With the development and improvement of the hydraulic fracturing method in recent years, natural gas has become abundant and relatively cheap. Methane is the main component of natural gas, and can be converted into a variety of more valuable chemicals, but these are generally expensive or inefficient, says Maria Flytzani-Stephanopoulos, a professor in the Department of Chemical and Biological Engineering at Tufts University. Figuring out a way to more easily “upgrade” methane into other products, such as methanol, has been a “‘holy grail’ for decades,” she says.
Now, as described in an article recently published in Nature (doi:10.1038/nature24640), Flytzani-Stephanopoulos and colleagues have developed a method to convert methane into either methanol or acetic acid under mild conditions, using oxygen gas and two different catalysts made of rhodium. This “is a remarkable step forward,” writes Ive Hermans, a chemistry professor at the University of Wisconsin–Madison, in a commentary accompanying the study.
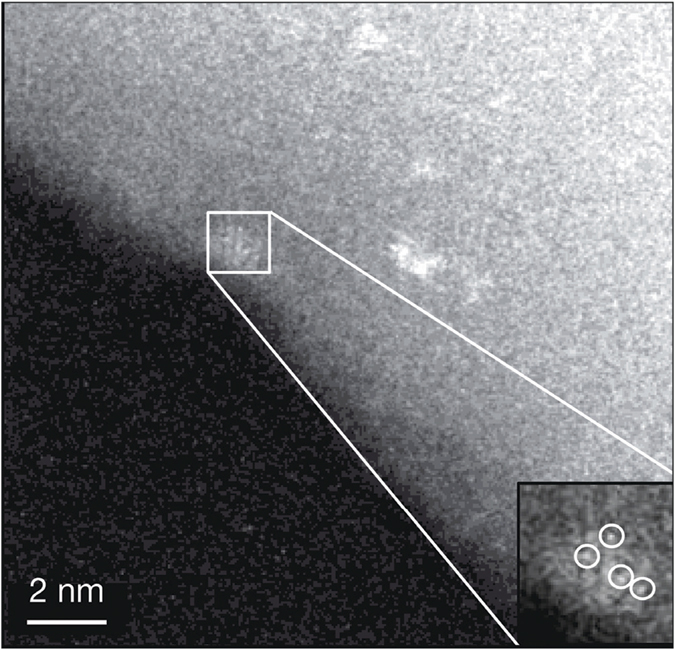
Aberration-corrected high-angle annular dark-field scanning transmission electron microscope image of a thin edge of an as-synthesized Rh-ZSM-5 flake. Isolated rhodium atoms are marked with white circles. Credit: Nature.
The catalysts consist of single atoms of rhodium distributed on a zeolite or titanium dioxide support, and the product of the reaction can be controlled by altering the acidity of the batch. Under acidic conditions, acetic acid is produced, whereas a more basic milieu leads to the formation of methanol. The reactions were carried out in a batch reactor, but the team is currently working to scale up and demonstrated stability of the process in a continuous flow reactor, Flytzani-Stephanopoulos says. They have also applied for a patent.
One current “upgrading” method converts methane into syngas, a mixture of carbon monoxide and hydrogen. This syngas can then be turned into methanol, liquid fuels, and other products through Fischer–Tropsch synthesis, but this process is energy-intensive, and would be more expensive were it not for the low cost of methane, notes Hermans, who was not involved in this study. This new process, however, gets around this two-step process.
The rhodium-zeolite catalyst produces “about 0.4 kilograms of acetic acid per kilogram of catalyst per hour, using methane, carbon monoxide, and oxygen at a total pressure of less than 30 bar, and water as a solvent,” Hermans notes. The formation rate is probably too low to make this particular reaction economically viable at the moment. But future work might be able to increase this rate or find other ways to make it economical.
“Understanding how the catalyst functions and what other metal cations may be similarly used alone or in combination will be a fertile research undertaking,” Flytzani-Stephanopoulos says. “The industrial impact depends on further catalyst optimization, already underway in our lab.”
The process produces low levels of acetic acid. This chemical could be used as a feedstock by oil-producing, or oleaginous, yeast to “produce lipids that can be further hydro-treated for green diesel and jet fuel production. In such a scheme we would be converting natural gas methane to diesel fuel in a two-stage system comprising a catalytic reactor for methane oxidation, and an aerobic fermentor for lipid synthesis,” Flytzani-Stephanopoulos says. This method allows utilization of the dilute acetic acid stream produced from the methane reactor without the need for expensive separation or concentration of the acid.
The study authors “propose an organometallic mechanism for the reaction, on the basis of the observed correlation between catalytic activity and the presence of isolated rhodium(I) species,” Hermans writes. “In the first step, a stable intermediate containing a Rh–CH3 group forms when the metal comes into contact with methane and oxygen. This intermediate then reacts with carbon monoxide to form acetic acid—presumably, because the carbon monoxide inserts itself into the Rh–CH3 bond to form an acetyl intermediate (Rh–COCH3), which is then hydrolysed to yield acetic acid.”
“On the basis of the observation that methanol, rather than acetic acid, forms when hydrogen ions are eliminated from the catalyst support, the authors hypothesize that the ions facilitate the insertion of carbon monoxide into Rh–CH3,” Hermans continues. “In the absence of those ions, an oxygen atom instead inserts into the Rh–CH3 intermediate to form a Rh–OCH3 group, but only in the presence of carbon monoxide molecules; hydrolysis of the Rh–OCH3 group then generates methanol. Carbon monoxide therefore acts as a co-catalyst in the conversion of methane to methanol.”
The researchers created the rhodium-zeolite particles by combining a mixture of the zeolite support and a solution of rhodium(III) nitrate. After impregnation, the samples were dried in a vacuum overnight, and then reduced in a mixture of hydrogen and helium at high temperatures. “This way we can anchor the rhodium as single isolated rhodium (I) cation species on the zeolite walls,” Flytzani-Stephanopoulos explains. Washing then removes unattached rhodium nanoparticles.
Creating the titanium catalyst involved deposition–precipitation, using an aqueous solution of Rh(III) nitrate. The desired amount of the rhodium solution was added dropwise into the titanium dioxide suspension, and heated under slightly basic conditions for three hours. The solids were then filtered and the slurry was exposed to UV radiation. “During the irradiation process, Rh cations are anchored on certain surface sites of the titanium dioxide,” and excess rhodium particles can be later washed off, Flytzani-Stephanopoulos says.
“Perhaps the most exciting aspect of this work is the discovery that the catalytically active sites are dispersed rhodium complexes, rather than rhodium nanoparticles, as many chemists might have expected,” Hermans writes. The study “therefore links homogeneous organometallic chemistry—which typically involves the reactivity of individual metal complexes—with solid-phase (heterogeneous) catalysis, and illustrates the importance of understanding catalysts at the atomic scale.”


