Background
CHD represents one-third of all major congenital anomalies, with a reported prevalence of 9 per 1000 live births [95% CI: 8.1-9.3]. During the past 50 years, there have been significant improvements in the medical and surgical management of CHD, with more children now reaching adulthood.Reference van der Linde, Konings and Slager 1 With improved survival comes an increasing burden of morbidity. In particular, growth failure during the first 2 years of life is considered to be a significant concern in infants with CHD.Reference Daymont, Neal, Prosnitz and Cohen 2 – Reference Marino and Magee 6 World Health Organisation definitions of persistent malnutrition in children include “stunting”, with a height for age⩽−2 z scores, and “underweight”, with a weight for age⩽−2 z score.Reference Joosten and Hulst 7 Persistent malnutrition in childhood is important as it has been linked to shorter adult height, increased all-cause mortality,Reference Ong, Hardy, Shah and Kuh 8 as well as poorer neurodevelopmental outcomes among young children with CHD.Reference Ravishankar, Zak and Williams 9
Stunting and becoming underweight are both dynamic processes of persistent malnutrition and are indicative of insufficient macronutrients and micronutrients to promote adequate growth.Reference Golden 10 The prevalence of persistent malnutrition at the time of CHD surgery is reportedly 30%,Reference Marino and Magee 6 , Reference Costello, Gellatly, Daniel, Justo and Weir 11 leading to poorer postoperative resilience and clinical outcomes including increased risk of cardiac arrest and infection,Reference Ross, Latham and Joffe 12 prolonged ICU stay,Reference Marino, Meyer and Johnson 13 and length of hospital stay.Reference Marino and Magee 6 , Reference Toole, Toole, Kyle, Cabrera, Orellana and Coss-Bu 14 In addition, infants with CHD who are underweight for age at the time of surgery also experience significant morbidity,Reference Oster, Ehrlich and King 15 , Reference Mitting, Marino, Macrae, Shastri, Meyer and Pathan 16 and those who are slow to gain weight postoperatively have increased mortality at 3 months of age.Reference Eskedal, Hagemo and Seem 17
Growth failure among infants is not just restricted to those with complex CHD lesions; infants with ventricular septal defects are often severely underweight at the time to surgery. As such, facilitating better growth before surgery has been seen as key to improving short- and longer-term outcomes,Reference Anderson, Iyer and Beekman 18 particularly as rapid catch-up growth after infancy is associated with negative metabolic sequela. By 2 years of age, many young children with CHD will have undergone surgery for their condition. However, a high-risk growth pattern has been defined as growth failure during the first 2 years of life with subsequent rapid catch-up growth between the ages of 2 and 7 years and 8 and 15 years.Reference Aguilar, Raff, Tancredi and Griffin 19 The current consequence of these growth patterns with respect to CHD is unknown, but it is speculated that increased adiposity in adults with CHD is associated with an increased risk of metabolic and cardiovascular disease later in life.Reference Smith-Parrish, Yu and Rocchini 20 – Reference Pasquali, Marino and Pudusseri 22 As a result, sustaining inherited growth patterns in infants with CHD before surgery, thereby avoiding rapid late catch-up postoperative growth, is fundamental to reducing long-term co-morbid complications.Reference Aguilar, Raff, Tancredi and Griffin 19
A number of quality improvement initiatives such as home monitoring programmes that aim to facilitate better growth during the months before surgery, particularly in those infants requiring a staged surgical approach, for example, univentricular physiology, have been implemented.Reference Anderson, Iyer and Beekman 18 , Reference Ghanayem, Tweddell, Hoffman, Mussatto and Jaquiss 23 – Reference Ghanayem, Hoffman and Mussatto 25 However, even within these well-established programmes, nutritional pathways describing principles to optimise nutritional support are not available.Reference Schidlow, Anderson and Klitzner 26 , Reference Anderson, Beekman and Kugler 27 Variations in nutrition practiceReference Oster, Ehrlich and King 15 , Reference Schidlow, Anderson and Klitzner 26 , Reference Anderson, Beekman and Kugler 27 may contribute to sub-optimal growth in the period leading up to surgery.Reference Anderson, Beekman and Kugler 27 Although there is a body of evidence around nutritional needs of infants with CHD, as well as a number of published algorithms with regard to nutritional support in the immediate postoperative period,Reference Wong, Cheifetz, Ong, Nakao and Lee 28 – Reference Scahill, Graham, Atz, Bradley, Kavarana and Zyblewski 31 to our knowledge none exist to support of infants in the months leading up to cardiac surgery. Variation in care across different units may contribute to differences in surgical outcomes, and there is a move towards standardising care aligned to defined standards to reduce the risks associated with variations in practice. In addition, lack of consensus regarding nutritional support in infants with CHD causes parental distress owing to conflicting messages.Reference Tregay, Brown, Crowe, Bull, Knowles and Wray 4 , Reference Tregay, Wray and Crowe 32 To address this gap, we aimed to develop a consensus-based nutritional pathway providing a structured approach for the nutritional care of infants with CHD awaiting surgical palliation or repair.
Methods
To develop the nutritional pathway to be used by paediatric dietitians, and other healthcare professionals, in the support of infants with CHD before surgery we used the modified Delphi consensus method described by Keller et alReference Keller, McCullough and Davidson 33 (Fig 1). Initially, we developed a set of principles to guide development of the nutritional pathway to help ensure that key objectives were met. Existing nutritional pathways or guidelines that had used a systematic evidence-based approach to nutritional support of infants during the perioperative periodReference Wong, Cheifetz, Ong, Nakao and Lee 28 , Reference Slicker, Hehir and Horsley 29 were modified following a focused literature search. The contributing literature is summarised in Supplementary material 1. The draft pathway was based on principles outlined in the Word Health Organizations Integrated Management of Childhood Illness. The aim was to provide a simple nutritional pathway based on a traffic light system of green (no concern), amber (some concern), and red (significant concern).Reference Heiby 34 The draft pathway was reviewed and refined by a small working group of investigators (L.V.M., N.J.D., C.S.K., M.L.D., J.E.R., M.J.J., and T.B.) before being presented at the first expert stakeholder meeting.
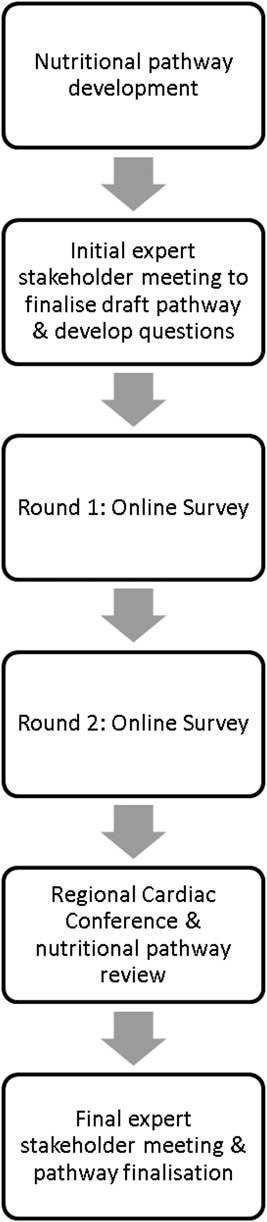
Figure 1 Process followed during modified Delphi consensus.
Step 1: First expert stakeholder meeting of British Paediatric Dietetic Paediatric Cardiology Interest Group
An expert stakeholder meeting was held with members of the British Dietetic Association (BDA) Paediatric Cardiology Interest Group who are also paediatric dietitians from Tertiary Surgical Cardiac Centres. The purpose of the meeting was to review and discuss the initial draft pathway and the planned consensus process in addition to gaining agreement behind the nutritional principles that had been incorporated from the available evidence.Reference Schidlow, Anderson and Klitzner 26 , Reference Wong, Cheifetz, Ong, Nakao and Lee 28 – Reference Karpen 30
Step 2: Development of Delphi statements and open-ended questions
After the first expert stakeholder meeting, changes were made to the draft pathway after which statements to be used in the two rounds of an online survey were developed. The survey contained 31 questions split into five sections, representing the layout of the nutritional pathway. For each question, participants were asked to indicate their level of agreement with a statement and responded using a 10-point scale, with 1 indicating strongly disagree to 10 indicating strongly agree, which included a neutral option. At the end of each section, participants were provided with the opportunity to include additional comments, within an open-ended text box (Supplementary material 2). Participant responses accounted for only one rating per question.
Stage 3: Two rounds of the online Delphi survey
The survey was created and distributed through a proprietary online platform hosted by the University of Southampton (iSurvey: https://www.isurvey.soton.ac.uk/). Members of the BDA Paediatric Cardiology Interest Group were invited to complete the first round of survey and sent a reminder after 3 weeks. Responses to each question were grouped into “disagreement” 1–4 and “agreement” 7–10. For analysis, consensus was defined as ⩾80% responses for each question as either “disagreement” or “agreement”.Reference Hsieh and Shannon 35 In round 2 of the survey, the questions remained unchanged, and participants were provided their own score from round 1 along with the cumulative scores from the rest of the group. They were invited to consider their score in comparison with the group score and offered the opportunity to modify their own score in light of this should they wish. It was made clear that even with the additional information provided participants did not have to change their opinion. Participants were given 4 weeks to complete the second round, as it was during high peak summer holiday season. Participants were informed that changes would be made to the draft pathway on the basis of consensus achieved after 2 rounds. Participants provided written consent as part of the Delphi survey.
Step 4: Regional conference: nutrition support in infants with CHD
As Paediatric Cardiac networks cover wide geographic areas, nutritional support is provided by paediatric dietitians working in a District General Hospital, as well as specialist centres. It was felt important to ensure that dietitians working in these hospitals agreed with the principles and content of the nutritional pathway in advance of the final expert meeting. Two months before the final expert meeting, clinical staff from NHS District General Hospitals (South Central Region, UK) were invited to attend a regional cardiac nutrition conference to discuss the modified pathway and achieve consensus with the nutrition principles outlined in the pathway across a wider group. Participants registered for the meeting were sent a copy of the modified nutritional pathway in advance. The morning session of the meeting was dedicated to presentations on nutritional support of infants with CHD, and set the scene for the development of the nutrition pathway. Participants registered for the meeting were sent a copy of the nutritional pathway in advance to be used as part of the afternoon facilitated discussion by M.J.J., who led the group through a point-by-point group discussion of the format and contents of the draft pathway. Paper copies were also printed for the day itself.
Stage 5: Second and final expert stakeholder meeting
A final face-to-face expert stakeholder meeting of BDA Paediatric Cardiology Interest Group was held whereby L.V.M. led the group through a point-by-point group discussion of the format and contents of the draft pathway, including areas of contention, with the aim of confirming the final version of the nutritional pathway for infants with CHD before surgery.
Results
Step 1: First expert stakeholder meeting of BDA Paediatric Cardiology Interest Group
In total, 10 expert dietitians from the BDA Paediatric Cardiology Interest Group and one physician attended the first stakeholder meeting (Table 1). During the point-by-point discussion, iterative changes were made to CHD conditions, with transposition of the great arteries move to higher nutritional risk, in addition to protein requirements for those with lower nutritional risk. By the conclusion of the meeting, all present agreed on the process of consensus in addition to the draft nutritional pathway.
Table 1 Characteristics of expert stakeholders and regional meeting of healthcare professionals.
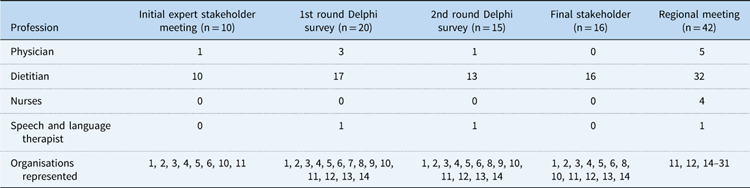
Specialist Cardiac Level 3 Centres: 1=Alder Hey Children’s Hospital NHS Foundation Trust; 2=Glasgow Children’s Hospital NHS Trust; 3=University Hospitals of Leicester NHS Trust; 4=Royal Brompton & Harefield NHS Foundation Trust; 5=Evalina Children’s Hospital NHS Foundation Trust; 6=Great Ormond Street Hospital for Children NHS Foundation Trust; 7=Newcastle Hospitals NHS Foundation Trust; 8=University Hospitals Bristol NHS Foundation Trust; 9=Leeds Teaching Hospitals NHS Trust; 10=Birmingham Children’s Hospital NHS Foundation Trust; 11=University Hospital Southampton NHS Foundation Trust, Others: 12=Our Lady’s Hospital, Dublin; 13=HCA Hospital, London; 14=Yeoville NHS District General Hospital; 15=St Peter’s NHS Hospital, Chichester; 16=Queen Alexandre NHS Foundation Hospital, Portsmouth; 17=Dorchester NHS Hospital; 18=Frimley NHS Hospital; 19=St. Mary’s NHS Hospital, Isle of Wight; 20=Kings College Hospital NHS Foundation Trust, London; 21=John Radcliffe NHS Foundation Trust, Oxford; 22=Stoke Mandeville NHS Foundation Trust Hospital; 23=Milton Keynes University Foundation Trust, Milton Keynes; 24=Reading NHS Foundation Hospital, Reading; 25=Worthing NHS Hospital, Worthing; 26=Bart Health NHS Foundation Trust, London; 27=Kings College Hospital NHS Foundation Trust, London; 28=Cardiff University Hospital Cardiff, Wales; 29=Barking NHS Hospital, London; 30=Royal Surrey County Hospital, Guildford; 31=Bromley Health Care, Bromley
Step 2: Development of Delphi statements and open-ended questions
On the basis of the initial draft guidelines, survey questions were created and the survey distributed to registered participants. The survey is detailed in Supplementary material 2.
The results for each question were exported to an excel file (csv) for review and analysis. Qualitative content was used for the comments, with minimal interpretation. All open-ended comments from rounds one and two were presented at the final expert stakeholder meeting to ensure that all opinions were accounted for.
Stage 3: Two-round online Delphi survey
An initial e-mail explaining the process and purpose of the Delphi consensus was sent to all members of the BDA Paediatric Cardiology Interest Group, who were asked to forward the e-mail onto other colleagues working in Paediatric Cardiology within their organisation who may be interested in participating. In all, 35 expert healthcare professionals expressed interest in completing the online survey. After their expression of interest, a 2nd e-mail was sent with a URL link to access the survey in addition to instructions for completion. Of the 35 registered, 20 completed round 1 (57%), including two clinicians and 18 dietitians from the BDA Paediatric Cardiology Interest Group. Of the 20 healthcare professionals who completed the first round, 15 (75%) completed round 2 (Table 2). Given the small number of specialist paediatric dietitians working in Tertiary Cardiac Surgical centres UK (n=18), the response rate for the survey was considered good as there was representation from each of the Tertiary Surgical Cardiac Centres. Of paediatric dietetic participants, one-third had more than 9 years of experience working with infants with CHD.
Table 2 Principles supporting the development of the nutrition pathway for infants with CHD before surgery.

After the first round, consensus was achieved regarding 76% of statements, and after round 2 this had increased to 96% (Table 3). Consensus had not been achieved regarding just three statements after round 2, namely infants with transposition of the great arteries have a high nutritional risk; an infant who does not vomit has a low nutritional risk; and infants will no longer require nutritional support 12 weeks after definitive surgery. These three statements were discussed at length during the final stakeholder meeting.
Table 3 Number and percentage of participant’s agreement with each statement between survey round 1 and 2.

Agreement scores 7–10; disagreement scores 1–4
Step 4: Regional conference: nutrition support in infants with CHD
In total, 42 participants took part in the Regional Conference: Nutrition support in infants with CHD including five clinicians, 32 paediatric dietitians, four nurses, and one speech and language therapist, working within the Southampton-Oxford Cardiology network. The afternoon session was dedicated to the nutritional pathway, whereupon the same moderator (M.J.J.) as for the first expert stakeholder meeting led those in attendance through a point-by-point discussion of the pathway, which provided the opportunity to make further iterative changes (Fig 2). Discussion focused on ensuring that the pathway contained guidance that could be implemented in the majority of settings. The meeting facilitator (L.V.M.) recorded minutes and used this to produce a final version of the pathway. The conference participants agreed with all components of the nutrition pathway, although the group recommended that the format of the screening questions outlined in Step 5 of the pathway – “Choosing a Nutrition Care Plan A, B and C” – be changed to an algorithm (Fig 3). Subsequent to the meeting, the investigators (L.V.M., C.S.K., and M.J.J.) developed a simple algorithm for this step (Fig 3), which was presented at the final stakeholder meeting.
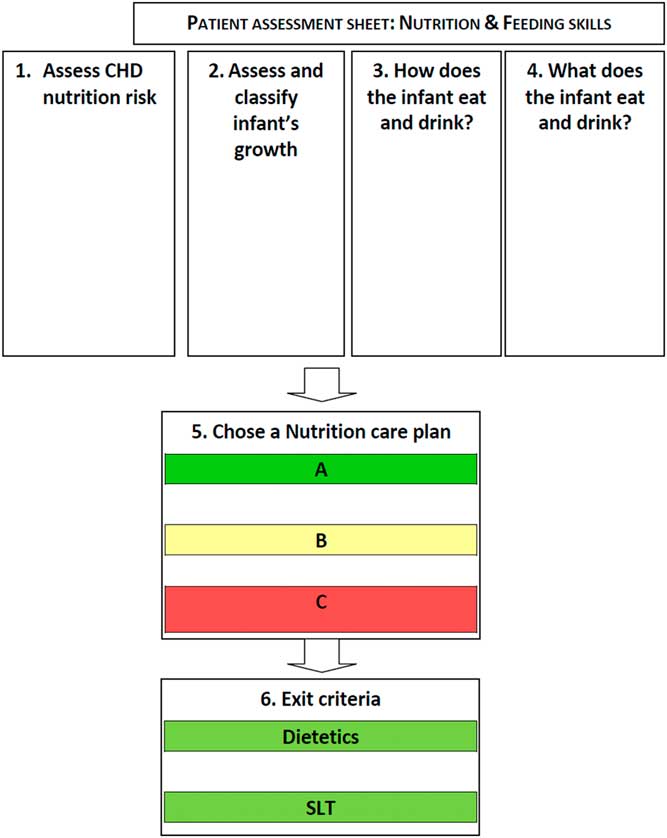
Figure 2 Nutritional pathway for infants with CHD before surgery. Nutrition Care Plan A, B, and C describe a package of nutritional care, in addition to exit criteria for dietetic and speech and language therapist (SLT) support (full nutritional pathway available in Supplementary material 3).
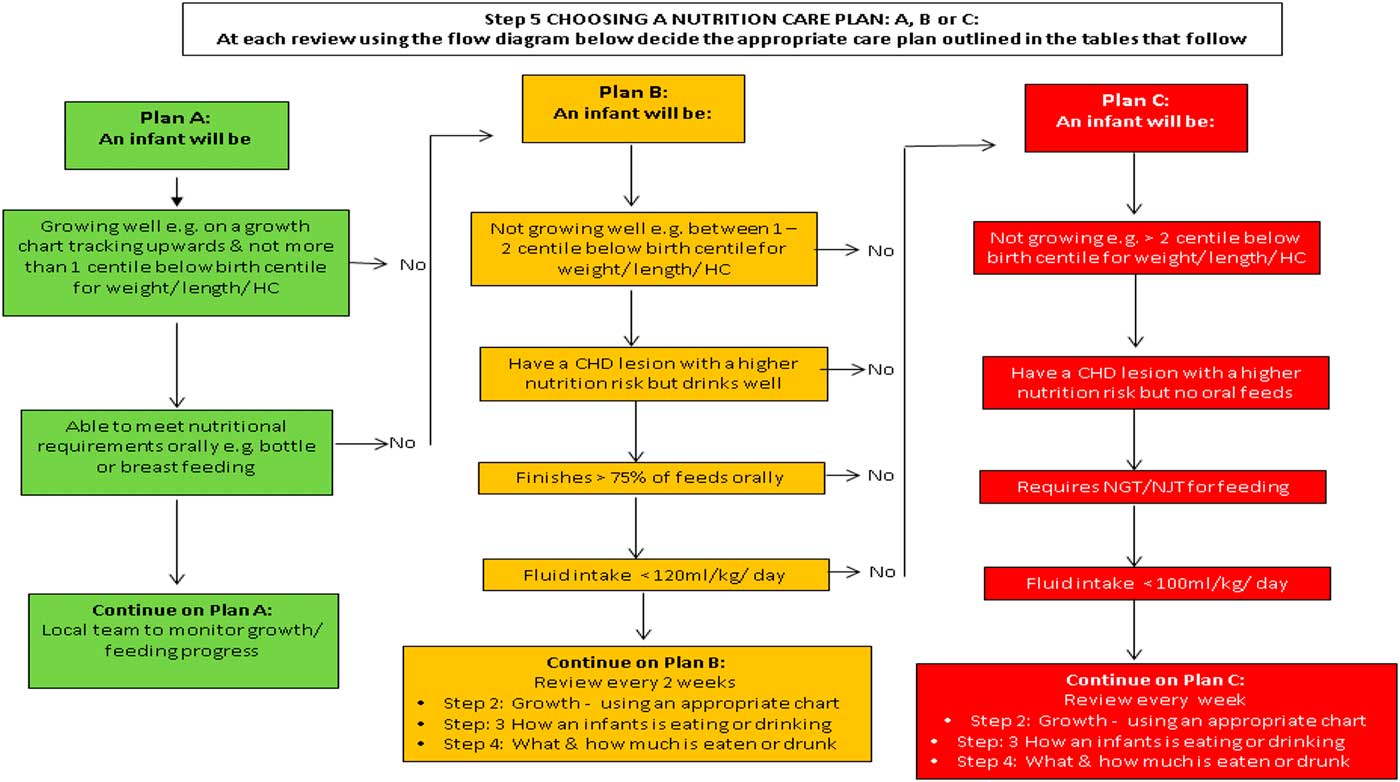
Figure 3 Step 5: Choosing a nutrition care plan: A, B or C (full nutritional pathway available in Supplementary material 3).
Stage 5: Second and final expert stakeholder meeting
The finalised nutritional pathway was presented at the final stakeholder meeting of the BDA Paediatric Cardiology Interest Group, attended by specialist dietetic representation from all but two of the Level 3 Cardiac centres. Dietitians from those two centres had participated in the online survey. The moderator (L.V.M.) led those in the meeting through a point-by-point discussion.
The three statements on which consensus had not been reached during the Delphi process were discussed, amended, and subsequently consensus was reached to permit inclusion in the final pathway: infants with transposition of the great arteries have a high nutritional risk; an infant who does not vomit has a low nutritional risk; and infants will no longer require nutritional support 12 weeks after definitive surgery.
The format change – that is, the use of an algorithm in place of a table for Step 5 “Choosing a Nutrition Care Plan A, B and C” – was discussed during the meeting. As only the format and not the information within had changed, the group agreed on the layout change. All participants at the meeting agreed on the content and format of the finalised pathway. The final pathway presented in Supplementary material 3 has since been endorsed by the British Dietetic Association.
Discussion
The best available evidence from the literature relating to nutritional support of infants with CHDReference Medoff-Cooper and Ravishankar 3 , Reference Tregay, Brown, Crowe, Bull, Knowles and Wray 4 , Reference Toole, Toole, Kyle, Cabrera, Orellana and Coss-Bu 14 , Reference Anderson, Beekman and Kugler 27 – Reference Scahill, Graham, Atz, Bradley, Kavarana and Zyblewski 31 , Reference Blasquez, Clouzeau and Fayon 36 – Reference Uzark, Wang and Rudd 69 was used to develop a nutritional pathway for infants with CHD. This was presented at an initial expert meeting involving the BDA Paediatric Cardiology Interest Group, taken through two rounds of an anonymous Delphi survey, discussed at a regional nutrition conference and finalised at a final expert BDA Paediatric Cardiology Interest Group meeting. Iterative changes were made throughout the process. At the end of this process, consensus around the nutrition principles within a nutritional pathway for infants with CHD awaiting surgery was achieved. This modified Delphi consensus process was inclusive of paediatric dietetic experts working in Tertiary Surgical Cardiac centres, as well as those working in District General Hospitals. Of those who registered to complete the Delphi survey, 17 dietitians completed the first round, of whom 80% went on to complete the second round, demonstrating a good level of engagement with the principles of the nutritional pathway. The timing of the survey – for example summer holidays – may have had an impact on participation. Consensus at the end of round 2 of the Delphi survey was achieved in all but three minor areas relating to reclassifying the nutritional risk of transposition of the great arteries, vomiting in infants, and the duration of follow-up post surgical repair. Importantly, the processes used in this project, particularly the regional conference, will have raised awareness and encouraged engagement in relation to the pathway, making successful implementation more likely going forward.
The overarching ambition of our wider quality improvement programme including the development of a nutritional pathway in infants with CHD before surgical repair/palliation was to reduce variation in nutrition management of infants with CHD; promote early referral to a paediatric dietitian/Speech and Language Therapist for feeding difficulties; reduce the prevalence of persistent malnutrition, as defined by WHO classifications, at the time of surgery; and improve clinical outcomes. Although there are a number of published algorithms with regard to nutrition support in the immediate postoperative intensive care period, until now none currently existed for the support of infants with CHD leading up to surgery.Reference Wong, Cheifetz, Ong, Nakao and Lee 28 – Reference Scahill, Graham, Atz, Bradley, Kavarana and Zyblewski 31 In an ideal setting, all infants with CHD at high risk of growth failure should be reviewed by a Paediatric Cardiac Dietitian weekly as part of a multidisciplinary team process. This action alone has been shown to improve growth among those with univentricular physiology.Reference Oster, Ehrlich and King 15 Currently, there is variable and often inadequate resource available within Paediatric Cardiac centres. Most units only have sufficient resource to provide nutritional support to inpatients, and on discharge patients are often referred to local dietetic services for ongoing nutrition support. A lack of consensus regarding optimal nutritional support for infants with CHD may contribute to the poor growth of infants awaiting surgery and have a negative impact on clinical outcomes.Reference Marino and Magee 6 , Reference Marino, Meyer and Johnson 13 , Reference Mitting, Marino, Macrae, Shastri, Meyer and Pathan 16 Therefore, improving growth before surgery is a priority. Persistent malnutrition has been widely described in infants with CHD including in cardiac centres in other countries.Reference Ravishankar, Zak and Williams 9 , Reference Costello, Gellatly, Daniel, Justo and Weir 11 , Reference Toole, Toole, Kyle, Cabrera, Orellana and Coss-Bu 14 , Reference Correia Martins, Lourenco and Cordeiro 70 – Reference Blasquez, Clouzeau and Fayon 73 We aimed to ensure that the principles of nutritional care within the pathway were as generic as possible to allow local adaptation within a variety of healthcare settings both nationally and internationally.
The causality of growth failure in this population group is multifactorial and includes increased metabolic requirements, malabsorption, and sub-optimal nutrition intake.Reference Medoff-Cooper, Naim, Torowicz and Mott 46 , Reference Blasquez, Clouzeau and Fayon 73 , Reference Medoff-Cooper, Irving and Marino 74 The Nutrition Care Plans A, B, and C were based on evidence suggesting that growth in children with complex CHD benefits from early intensive nutrition support,Reference Vogt, Manlhiot, Van Arsdell, Russell, Mital and McCrindle 75 making use of energy- and nutrient-dense formulas where necessary.Reference Medoff-Cooper and Ravishankar 3 , Reference Medoff-Cooper, Naim, Torowicz and Mott 46 Within the literature, recommendations for nutrition support suggest that growth will be achieved with a calorie intake of 90–110 kcal/kg, ensuring an optimal protein–energy ratio of 9–12%Reference Golden 10 and sufficient intake of micronutrients.Reference Medoff-Cooper and Ravishankar 3 , Reference Golden 10 , Reference Wong, Cheifetz, Ong, Nakao and Lee 28 , Reference Slicker, Hehir and Horsley 29 , Reference Keller, McCullough and Davidson 33 Although energy expenditure in infants with CHD has not been shown to be increased,Reference Irving, Medoff-Cooper and Stouffer 61 , Reference Trabulsi, Irving and Papas 68 there is evidence that additional energy and protein is required to support catch-up growth.Reference Golden 10 Achieving sufficient intake is often affected by vomiting, reflux, ability to sustain feeding for long enough before tiring, and early satiety.Reference Blasquez, Clouzeau and Fayon 73
As most infants with CHD are followed up by local dietetic services rather than at a specialist centre, it was imperative to achieve wide stakeholder engagement and agreement to the nutrition principles within the nutritional pathway. This was achieved during a regional nutrition conference. All of the participants attending the conference agreed with the content of the nutritional pathway, but suggested a format change for Step 5 “Choosing a Nutrition Care Plan A, B or C” within the guidelines. This amendment was made before presenting the pathway at the final expert meeting. During the final stakeholder meeting, each of the points was discussed until consensus was achieved.
Some qualitative comments revealed concern regarding the use of nut butters, recommended in Nutrition Care Plan B and C, in early weaning foods, and allergic risk. However, recent studies suggest that although there are insufficient data to demonstrate that early introduction of peanut into infants’ diets – between 4 and 6 months of age – would reduce risk of developing a peanut allergy,Reference Fleischer 76 early introduction of peanuts is not considered unsafe 77 and as nut butters are a nutrient-dense food source the recommendation to fortify complementary foods with them has been included within the pathway as there is an extensive body of research considering their use in the form of Ready-to-Use Therapeutic Foods.Reference Manary, Ndkeha, Ashorn, Maleta and Briend 78
Other work from our centre suggests that a nutritional pathway can be readily and accurately implemented in a healthcare setting improving nutritional care, growth, and clinical outcomes in vulnerable patient populations.Reference Johnson, Leaf and Pearson 79 , Reference Johnson and May 80 The next stage of this quality improvement work is to implement the described nutritional pathway within a feasibility study. Part of this will include consideration of whether monitoring nutrition intake and growth using a digital home monitoring program is easy, feasible, and acceptable for parents and healthcare professionals (Fig 3; Supplementary material 3). We will use qualitative and quantitative methodsReference Johnson and May 80 to define the outcomes needed for a larger multicentre study to evaluate whether this approach does actually improve growth among infants with CHD before surgery.
There are a number of limitations to this work, the principal one being that consensus processes have inherent bias and a heavy reliance on the opinions of experts. There is also no standardised methodology for completing modified Delphi or Delphi processes and as such the recommended sample size and required response rate varies. The challenge with having a small group of experts within one field is that their opinions may show little variability, limiting the range of options considered in achieving consensus. A larger group of experts are likely to deliver a broader range of expertise, in turn making it more challenging to achieve consensus.Reference Hsu and Sandford 81 Paediatric dietitians are usually the key healthcare professionals involved in the nutritional care of infants with CHD, and thus using their nutritional expertise for this modified Delphi process was appropriate. A total of 52 dietitians provided some input whether as part of the BDA meetings, online survey, or regional stakeholder meeting. This suggests there was high stakeholder engagement with the contents of the nutritional pathway and the need to standardise nutritional practices for this vulnerable cohort. They had a range of experience of Regional and Tertiary level 3 Cardiac centres, which ensured that the views of a wide range of opinions was taken into account. As the literature used for the development of the nutritional pathway was based on international research and practice, it is anticipated that the principles presented within the pathway are transferable to other healthcare systems.
Conclusion
We have developed the first comprehensive, consensus-based Nutrition Pathway to guide nutritional support for infants with CHD before surgery and optimise growth in these vulnerable patients. Consensus regarding the format and content of the guideline was achieved among healthcare professionals working at specialist paediatric cardiac centres and at local district hospitals. We intend to implement the nutritional pathway in a feasibility study to determine whether it is practical to use and whether the pathway better supports growth in infants with CHD before surgery.
Acknowledgements
The authors thank the members of the British Dietetic Association – Paediatric Cardiology Interest Group – for their assistance and support with this project, as well as Emma Gentles, Glasgow Children’s Hospital, Laura Flannigan, Glasgow Children’s Hospital (Teleconference), Alyshah Keshani, Great Ormond Hospital for Sick Children (GOSH), Dr Graeme O’Connor, GOSH, Sam Armstrong, Birmingham Children’s Hospital, Marianne Croft, Alderhey Children’s Hospital, Amber Greene, Leicester Children’s Hospital, Jason Barling, Bristol Children’s Hospital, Neam Al Mossawi, HCA Health Care, Harley Street, Julia Hopkins, Evalina Children’s Hospital, Meredith Purvis, Evalina Children’s Hospital, Anne Grimsley, Belfast Children’s Hospital, David Hopkins, Yoeville Hospital, Anne Marie Shime, Our Lady’s Children Hospital, Dublin, Amy Calvert, Brompton & Harefield Hospital, Shelina Meah, St. Mary’s Imperial College London, Lisa Sheridan (Birmingham Children’s Hospital) Kalpana Hepani (Evalina Children’s Hospital). The authors also thank Dr Philippa Thomas, MBChB, BSC (Hons) Paediatric Specialty Trainee, Health Education Wessex, for her help with developing the Algorithm: Step 5. Authors’ Contribution: L.V.M. formulated the original idea and wrote the initial nutritional pathway using the best available evidence, collated the Delphi consensus, and drafted the manuscript. L.V.M., N.J.D., C.S.K., M.L.D., J.E.R., T.B., and M.J.J. made iterative changes to the pathway at various time points during the Delphi process. M.J.J. facilitated the discussion during the expert meetings. T.R., A.-S.E.D., N.J.H., and M.J.J. contributed to revising the manuscript for important intellectual content, and all authors provided final approval of the version to be submitted.
Financial Support
This report is independent research arising from an Integrated Clinical Academic Clinical Lectureship, Luise Marino – ICA-CL-2016-02-001 supported by the National Institute for Health Research and Health Education England. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, Health Education England, or the Department of Health.
Conflicts of Interest
None.
Ethical Standards
The need for ethical approval was waived by a local ethics committee. Consent was given by those who participated in the anonymous on line survey.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951118000549









