PE.Reference Wan, Quinlan, Agnelli and Eikelboom 23 - Reference Le Conte, Huchet and Trewick 26 The risk of death is reduced from 19.0% to 9.4% (absolute risk reduction [ARR] 9.6%), as compared with anticoagulation alone.Reference Wan, Quinlan, Agnelli and Eikelboom 23 The most well-studied and widely recommended systemic thrombolytic regimen for patients with PE who have a pulse is alteplase, 100 mg IV over two hours.Reference Kearon, Akl and Comerota 22 , Reference Lankeit and Konstantinides 27 This regimen has been associated with earlier improvements in total pulmonary resistance, as compared with both older agents such as streptokinase (given over 12–24 hours)Reference Meneveau, Schiele and Metz 28 or shorter infusions of alteplase of 0.6 mg/kg over 15 minutes.Reference Sors, Pacouret and Azarian 29 However, clinical outcomes and complication rates were similar across patient groups.Reference Meneveau, Schiele and Metz 28 , Reference Sors, Pacouret and Azarian 29
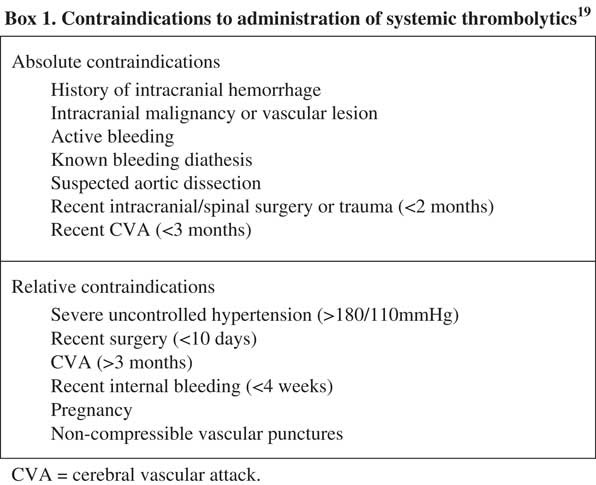
Figure 4 Contraindications to administration of systemic thrombolyticsReference Sharifi, Berger and Beeston 19
Thrombolytic therapy carries a risk of major bleeding, including intracranial hemorrhage (ICH), with a number needed to harm of 11–176 quoted in the literature.Reference Chatterjee, Chakraborty and Weinberg 7 , Reference Wan, Quinlan, Agnelli and Eikelboom 23 In patients without absolute contraindications, the overall incidence of ICH after thrombolytic therapy was 0.9%–3%.Reference Goldhaber, Visani and De Rosa 4 , Reference Stein, Matta, Steinberger and Keyes 30 , Reference Konstantinides and Marder 31 Approximately 20% of patients would experience a major hemorrhageReference Goldhaber, Visani and De Rosa 4 , Reference Fiumara, Kucher, Fanikos and Goldhaber 32 ; gastrointestinal is most common, followed by retroperitoneal and intracranial.Reference Fiumara, Kucher, Fanikos and Goldhaber 32 Predictors of major bleeding are relatively understudied but may include the need for vasoactive agents, malignancy, diabetes, recent bleeding, renal failure, dual anti-platelet therapy, and elevated international normalized ratio (INR).Reference Fiumara, Kucher, Fanikos and Goldhaber 32 Bleeding risk is likely lower in those under age 65.Reference Chatterjee, Chakraborty and Weinberg 7
Because of this bleeding risk, recent literature has focused on the safety and efficacy of reduced doses of thrombolytics for the treatment of PE. A randomized trial comparing 50 mg per two hours of rt-PA with 100 mg per two hours of rt-PA demonstrated similar improvements in hemodynamic parameters, with significantly fewer bleeding episodes in the 50 mg group.Reference Wang, Zhai and Yang 33 A subsequent prospective cohort of 98 patients with a PE (14 with a “severe PE”) was treated with 50 mg of IV alteplase over two hours, followed by unfractionated heparin and rivaroxaban, and no in-hospital deaths or bleeding episodes were reported.Reference Sharifi, Bay, Schwartz and Skrocki 34 These dosing regimens may offer acceptable safety and efficacy in the treatment of patients with PE who have a pulse.
If systemic thrombolysis is contraindicated, alternative treatment options do exist. Depending on local resources and expertise, surgical thrombectomy or catheter-directed therapies may be appropriate. Recently published guidelines also recommend considering these modalities if systemic thrombosis has failed.Reference Konstantinides, Torbicki and Agnelli 5 , Reference Kearon, Akl and Comerota 22 The published risk of death with surgical thrombectomy is 5.3%–27%, perhaps as high as 46% in the elderly.Reference Fukuda, Taniguchi, Fukui, Minakawa, Daitoku and Suzuki 35 - Reference Stein and Matta 37 Published evidence supporting catheter-directed therapies in high-risk patients, including intravascular thrombolytics, suction, ultrasound-assisted, or rotational embolectomy, does not clearly favour one approach over others.Reference Tapson 38 The incidence of hemorrhagic and mechanical complications (e.g., dissection, perforation, and arteriovenous [AV] fistulization) is approximately 10%.Reference Kuo, Gould and Louie 39 Published rates of ICH are lower than those observed with systemic lysis (<1%).Reference Mostafa, Briasoulis, Telila, Belgrave and Grines 40 IVC filter placement is not routinely recommended for any patients with PE but may be considered in those with absolute contraindications to ongoing anticoagulation, including a major hemorrhage.Reference Konstantinides, Torbicki and Agnelli 5 , Reference Kearon, Akl and Comerota 22 , Reference Walter, Moores and Jiménez 41
In pulseless patients with PE, proposed thrombolytic regimens have included:
∙ tenecteplase 50 mg IV bolusReference Perrott, Henneberry and Zed 42
∙ alteplase ~80 mg IV bolus (dose chosen by treating physician)Reference Er, Nia and Gassanov 1
∙ alteplase 50 mg IV bolus, followed by 50 mg 30 minutes laterReference Ruiz-Bailén, Aguayo-de-Hoyos and Serrano-Córcoles 43
∙ alteplase 50 mg IV bolusReference Sharifi, Berger and Beeston 19
∙ alteplase 0.6–1.0 mg/kg (maximum dose 100 mg) IV bolusReference Janata, Holzer and Kürkciyan 44
As in the treatment of high-risk PE with a pulse, alteplase is the thrombolytic treatment of choice for PE in cardiac arrest.Reference Er, Nia and Gassanov 1 , Reference Sharifi, Berger and Beeston 19 , Reference Perrott, Henneberry and Zed 42 - Reference Janata, Holzer and Kürkciyan 44 Its recommended shorter infusion time is more practical in critical illness and may promote more rapid clot lysis, as compared with the prolonged infusions of older agents such as streptokinase and urokinase.Reference Kearon, Akl and Comerota 22 , Reference Tapson 38 , Reference Walter, Moores and Jiménez 41 While case reports and case series can only provide limited quality and potentially biased evidence supporting specific drug regimens, the available literature does confirm the administration of rapid IV bolus alteplase (i.e., over minutes, rather than two hours) had encouraging outcomes in patients who experienced a cardiac arrest due to PE. Successful thrombolysis with tenecteplase, using weight-based dosing protocols extrapolated from the myocardial infarction literature, has also been reported in small numbers of cardiac arrest patients with suspected PE.Reference Logan, Pantle, Huiras, Bessman and Bright 10 , Reference Livaditis, Paraschos and Dimopoulos 11 The PEITHO study provided higher-level evidence for the use of bolus tenecteplase in PE, though this randomized controlled trial recruited only intermediate-risk patients.Reference Meyer, Vicaut and Danays 45 While its recommended administration as a rapid IV bolus makes it attractive for use during CPR, tenecteplase is not currently approved for this indication.
Traditionally, concern about hemorrhage precipitated by prolonged CPR has limited the use of systemic thrombolytics in arrested patients.Reference Janata, Holzer and Kürkciyan 44 , Reference Li, Fu and Jing 46 - Reference Newman, Greenwald and Callaway 47 Alteplase has the shortest half-life of all thrombolytics (4–10 minutes) and requires the presence of fibrin for activation; this may reduce the risk of bleeding complications by limiting the duration and extent of non-pathologic clot lysis.Reference Logan, Pantle, Huiras, Bessman and Bright 10 Furthermore, a retrospective review of 66 patients with confirmed PE and cardiac arrest, treated with systemic thrombolytics, did not find an increased incidence of bleeding if the duration of CPR exceeded 10 minutes.Reference Janata, Holzer and Kürkciyan 44 In patients who arrested with a high suspicion of PE and contraindication to thrombolysis (Figure 4), a clinician must decide whether to treat based on the individual patient’s presentation, goals of care, and perceived risk of hemorrhage in each case.Reference Tapson 38
CONCLUSION
We administered a 100 mg bolus of alteplase to a young, previously well patient with a high-risk PE. This treatment was associated with rapid ROSC, sustained hemodynamic stability, and neurologically intact survival after cardiac arrest, but also with a major hemorrhage requiring intervention. PoCUS may prove especially valuable in making a prompt working diagnosis in critically ill patients. Optimal thrombolytic agent and dosing in cardiac arrest remain unclear.
Competing interests: None declared.






