Hypertension is a major health concern worldwide, and studies have shown an association between high-salt (HS) diets and hypertension(Reference Kotchen and McCarron1–Reference Polonia, Martins and Pinto4). Obesity is the main risk factor for primary hypertension, increasing cardiovascular mortality worldwide and hypertension-related deaths(Reference Hall and Hall5–Reference Kung and Xu7). Child obesity and weight gain during infancy have been associated with high blood pressure (BP), high fasting TAG levels and other metabolic risk factors independent of birth weight(Reference Ekelund, Ong and Linné8). These findings suggest that nutritional or environmental factors during infancy can raise the risk of obesity and CVD later in life.
It has been demonstrated that HS diets stimulate hyperphagic conditions in both humans and animals, increase subcutaneous fat mass and serum leptin and predispose to diabetes and obesity(Reference Kitada, Daub and Zhang9–Reference Libuda, Kersting and Alexy12). Moreover, it is well defined that HS intake is the most common cause for high BP, which can lead to cardiovascular alterations and pathologies(Reference He, Li and MacGregor13). According to the large reports that HS intake increases the risks of hypertension and deaths from CVD, several societies worldwide recommend consumption between 3·75 and 6 g/d; however, daily salt intake is higher than 10 g/d in various regions of the world(Reference He, Li and MacGregor13,Reference Brown, Tzoulaki and Candeias14) . Recently, Li et al. (Reference Li, Jin and Yu15) showed that overweight and obese individuals are prone to consuming HS diets, increasing the risk of pathologies such as hypertension. Effects of HS intake in utero and early life on cardiovascular functions have been explored, revealing the deleterious effects of HS on cardiovascular parameters(Reference Moreira, da Silva and Silveira16,Reference Ding, Lv and Mao17) .
Cardiovascular homoeostasis involves complex mechanisms, including hormonal, neuronal and other regulation factors. The role of the renin–angiotensin system (RAS) in the development of cardiac hypertrophy and fibrosis induced by a HS diet is well established(Reference Yamazaki, Komuro and Shiojima18). In addition, a higher cardiac angiotensin II (AngII) signalling was induced by HS diets(Reference Ferreira, Katayama and Oliveira19,Reference Katayama, Pereira and Dopona20) . Angiotensin type-1 receptor (AT1) expression is increased in the heart of rats fed a HS diet that develop cardiac hypertrophy, fibrosis and increased oxidative stress(Reference Katayama, Pereira and Dopona20,Reference Baker21) . In addition, obesity and HS diet also impair baroreflex sensitivity, which is positively correlated with the development of hypertension(Reference Moreira, da Silva and Silveira16,Reference La Rovere, Pinna and Raczak22,Reference Skrapari, Tentolouris and Perrea23) . Likewise, obesity induces raised serum AngII levels, through increased Angiotensinogen gene expression, that is correlated with obesity-induced hypertension(Reference Kalupahana, Massiera and Quignard-Boulange24,Reference Yiannikouris, Gupte and Putnam25) . On the other hand, the activation of AngII type-2 receptor by the Ang II, and the activation of the MAS receptor by the angiotensin-(1-7), has opposite effects to those observed by activation of AT1 receptor(Reference Xu, Carretero and Liu26,Reference Xu, Costa-Goncalves and Todiras27) . However, the effects presented by the majority of the studies evaluated the chronic effects of continuous HS diets, without considering the effects of early-life HS exposure on cardiovascular health at adulthood.
The present study hypothesised that obesity, induced by postnatal early overnutrition (PO), together with a HS diet during puberty negatively programme cardiovascular function, which can be associated with cardiac hypertrophy, fibrosis and changes in cardiac RAS and antioxidant defence in adult rats. Therefore, we aimed to assess the late effects of HS during puberty in both obese and non-obese rats on cardiovascular parameters, including body composition, baroreflex index, heart morphology and cardiac RAS receptors.
Materials and methods
Ethical approval
The handling of animals and experimental procedures were performed according to ARRIVE guidelines, the rules of the National Council of Animal Experiments Control and the Brazilian Society of Science in Laboratory Animals and approved by the Ethics Committee on Animal Use of Federal University of Goiás – CEUA/UFG (protocol no. 051/2017).
Experimental design and treatment
Male and female Wistar rats (70-d old), provided by the Central Animal Facility House of the Federal University of Goiás, were housed in the Animal Facility House of the Department of Physiological Sciences of the Federal University of Goiás in polypropylene cages (45 × 30 × 15 cm), maintained on a 12 h light–12 h dark cycle (07.00 hours lights on) and controlled temperature (22·0°C ± 2°C). After 1 week of adaptation, the animals were mated in a ratio of two females (n 24) to each male (n 12). Pregnant rats were accommodated in individual cages throughout the pregnancy period. At delivery (PN1), pregnant rats were divided into two groups: normal litter (NL – twelve dams) and small litter (SL – twelve dams). At third postnatal day (PN3), NL were adjusted to nine pups (six males and three females) and SL litters were adjusted to three pups (three males), and these numbers were maintained throughout the lactation period. At weaning (PN21), offspring were housed in polypropylene cages (three animals/cage). The offspring from both groups were fed with standard rodent chow (Nuvilab), containing 63 g/kg carbohydrate, 23·7 g/kg protein, 4·1 g/kg fat and 0·27 g/kg NaCl, and had unlimited access to food and water.
At PN30, NL and SL offspring were subdivided into two groups: NL plus HS solution (NL + HS – eighteen animals from six different litters) and SL plus HS solution (SL + HS – eighteen animals from six litters). Animals from HS groups were treated with 0·3 m NaCl from PN30 until PN60, in drinking water, while to the respective control groups (NL and SL – eighteen animals from six different litters per group) was provided tap water. After the treatment period, tap water was provided to both groups until PN120. Food intake, liquid intake and body weight were evaluated every 5 d from PN30 until PN120.
Tail systolic blood pressure measurement
At PN60, PN90 and PN120, systolic BP (SBP) was measured by tail plethysmography using a tail-cuff sphygmomanometer (Harvard Apparatus) in conscious rats (n 10–15 per group) pre-warmed at approximately 37°C to promote caudal artery dilatation. Data were digitised at a frequency of 2 kHz using an analogue-digital converter (PowerLab, ADInstruments).
Cardiovascular parameters measurement
At PN120, a batch of animals (n 8–12/group) were anaesthetised (Ketamine 100 mg and Xylazine 10 mg/kg of body weight; Syntec), submitted to the implant of a polyethylene catheter into the abdominal aorta through the right femoral artery for pulsatile arterial pressure recording, and an implant of another catheter into vena cava through the right femoral vein for drug administration. After 24 h, cardiovascular parameters were recorded in conscious animals. BP signal was obtained by connecting the arterial catheter to a pressure transducer (MLT0699, ADInstruments) coupled to an analogue amplifier (Bridge Amp, FE221, ADInstruments). Data were acquired at a frequency of 2 KHz using an analogue-digital converter (PowerLab 4/25, ML845, ADInstruments). SBP, diastolic BP, mean arterial pressure (MAP) and heart rate (HR) were obtained from the pulsatile arterial pressure, performed by LabChart software (LabChart 7 v7.3.7; ADInstruments). Basal MAP, SBP, diastolic BP and HR were assessed after a 30-min period for stabilisation.
Baroreflex and chemoreflex assessment
After baseline recordings, animals received intravenous bolus injection (0·1 ml) of three doses of phenylephrine, an adrenergic agonist (1·0, 1·5 and 2·0 μg; Sigma-Aldrich), or sodium nitroprusside, a nitric oxide donor (10·0, 15·0 and 20·0 μg; Sigma-Aldrich), to evaluate the baroreflex sensitivity. Baroreflex index was evaluated by phenylephrine bradycardic effect and sodium nitroprusside tachycardic effect. The quantitative analysis of the baroreceptor sensitivity was performed by the ratio of ΔHR:ΔMAP and expressed in Δbpm/ΔmmHg.
Chemoreflex was evaluated by intravenous bolus injection (0·1 ml) of a single dose of potassium cyanide (4·0 μg; Sigma-Aldrich), after baseline MAP and HR recordings and baroreflex assessment. The quantitative analysis of the chemoreceptor sensitivity was performed by the ratio of ΔHR:ΔMAP and expressed in Δbpm/ΔmmHg.
Euthanasia and sample collection
After BP recording, the animals were anaesthetised with sodium thiopental (20 mg/kg of body weight, intravenous; Thiopentax, Cristalia) and euthanised for heart, tibia and white adipose tissue samples (periepididymal, retroperitoneal, mesenteric and inguinal) collection. Heart samples were weighed, the apex was stored at –80°C for Western blot analysis and middle part of the heart fixed in 10 % formaldehyde for histological analysis. Tibia samples were measured, and white adipose tissue samples were weighed. Heart mass index was calculated by the ratio of heart mass:tibia length, and the results were expressed as g/cm.
Histological analysis
Paraffin-embedded heart samples were sectioned in non-serial cuts of 6 μm thickness and stained with haematoxylin–eosin, to cardiomyocyte diameter measurement, and picrosirius red to perivascular and interstitial fibrosis measurement. The morphometric analyses were performed using digital images (TIFF 24-bit colour, 2560 × 1920 pixels) obtained with a digital camera coupled to a light microscope (ICC50 HD plus DM500, Leica Microsystems). The diameter of twenty cardiomyocytes was measured using twenty digital images (×1000 magnification) from each animal (n 5 animals/group). Perivascular and interstitial fibrosis were measured using twenty and twenty-five digital images (×400 and ×100 magnification), respectively, from each animal (n 5 animals/group). Perivascular fibrosis index was determined by the ratio of total fibrosis area to the area of vessel lumen. Interstitial fibrosis was analysed by stereology, using a grid made up of 300 test points, and interstitial fibrosis percentage was estimated by the ratio between the number of points marked by collagen and total test points. Cardiomyocytes diameter and perivascular fibrosis index analyses were performed using ICY software (Institut Pasteur). Interstitial fibrosis was done using Image Pro Plus version 6 (Media Cybernetics).
Western blot
Left ventricle samples (n 4 animals from different litters to each group) were processed as previously described(Reference Junior, Cavalcante and Ferreira28). Briefly, samples were griound in ice-cold lysis buffer, the lysates were centrifuged and the supernatants collected. The supernatants were quantified, and equal amounts of protein were denatured in Laemmli buffer. Aliquots containing 40 μg of protein were submitted to separation by SDS-PAGE and then transferred to nitrocellulose membranes. Membranes were incubated with specific antibodies for proteins of interest (Table 1), according to manufacturer instructions, and chemiluminescence was detected by an image documentation system (ImageQuant LAS 4000 series, GE Healthcare). The intensity of the bands was quantified by relative optical density using FIJI software (ImageJ, National Institutes of Health). Glyceraldehyde-3-phosphate dehydrogenase was used as load control.
Table 1. List of antibodies used for Western immunoblotting
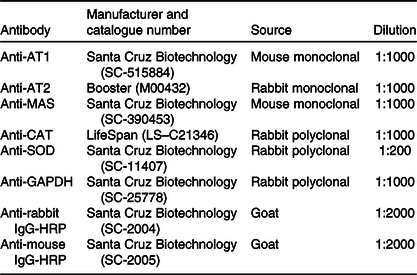
AT1, angiotensin type-1 receptor; AT2, angiotensin II type-2 receptor; MAS, MAS receptor; CAT, catalase; SOD, superoxide dismutase 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HRP, horseradish peroxidase.
Statistical analysis
Our main interest was to detect how BP would behave in the presence of both factors, obesity and high Na consumption. For this, a minimum variation of 10 % in mean BP was estimated, based on the previous studies of our group as effect of those interventions(Reference Moreira, da Silva and Silveira16,Reference Junior, Cavalcante and Ferreira28) . For a power of 80 % and a significance level of 5 %, this allows detection of a difference of 14 % between the groups, with a minimum sample size of eight rats/group.
Statistical analyses and graph construction were done using GraphPad Prism software (version 6, GraphPad Software, Inc.). The results are expressed as mean values with their standard errors. Two-way ANOVA, with Tukey’s post hoc test, was used to evaluate body weight, food and liquid intake from PN30 until PN120. The other data were analysed by one-way ANOVA, with Tukey’s post hoc test. The level of significance was set at P < 0·05.
Results
Effect of postnatal early overnutrition and high salt intake during puberty on food and liquid intake and body composition
Fig. 1(a) shows the evolution of body weight. As expected, SL rats had greater body weight gain from PN30 until PN120 compared with NL rats (P < 0·05; Fig. 1(a)). However, NLHS rats showed less body weight gain from PN45 until PN75 compared with NL rats (P < 0·05; Fig. 1(a)). In addition, SLHS rats showed less body weight gain from PN40 until PN120 compared with SL rats (P < 0·05; Fig. 1(a)).
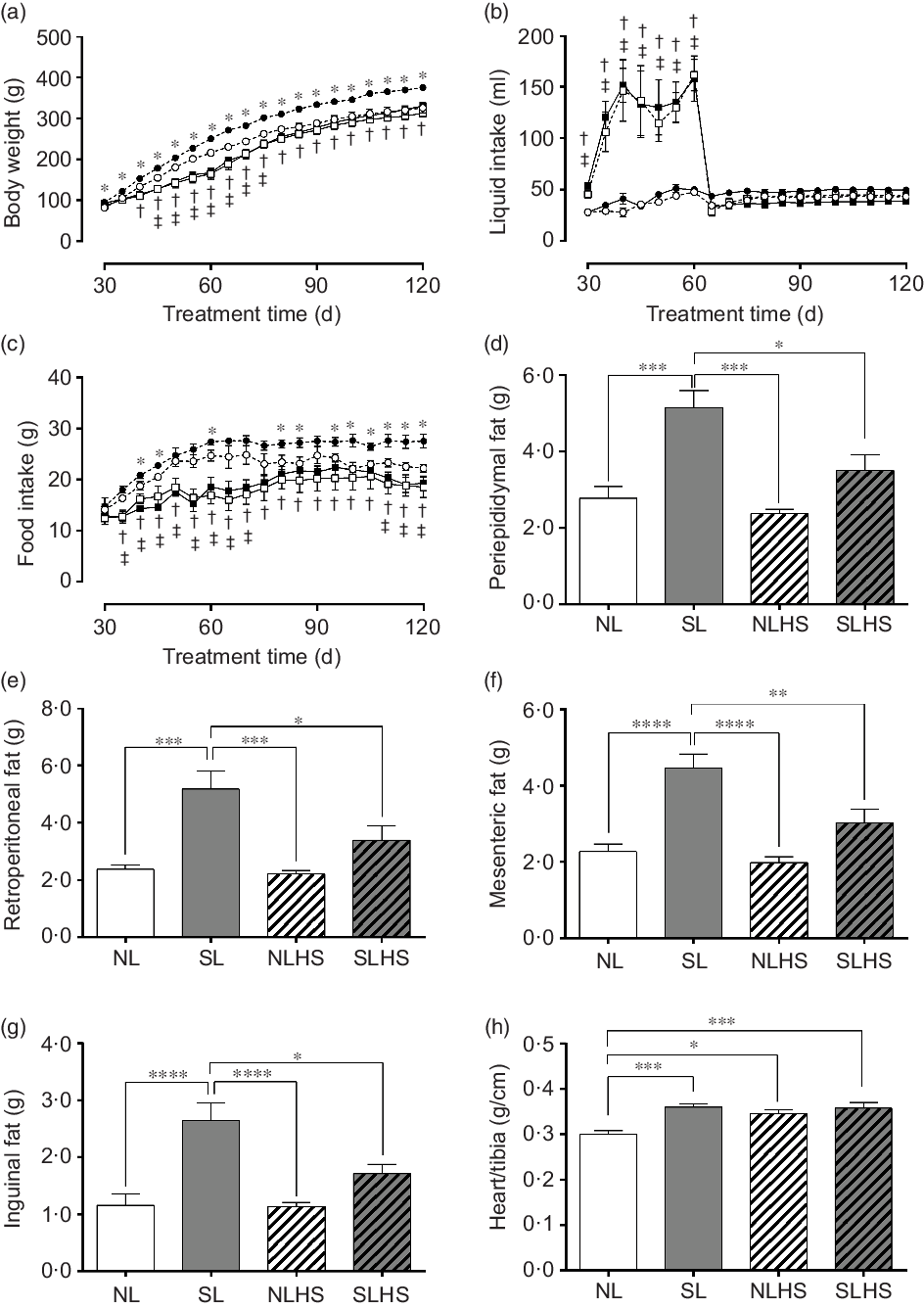
Fig. 1. Effect of postnatal early overnutrition and high salt (HS) intake during puberty on food and liquid intake and body composition. Body weight (a), liquid intake (b) and food intake (c). Periepididymal (d), retroperitoneal (e), mesenteric (f) and inguinal (g) white adipose tissue (WAT) mass. Heart/tibia index (h). Data are mean values with their standard errors (n 12–15). To compare the experimental groups, two-way ANOVA followed by Tukey’s post hoc test (a–c) was used. NL, normal litter; SL, small litter; NLHS, NL plus HS; SLHS, SL plus HS. One-way ANOVA (d–g); * P < 0·05, ** P < 0·01, *** P < 0·001, **** P < 0·0001, † SL v. SLHS (P < 0·05); ‡ NL v. NLHS (P < 0·05). (a)  , NL;
, NL;  , SL;
, SL;  , NLHS;
, NLHS;  , SLHS. (b)
, SLHS. (b)  , NL;
, NL;  , SL;
, SL;  , NLHS;
, NLHS;  , SLHS. (c)
, SLHS. (c)  , NL;
, NL;  , SL;
, SL;  , NLHS;
, NLHS;  , SLHS.
, SLHS.
During the HS treatment period, liquid intake was significantly higher in the groups NLHS and SLHS in relation to NL and SL, respectively (P < 0·05; Fig. 1(b)). However, from PN65 until PN120, both NLHS and SLHS groups returned to normal levels of liquid intake (Fig. 1(b)). SL animals showed greater food intake compared with NL animals (P < 0·05; Fig. 1(c)). However, NLHS and SLHS animals showed reduced food intake in relation to NL and SL, respectively (P < 0·05; Fig. 1(c)). The SL group had greater periepididymal (P < 0·001; Fig. 1(d)), retroperitoneal (P < 0·001; Fig. 1(e)), mesenteric (P < 0·0001; Fig. 1(f)) and inguinal (P < 0·0001; Fig. 1(g)) white adipose tissue mass compared with the NL group. Nevertheless, SLHS animals showed significantly lower adiposity (P < 0·05; Fig. 1(d–g)) compared with SL animals. HS intake did not change body adiposity in the NL group at adulthood (Fig. 1(d–g)). Furthermore, the SL, NLHS and SLHS groups had greater heart mass index as compared with the NL group (P < 0·05; Fig. 1(h)).
Effect of postnatal early overnutrition and high salt intake during puberty on systolic blood pressure
Fig. 2 shows SBP measured by tail-cuff plethysmography. SL and SLHS animals presented higher values of SBP at PN60 (P < 0·05; Fig. 2(a)), PN90 (P < 0·05; Fig. 2(b)) and PN120 (P < 0·01; Fig. 2(c)) compared with NL animals. SBP was not different in NLHS animals compared with NL animals (Fig. 2(a–d)).

Fig. 2. Effect of postnatal early overnutrition and high salt (HS) intake during puberty on systolic blood pressure (SBP). SBP at postnatal day 60 (PN60) (a), PN90 (b) and PN120 (c). Data are mean values with their standard errors (n 10–15). To compare the experimental groups, one-way ANOVA followed by Tukey’s post hoc test was used. NL, normal litter; SL, small litter; NLHS, NL plus HS; SLHS, SL plus HS. * P < 0·05, ** P < 0·01, *** P < 0·001.
Effect of postnatal early overnutrition and high salt intake during puberty on cardiovascular parameters
Fig. 3 shows cardiovascular parameters evaluated in conscious animals at PN120. The SL, NLHS and SLHS groups showed greater SBP in relation to the NL group (P < 0·01; Fig. 3(a)). Similarly, diastolic BP and MAP were higher in SL, NLHS and SLHS rats compared with the NL group (P < 0·01; Fig. 3(b and c), respectively). Only the SLHS group showed increased HR compared with NL and SL groups (P < 0·05; Fig. 3(d)).
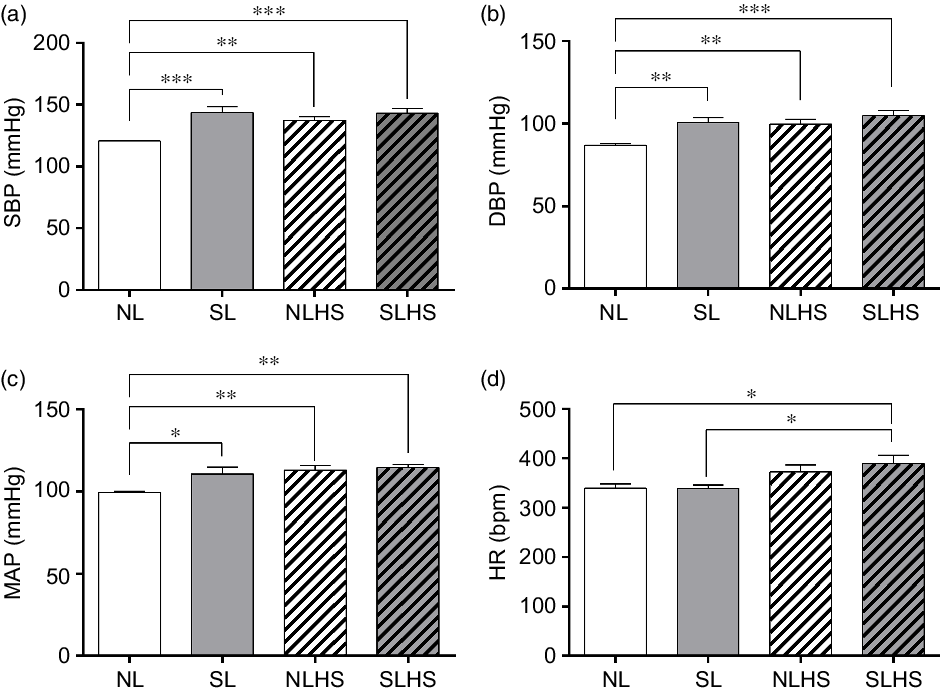
Fig. 3. Effect of postnatal early overnutrition and high salt (HS) intake during puberty on cardiovascular parameters. Systolic blood pressure (SBP) (a), diastolic blood pressure (DBP) (b), mean arterial pressure (MAP) (c) and heart rate (d) at postnatal day 120. Data are mean values with their standard errors (n 8–12). To compare the experimental groups, one-way ANOVA followed by Tukey’s post hoc test was used. NL, normal litter; SL, small litter; NLHS, NL plus HS; SLHS, SL plus HS. * P < 0·05, ** P < 0·01, *** P < 0·001.
Effect of postnatal early overnutrition and high salt intake during puberty on baroreflex and chemoreflex
Both NLHS and SLHS animals showed a decrease in the bradycardic index induced by phenylephrine compared with NL animals (P < 0·05; Fig. 4(a)). No changes in the bradycardic index were observed in SL rats (Fig. 4(a)). On the other hand, tachycardic index induced by sodium nitroprusside was lower in the SL and NLHS groups when compared with the NL group (P < 0·05; Fig. 4(b)). Chemoreflex index induced by potassium cyanide was higher in SL and NLHS animals compared with NL animals (P < 0·05; Fig. 4(c)). SLHS animals did not present differences in tachycardic and chemoreflex indexes compared with the SL group.
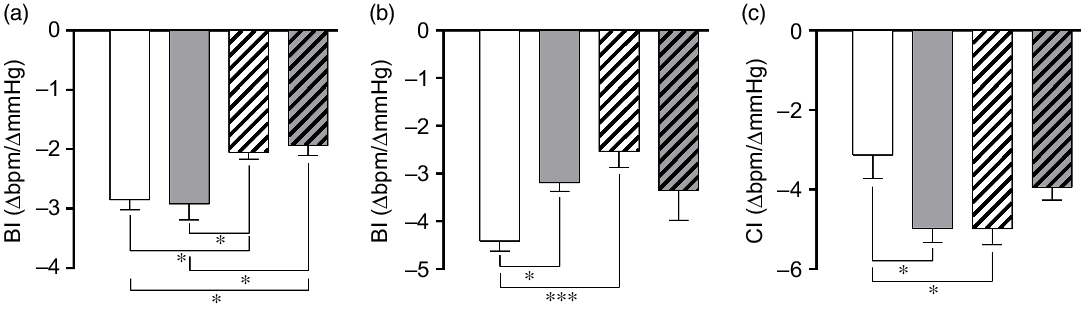
Fig. 4. Effect of postnatal early overnutrition and high salt (HS) intake during puberty on baroreflex and chemoreflex. Bradycardic baroreflex index (BI) induced by phenylephrine (a), tachycardic BI induced by sodium nitroprusside (b) and chemoreflex index (CI) induced by potassium cyanide (c). Data are mean values with their standard errors (n 8–12). To compare the experimental groups, one-way ANOVA followed by Tukey’s post hoc test was used. NL, normal litter; SL, small litter; NLHS, NL plus HS; SLHS, SL plus HS. * P < 0·05, *** P < 0·001. (a)  , NL;
, NL;  , SL;
, SL;  , NLHS;
, NLHS;  , SLHS. (b)
, SLHS. (b)  , NL;
, NL;  , SL;
, SL;  , NLHS;
, NLHS;  , SLHS. (c)
, SLHS. (c)  , NL;
, NL;  , SL;
, SL;  , NLHS;
, NLHS;  , SLHS.
, SLHS.
Effect of postnatal early overnutrition and high salt intake during puberty on cardiac morphology
Fig. 5 presents cardiac morphology analysis. SL, NLHS and SLHS animals showed higher cardiomyocyte diameter (P < 0·0001; Fig. 5(b)) compared with NL animals. In addition, quantitative analysis shows significant increase of the perivascular fibrosis index (P < 0·0001; Fig. 5(d)) and interstitial fibrosis (P < 0·05; Fig. 5(f)) in the left ventricle from SL, NLHS and SLHS animals compared with NL animals.
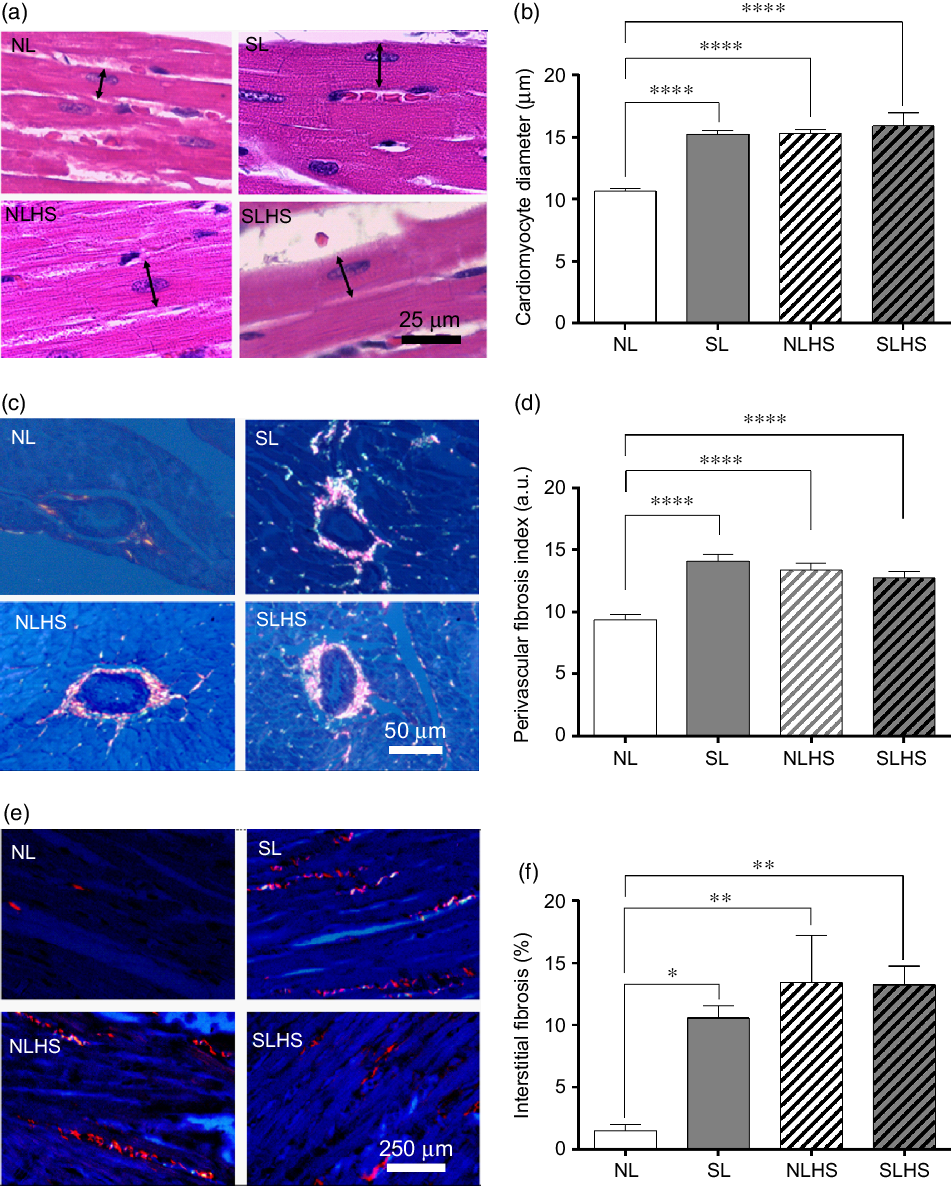
Fig. 5. Effect of postnatal early overnutrition and high salt (HS) intake during puberty on cardiac morphology. Representative photomicrographs (×1000 magnification, scale bars = 25 µm) showing cardiomyocyte sections stained with haematoxylin–eosin (a), and the quantitative analyses of cardiomyocyte diameter measurements (b). Representative photomicrographs (×400 magnification, scale bars = 50 µm) showing transversal sections of ventricular vessels stained with picrosirius red (c) and the quantitative analyses of perivascular fibrosis index (d). Representative photomicrographs (×100 magnification, scale bars = 250 µm) showing longitudinal cardiomyocytes sections from left ventricles stained with picrosirius red (e) and the quantitative analyses of interstitial fibrosis (f). Data are mean values with their standard errors (n 5). To compare the experimental groups, one-way ANOVA followed by Tukey’s post hoc test was used. NL, normal litter; SL, small litter; NLHS, NL plus HS; SLHS, SL plus HS; a.u., arbitrary units. * P < 0·05, ** P < 0·01, **** P < 0·0001.
Effect of postnatal early overnutrition and high salt intake during puberty on angiotensin receptors expression in the heart
Angiotensin receptors in the heart were evaluated by Western blot analysis in left ventricle samples. There were no significant changes in AT1 receptor protein expression (Fig. 6(a)). SLHS rats had less AngII type-2 receptor expression in the heart when compared with all other groups (P < 0·05; Fig. 6(b)). No change was observed in the MAS receptor expression (Fig. 6(c)).

Fig. 6. Effect of postnatal early overnutrition and high salt (HS) intake during puberty on angiotensin receptors expression in the heart. Western blot analyses of angiotensin type-1 receptor (AT1) (a), AngII type-2 receptor (AT2) (b) and MAS (c) receptor expression. Representative immunoblots are shown below the graphs. Data are mean values with their standard errors (n 4). To compare the experimental groups, one-way ANOVA followed by Tukey’s post hoc test was used. NL, normal litter; SL, small litter; NLHS, NL plus HS; SLHS, SL plus HS; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. * P < 0·05, ** P < 0·01.
Effect of postnatal early overnutrition and high salt intake during puberty on catalase and superoxide dismutase 1 expression in the heart
To evaluate the effect of PO and HS on antioxidant defence, we evaluated catalase and superoxide dismutase 1 enzymes expression in the heart. As shown in Fig. 7(a), catalase protein expression levels remain unchanged. On the other hand, superoxide dismutase 1 protein expression levels were increased in SL and NLHS animals compared with the NL group (P < 0·05; Fig. 7(b)). However, no difference was observed in superoxide dismutase 1 expression in the heart from SLHS animals (Fig. 7(c)).
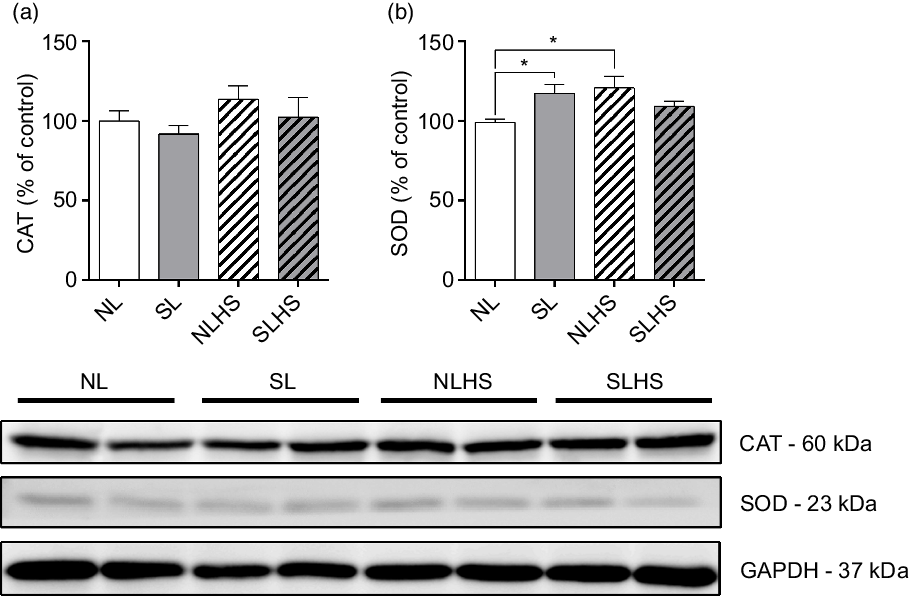
Fig. 7. Effect of postnatal early overnutrition and high salt (HS) intake during puberty on catalase (CAT) and superoxide dismutase 1 (SOD) expression in the heart. Western blot analyses of CAT (a) and SOD (b) enzyme expression. Representative immunoblots are shown below the graphs. Data are mean values with their standard errors (n 4). To compare the experimental groups, one-way ANOVA followed by Tukey’s post hoc test was used. NL, normal litter; SL, small litter; NLHS, NL plus HS; SLHS, SL plus HS; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. * P < 0·05.
Discussion
HS intake is common in Western diets contributing in particular to hypertension and CVD onset. In this paper, we showed for the first time that rats fed HS during puberty were programmed to develop hypertension, reduced baroreflex sensitivity, higher chemoreflex index and cardiac remodelling at adulthood. Our data show that, even after 60 d of recovery, animals fed a HS diet present cardiovascular dysfunctions. Thus, puberty is a critical period for cardiovascular programming, and nutritional disturbances in this period lead to permanent changes in the cardiovascular system.
As observed in our previous study, the SL group presented increased body weight and food intake than their counterparts(Reference Junior, Cavalcante and Ferreira28). However, high Na intake lowered weight gain in both NLHS and SLHS throughout HS exposure, due to reduced food intake in this period, as previously reported by Moreira et al.(Reference Moreira, da Silva and Silveira16). Interestingly, after the HS exposure, both NLHS and SLHS increased food consumption to same levels than NL. This result indicates that HS exposure during puberty mitigates the hyperphagia presented by obese animals.
At adulthood, fat depots were increased in SL compared with NL, but not in SLHS compared with NLHS. Nonetheless, the SLHS group had lower adiposity compared with SL, reinforcing the negative influence of HS exposure on food intake throughout the experimental period. In addition, animals fed a HS diet had lower body weight during treatment and less adiposity at adulthood. Interestingly, it has been shown that HS intake induces adipocyte hypertrophy and reduces the number of these cells from both obese and non-obese animals(Reference Dobrian, Schriver and Lynch29). Conversely, it was shown that HS intake causes energy metabolism changes, increasing hepatic ketogenesis and muscle catabolism induced by glucocorticoids, reducing adiposity and muscular mass(Reference Kitada, Daub and Zhang9). On the other hand, Lanaspa et al.(Reference Lanaspa, Kuwabara and Andres-Hernando30) demonstrated that HS causes hyperphagia by endogenous fructose production leading to hypothalamic leptin resistance and weight gain. However, the effects observed in the body composition of SLHS are not due to chronic exposure to HS, but due to hypothesised metabolic programming in puberty, by the HS exposure in this period, which reinforces the needed nutritional care in early life.
It is well known that impaired baroreflex sensitivity predisposes to hypertension and that HS intake impairs this parameter in lean and obese individuals(Reference He, Li and MacGregor13,Reference Moreira, da Silva and Silveira16,Reference La Rovere, Pinna and Raczak22,Reference Skrapari, Tentolouris and Perrea23,Reference Lanaspa, Kuwabara and Andres-Hernando30,Reference Fardin, Oyama and Campos31) . In our study, in vivo measurements of BP reveals that obesity and high Na intake or that combination can raise BP levels at adulthood; however, only the SLHS group presented basal tachycardia compared with SL and NL. In this sense, bradycardic baroreflex index, in response to phenylephrine injection, was impaired in both groups exposed to hypertonic solution compared with their respective controls. However, when the tachycardic baroreflex response to sodium nitroprusside was analysed, obesity and hypertonic solution treatment was detrimental, but there was no worsening of association of the two factors. It has been described that baroreceptor function can be altered by increased circulating NEFA and humoral molecules, such as AngII, insulin and leptin(Reference Arnold, Shaltout and Gallagher32–Reference Ricksten and Thorén34). Here, we hypothesised that constantly higher BP in the SL group and HS-fed animals leads to baroreceptor desensitisation, as observed in spontaneously hypertensive rats. Thus, our study showed that HS intake during puberty was more deleterious to baroreceptor reflex sensitivity than obesity.
In a similar way, peripheral hypoxia activates chemoreceptors, increasing BP and pulmonary ventilation, leading to increased perfusion to peripheral tissues(Reference Kara, Narkiewicz and Somers35). Previous study demonstrated that patients with hypertension have a marked increase in sympathetic and ventilatory response to hypoxia(Reference Somers, Mark and Zavala36). In the present study, chemoreflex index was impaired by obesity and hypertonic solution treatment, but not aggravated by the combination of these variables.
Understanding baroreceptor response is very important because their sensitivity can be negatively associated with CVD development. Fardin et al.(Reference Fardin, Oyama and Campos31) showed that baroreceptor dysfunction might be a predictor of sympathoexcitation and hypertension associated with obesity(Reference Fardin, Oyama and Campos31). Unfortunately, in this work, we did not evaluate sympathetic activity; however, other mechanisms may be involved in the changes we observe here.
Furthermore, obese people are prone to an HS intake due to a reduced salt sensitivity and a higher salt preference, contributing to the development of pathologies, such as hypertension(Reference Li, Jin and Yu15). In addition, the consumption of HS diets is related to diastolic dysfunction of the left ventricle in hypertensive and normotensive individuals. In this sense, Matsui et al.(Reference Matsui, Ando and Kawarazaki37) reported that diastolic stiffness can be influenced by several factors, but is mainly affected by the structural components of the myocardium. Similarly, obese individuals develop ventricular hypertrophy, which is positively correlated with high BP, BMI and lower O saturation(Reference Avelar, Cloward and Walker38,Reference Aurigemma, de Simone and Fitzgibbons39) . Animal models also develop left ventricular hypertrophy due to metabolic programming. It is well established that PO induces cardiac remodelling due to increased oxidative stress and modified gene expression in the heart(Reference Junior, Cavalcante and Ferreira28,Reference Moreira, Teixeira Teixeira and da Silveira Osso40–Reference Habbout, Guenancia and Lorin42) . Here, we analysed the heart morphology, revealing that obesity, HS or the combination of these factors causes heart hypertrophy, with increased cardiomyocyte diameter and fibrosis.
Morphological changes in the heart lead to functional disturbances. It is well known that cardiac wall thickness is an important determinant of left ventricular diastolic functionality. Concentric left ventricular hypertrophy is commonly found in obese individuals(Reference Aurigemma, de Simone and Fitzgibbons39). Brooks et al. (2010) demonstrated that cardiomyocyte hypertrophy is associated with compromised heart relaxation during diastole(Reference Brooks, Shen and Conrad43). Furthermore, another study demonstrated that the prevalence of ventricular cardiomyocyte hypertrophy can be substantially increased by obesity and hypertension convergence(Reference Avelar, Cloward and Walker38). Here, the SL, NLHS and SLHS groups had cardiac hypertrophy and greater cardiomyocyte diameter.
It has been demonstrated that the hearts from obese animal models have increased fibrosis around the coronary vessels, as well as deposit of collagen in the interstitial area(Reference Junior, Cavalcante and Ferreira28,Reference Carroll and Tyagi44) . Further, cardiac fibrosis is another important factor to diastolic dysfunction(Reference Collier, Watson and van Es45). In this sense, Brilla et al.(Reference Brilla, Janicki and Weber46) showed that hypertension associated with myocardial fibrosis in hypertensive and normotensive animals leading to systolic and diastolic dysfunctions, and also, Gao et al. (Reference Gao, Han and Zhou47) revealed that HS intake accelerates cardiac remodelling in spontaneously hypertensive rats. In the present study, quantitative analysis of fibrosis shows significant increase of the perivascular and interstitial collagen deposition in the left ventricle from the HS and SL groups, as well as in the SLHS group, revealing that high Na intake during puberty also accelerates cardiac remodelling through metabolic programming in this period.
Nakamura et al.(Reference Nakamura, Kataoka and Fukuda48) demonstrated that the RAS regulates fibrosis in aldosterone/salt-induced rats by oxidative stress and the up-regulation of the cardiac AT1 receptor expression(Reference Nakamura, Kataoka and Fukuda48). Additionally, the up-regulation of TLR4, a receptor for pathogen-associated molecular patterns which are involved with increased ROS production through raised NADPH oxidase activity, by the AngII in spontaneously hypertensive rats contributes for the maintenance of hypertension(Reference De Batista, Palacios and Martín49). Here, we did not observe changes in AT1 expression. However, AngII type-2 receptor expression was reduced in adult rats from the SLHS group, suggesting that the cardiometabolic imprint caused by HS intake and obesity in puberty, together, affected the RAS in these animals. Moreover, increased superoxide dismutase 1 expression was observed in the hearts from SL and NLHS animals, and this result is in agreement with the cardiac fibrosis observed in these animals, which suggest raised levels of ROS formation, as previously described by other studies(Reference Junior, Cavalcante and Ferreira28,Reference Mayyas, Alzoubi and Al-Taleb50) .
Our results emphasise the effects of PO and the HS intake during important periods of development, such as puberty. Thus, early-life nutritional disorders increase the risk of adverse health outcomes in adults, by increasing susceptibility to new stressful insults. Overall, we conclude that the responses of newborn offspring to PO and juveniles to a HS diet lead to a significant change in cardiovascular homoeostasis in adult rats. The major contributions of this paper are the elucidation of the deleterious effects of nutritional disorders, during critical periods, for cardiometabolic programming observable in adult life, such as high BP, impairment of baroreflex and chemoreflex function and cardiac remodelling.
Acknowledgements
This work was supported by the Brazilian foundations: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG).
G. B. R., C. H. C. and R. M. G. designed the research; G. B. R., L. C. C., A. B. S. M., M. D. F. J., R. A. C., L. A. F., F. D. T. and P. R. A. N. conducted the research; M. R. N. C., G. R. P., P. C. F. M. and R. M. G. provided essential materials; G. B. R., M. D. F. J., P. R. L., P. C. F. M. and R. M. G. analysed the data; G. B. R., M. D. F. J. and R. M. G. wrote the paper and R. M. G. had primary responsibility for final content. All authors read and approved the final manuscript.
There are no conflicts of interest.











