Organochlorines (OC) are chemical products that were widely used after World War II as insecticides and in industry. In the 1960s, their adverse effects for the environment and human health began to be known, and in the 1970s their use was banned in most industrialised countries. However, because they are resistant to degradation, many persistent organic pollutants continue to be present in most food chains worldwide(Reference Porta, Puigdomenech and Ballester1). Furthermore, because of their lipophilicity, OC accumulate in adipose tissue of organisms. Being at the top of the food chain, man is contaminated via food, in the infancy from breast milk(Reference Porta2) and later from animal products such as fish, meat and dairy products(Reference Travis, Arms, Lave and Upton3, Reference Mullerova and Kopecky4).
The dietary consumption of meat and other animal products differs among individuals. Diet may vary according to religion, because of particular health problems or for ecological beliefs. Among individuals adhering to different dietary patterns, vegetarians may be defined as individuals who do not eat meat. However, among self-defined vegetarians, some exclude only red meat, while others do not eat any flesh food, including fish or poultry(Reference Weinsier5, Reference Haddad and Tanzman6). Some vegetarians do not eat any animal products, including dairy products, eggs and honey, and are defined as vegans(Reference Haddad and Tanzman6). As vegans do not eat any animal products which are the main source of OC for man, their exposure to these compounds is theoretically lower than that of non-vegetarians. Accordingly, some studies have already shown that OC concentration is lower in breast milk or in the adipose tissue of vegetarians than in omnivores(Reference Hergenrather, Hlady and Wallace7–Reference Noren9). However, to our knowledge, the differences in plasma OC concentration have not been studied yet between real vegans and omnivores. It can thus be hypothesised that vegans would have a lower plasma OC concentration than omnivores.
As in vegetarians and omnivores, OC plasma and tissue concentrations have been compared in individuals who differed in their weight status. Hue et al. (Reference Hue, Marcotte and Berrigan10) showed that, at steady-state weight, obese and morbidly obese individuals present similar plasma concentration of OC to lean subjects. They also demonstrated that total plasma OC concentration is related to age and not to BMI(Reference Hue, Marcotte and Berrigan10), supporting the suggestion that adipose tissue could have a protective role, keeping the lipophilic pollutants away from the organs(Reference Chevrier, Dewailly and Ayotte11). However, when obese individuals engage in a weight-loss programme, the body load of OC becomes more detectable in response to body fat loss which favours a significant rise of blood and subcutaneous adipose tissue concentrations(Reference Chevrier, Dewailly and Ayotte11–Reference Imbeault, Chevrier and Dewailly13). A recent study showed that plasma OC concentrations were about 388 % greater in obese subjects at 1 year after biliopancreatic diversion surgery compared with lean controls(Reference Hue, Marcotte and Berrigan14). The increased concentration of plasma OC induced by weight loss may have several adverse consequences on health. In fact, exposure to persistent organic pollutants suppresses the immune system, thereby increasing the risk of acquiring several human diseases. They are known to alter thyroid(Reference Zoeller15) and reproductive function(Reference Frazier16) in both males and females and to increase the risk of developing cancer(Reference Clapp, Jacobs and Loechler17), diabetes(Reference Lee, Lee and Song18–Reference Vasiliu, Cameron and Gardiner20), Parkinson's disease(Reference Hatcher, Pennell and Miller21), cardiovascular disease and liver disease(Reference Carpenter22). Women are at high risk of giving birth to infants of low birth weight, who are at high lifetime risk for several diseases(Reference Windham and Fenster23). In addition, the increase in plasma OC concentrations can induce thermogenic adaptations promoting weight regain after a weight loss. It is indeed associated with an accentuation of the decrease in plasma triiodothyronine (T3) concentrations(Reference Pelletier, Doucet and Imbeault24) and skeletal muscle oxidative enzymes(Reference Imbeault, Chevrier and Dewailly13). Moreover, the main predictor of the enhanced fall in resting(Reference Pelletier, Doucet and Imbeault24) and sleeping(Reference Tremblay, Pelletier and Doucet25) metabolic rate observed after weight loss was found to be the change in total plasma OC concentration. It is thus clear that the adverse consequences produced by OC pollutants may aggravate health and the obesity epidemic.
Up to now, the ingestion of a non-absorbable dietary fat substitute is the only strategy that has been shown to accelerate the body clearance of OC or analogous compounds(Reference Jandacek and Tso26). In two individuals acutely contaminated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the intake of olestra-containing potato chips accelerated by 8- to 10-fold the clearance of TCDD(Reference Geusau, Tschachler and Meixner27). Furthermore, over 2 years of an olestra-containing diet (20 g/d) leading to a weight loss of 18 kg, the OC aroclor 1254 in the adipose tissue of an obese diabetic male dramatically decreased from 3200 mg/kg to 56 mg/kg(Reference Redgrave, Wallace and Jandacek28). This is concordant with two recent studies reporting that olestra induced a significant faecal loss of hexachlorobenzene in precontaminated animals(Reference Jandacek, Anderson and Liu29) and that sucrose polyester enhanced disposal of 2,2′,4,4′tetrabromodiphenyl ether in rats through interruption of enteropathic circulation(Reference Meijer, Hafkamp and Bosman30). However, since these observations were made in animal models or in a context of severe human contamination, uncertainty exists as to what extent olestra could reduce the level of OC in obese individuals exposed to habitual weight-loss programmes inducing a small to moderate decrease in the lipid dilution space for OC.
Hence, the main aim of study 1 was to compare plasma OC concentrations between vegans and omnivores. In addition, the objective of study 2 was to verify whether olestra can prevent the increase in plasma OC concentration that is generally observed in response to a weight-loss programme. The main preoccupation underlying these two pilot studies was to evaluate the potential of some nutritional approaches to prevent or reduce the body load of OC in humans.
Experimental methods
Study 1
Nine vegan subjects (six women and three men) aged 28–72 years participated in the present study. To be eligible for the study, vegans had to have followed a vegan diet for at least 4 years. Of the nine vegans who participated in the study, three were also crudivores, i.e. they ate only raw food or food baked at a maximal temperature of 43°C. Their main food sources were fruits and vegetables, raw nuts and germinated grains. Fifteen omnivores (eleven women and four men) aged 24–68 years also participated in the study. All subjects had to be free of any disease that could affect the studied variables. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Laval University Ethics Committee. Written informed consent was obtained from all subjects.
Body weight and height were measured according to standardised procedures recommended at the Airlie Conference(Reference Lohman, Roche and Martorel31). BMI was calculated as body weight divided by height squared (kg/m2). To determine body fat mass, body density was first measured by the hydrostatic weighing technique. The equation of Siri(Reference Siri32) was then used to derive the percentage of body fat from density. The pulmonary residual volume required for this calculation was measured by the He dilution technique(Reference Meneely and Kaltreider33). The percentage of body fat was multiplied by body weight to obtain body fat mass; fat-free mass was then calculated as the difference between body weight and body fat mass.
RMR was determined by indirect calorimetry after an overnight fast. Following a 15 min resting period, expired gases were collected through a mouthpiece for 15 min while the subject had his nose clipped. A non-dispersive IR analyser (Uras 10 E; Hartmann & Braun, Frankfurt, Germany) was used to measure the O2 and CO2 concentrations. The pulmonary ventilation was determined with a S-430A measurement system (KL Engineering, Ventura, CA, USA). The energy equivalent of O2 volume was calculated by the Weir formula(Reference Weir34).
Serum total T3 and free thyroxine (fT4) concentrations were determined by heterogeneous competitive immunoassay (Bayer Immuno 1™ System; Bayer Corp., Tarrytown, NY, USA). Detection limits were 0·09 nmol/l and 1·3 pmol/l for T3 and fT4, respectively.
The concentrations of eleven chlorinated pesticides (β-hexachlorocyclohexane (β-HCH), p, p′-dichlorodiphenyldichloroethane (p, p′-DDE), p, p′-dichlorodiphenyltrichloroethane (p, p′-DDT), hexachlorobenzene, mirex, aldrin, α-chlordane, γ-chlordane, oxychlordane, cis-nonachlor, trans-nonachlor), fourteen polychlorinated biphenyls (PCB), i.e. congeners with International Union of Pure and Applied Chemistry (IUPAC) nos. 28, 52, 99, 101, 105, 118, 128, 138, 153, 156, 170, 180, 183 and 187, and one commercial mixture of PCB (aroclor 1260) were determined in plasma at the Quebec Toxicological Center. Blood samples were centrifuged to extract plasma (2 ml) which was then cleaned up on deactivated Florisil columns. Samples were eluted with methylene chloride–hexane (25:75, v/v) and concentrated to a final volume of 100 μl. Samples were then analysed on an HP-5890 series II gas chromatograph with dual-capillary columns and dual 63Ni electron detectors. Peaks were identified by their relative retention times obtained on the two columns using a computer program developed by the Quebec Toxicological Center. Total and free cholesterol (TC and FC), TAG and phospholipid (PL) plasma concentrations were also determined by enzymic methods on a Technicon automatic analyser (RA-500; Bayer Corp.) with test packs. Plasma total lipids were then calculated with the following summation method: total lipids = 1·677(TC − FC) + FC + TAG + PL(Reference Patterson, Isaacs and Alexander35). Depending on the lipid content, detection limits varied from 0·02 to 0·3 μg/l. The OC concentrations are expressed in μg/l of plasma and in μg/kg of blood lipids to correct for the differences in total plasma lipids between individuals.
Study 2
For this study, we took advantage of the results obtained during the first 3 months of a 9-month parallel-arm, controlled feeding protocol which was named ‘The Ole Study’. Thirty-seven overweight/obese (BMI 27–35 kg/m2), healthy and sedentary men, aged 21–60 years, completed this project which was performed at the Pennington Biomedical Research Center (Baton Rouge, LA, USA), according to previously described procedures(Reference Bray, Lovejoy and Most-Windhauser36). As described in the next paragraph, the subjects were categorised in three groups differing by the nature of the dietary regimen to which they were exposed. In addition, this description indicates that one group was subjected to an olestra supplementation whereas the other two groups did not consume this supplement.
The Ole Study(Reference Bray, Lovejoy and Most-Windhauser36) was aimed at evaluating the effect on body weight, body fat, lipids, glucose and insulin of a fat-reduced diet and a diet in which dietary fat was replaced by olestra, which cooks and has the mouth-feel of normal fats but cannot be digested in the intestine(Reference Peters, Lawson and Middleton37). Subjects were randomly assigned to one of three diets: a standard diet aimed at maintaining a weight-stable state (33 % fat; n 13), a fat-reduced diet (25 % digestible fat; n 14), or a fat-substituted diet (one-third of dietary fat replaced by olestra to achieve a diet containing 25 % metabolisable fat; n 10). The energy level of the fat-substituted and the fat-reduced diets was designed to be 11 % less than what was determined during the run-in phase. This was accomplished by reducing the number of unit foods and the basal diet energy level. However, the subjects were allowed to request additional snack packs if they felt hungry or reduce the number of unit foods consumed if they were too full. Subjects in the standard group lost an unexpected amount of body weight and fat mass, even if the foods provided were intended to maintain body weight. Bray et al. (Reference Bray, Lovejoy and Most-Windhauser36) suggested that this phenomenon may reflect the fact that even the 33 % fat diet given during the run-in period provided less energy as fat than the subjects' pre-study diets, which was estimated to be close to 39 %. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Pennington Institutional Review Board. Written informed consent was obtained from all subjects.
Body weight and fat mass were measured at baseline and after 3 months of intervention by dual-energy X-ray absorptiometry with a Hologic QDR 2000 absorptiometer (Hologic Inc., Waltham, MA, USA). Blood samples were also taken at baseline and after 3 months of intervention and OC concentrations were measured as described in study 1. However, because no weight-stabilisation period was done before blood samples were taken, we assumed that body concentrations of OC were not in a state of equilibrium. Porta et al. (Reference Porta, Jariod and Lopez38) studied alternative ways of correcting serum concentrations of OC compounds other than the OC:total lipids ratio in patients who were in a state of body dis-equilibrium. They suggested that it is unwarranted to routinely correct OC by total lipids and offered alternatives such as no correction for total blood lipids. In light of this evidence, our statistical analyses were performed with the OC concentrations expressed as mass of OC per volume of plasma (μg/l).
Statistical analysis
In study 1, Student's t test was used to compare the means of descriptive characteristics between vegans and omnivores. In addition, the OC concentrations were compared between the two groups. Student's t test was applied when one non-detectable entry or less was present and Fisher's exact test was used when more than one non-detectable entry was present. P values were adjusted for age and BMI. Non-detectable results were given half the detection limit for statistical considerations. Finally, associations between body fat mass and total OC concentration were assessed for all the participants. This was also the case for the determination of the relationships between T3, fT4 or age and total OC concentration.
In study 2, one-way ANOVA was used to compare baseline age, body weight, fat mass and OC concentrations (P values adjusted for age, body weight and fat mass), as well as changes in body weight, fat mass and OC concentrations (P values adjusted for age, Δ body weight and Δ fat mass) after 3 months of intervention. Post hoc t tests were used to test for differences between each group if an ANOVA was significant. To further assess differences of changes in OC concentrations, the two non-olestra diets (standard diet and fat-reduced diet) were compared with the olestra diet (fat-substituted diet) using the appropriate contrast statement with SAS Mixed procedures (P values adjusted for age, Δ body weight and Δ fat mass). Finally, the changes in fat mass were correlated to the changes in OC concentrations between the control group (standard+fat-reduced group) and the fat-substituted group. The slopes and intercepts of the regression lines were compared between both groups using SAS GLM procedures. All statistical analyses were performed with the SAS software version 9.1 (SAS Institute, Inc., Cary, NC, USA). Data are given as mean values and standard deviations. Statistical significance was set at P < 0·05.
Results
Study 1
Table 1 presents subjects' characteristics for vegans and omnivores. Vegan subjects recruited had been practising a vegan diet for a mean of 10·2 (sd 4·8) years. They tended to be older and leaner than omnivores, with a lower body weight, BMI, percentage body fat and fat mass, but not to a statistically significant extent.
Table 1 Characteristics of participants involved in study 1
(Mean values and standard deviations)
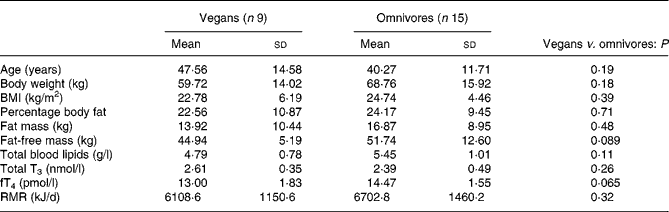
T3, triiodothyronine; fT4, free thyroxine.
There were nine OC compounds that were completely undetectable in each group (aldrin, α-chlordane, γ-chlordane, cis-nonachlor and PCB nos. 52, 101, 105, 128 and 183). Concentrations of the seventeen other pollutants were considered for statistical analyses. With age and BMI taken into account for potential confounders, the plasma concentration of four OC compounds (expressed in μg/l) was significantly lower in vegans compared with omnivores (Table 2). Furthermore, PCB 99 (P = 0·033) was the only OC to be less detectable in the vegans than in the omnivores (see Table 3). However, when values were expressed in μg/kg blood lipids, a difference was found for PCB 99 only (P = 0·023; Table 4). Finally, in Table 2, it is to be noted that the adjusted means for PCB 180 were − 0·029 (sd 0·046) and 0·012 (sd 0·034) μg/l for vegans and omnivores, respectively, and that they were significantly different (P < 0·05) despite the apparent equality of non-adjusted means.
Table 2 Plasma organochlorine concentrations (μg/l) in study 1
(Mean values and standard deviations)
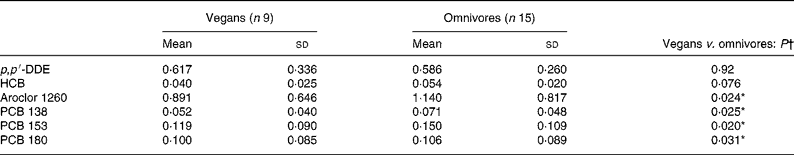
p, p′-DDE, p, p′-dichlorodiphenyldichloroethane; HCB, hexachlorobenzene; PCB, polychlorinated biphenyl.
* P < 0·05.
† Analysed by Student's t test. P values are adjusted for age and BMI.
Table 3 Detectable and non-detectable (ND) plasma organochlorines (no. of entries) in study 1
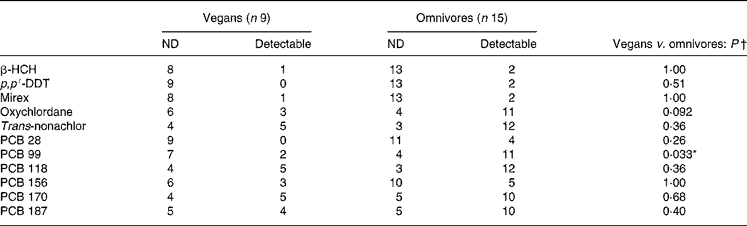
β-HCH, β-hexachlorocyclohexane; p, p′-DDT, p, p′-dichlorodiphenyltrichloroethane; PCB, polychlorinated biphenyl.
* P < 0·05.
† Analysed by Fisher's exact test.
Table 4 Plasma organochlorine concentrations (μg/kg blood lipids) in study 1
(Mean values and standard deviations)

β-HCH, β-hexachlorocyclohexane; p, p′-DDE, p, p′-dichlorodiphenyldichloroethane; p, p′-DDT, p, p′-dichlorodiphenyltrichloroethane; HCB, hexachlorobenzene; PCB, polychlorinated biphenyl.
* P < 0·05.
† Analysed by Student's t test. P values are adjusted for age and BMI.
Correlation analyses were performed by combining the values of the two groups of subjects. A significant positive association was observed between fat mass and total plasma OC concentration (r 0·37; P < 0·05). Age was also positively correlated with total plasma OC concentration (r 0·63; P < 0·01). Besides, plasma T3 concentration was negatively related with total plasma OC concentration (r − 0·48; P < 0·05). However, no significant association was found between plasma fT4 and total plasma OC concentration.
Study 2
There were seven OC compounds that were completely undetectable in both groups (aldrin, α-chlordane, γ-chlordane, and PCB nos. 52, 101, 105 and 128). Concentrations of the nineteen other pollutants were considered for statistical analyses. The baseline characteristics (before treatment) of the subjects are shown in Table 5. Age, body weight and fat mass were not significantly different between the groups. After correction for age, body weight and fat mass, plasma OC concentrations were not significantly different between the groups. Table 6 shows the changes in body weight, fat mass and plasma concentration of detectable pollutants after 3 months of weight-loss intervention. All groups showed significant reductions in body weight and fat mass, which were not significantly different between the groups. One-way ANOVA showed a difference between the changes in OC concentrations for β-HCH, which decreased in the fat-substituted group and increased in the two other groups (P = 0·045). Post hoc t tests demonstrated a significant difference between the fat-substituted group and the fat-reduced group (P = 0·017), a borderline difference between the fat-substituted and the standard groups (P = 0·050), and no difference between the two non-olestra diets (P = 0·64). However, the contrast analysis (data not shown) did not show a significant difference between the two non-olestra diets and the fat-substituted diet. Indeed, change in mirex concentrations (which increased significantly less in the fat-substituted group than in the two other groups) was the only significant contrast (P = 0·029). However, as stated earlier, the one-way ANOVA did not demonstrate a significant difference between the three groups for this compound.
Table 5 Baseline characteristics of the participants involved in study 2
(Mean values and standard deviations)

β-HCH, β-hexachlorocyclohexane; p, p′-DDE, p, p′-dichlorodiphenyldichloroethane; p, p′-DDT, p, p′-dichlorodiphenyltrichloroethane; HCB, hexachlorobenzene; PCB, polychlorinated biphenyl.
† P values for differences in organochlorine concentrations are adjusted for age, body weight and fat mass.
Table 6 Comparison of changes in weight, fat mass and plasma organochlorine concentrations between the standard, the fat-reduced and the fat-substituted groups in study 2
(Mean values and standard deviations)

β-HCH, β-hexachlorocyclohexane; p, p′-DDE, p, p′-dichlorodiphenyldichloroethane; p, p′-DDT, p, p′-dichlorodiphenyltrichloroethane; HCB, hexachlorobenzene; PCB, polychlorinated biphenyl.
a,b Mean values with unlike superscript letters were significantly different (P = 0·017).
* P < 0·05.
† P values for differences in Δ organochlorine concentrations are adjusted for age, Δ body weight and Δ fat mass.
‡ The deltas (Δ) are equal to the values after 3 months of treatment minus the values before treatment.
Finally, as complementary analyses, we combined the standard and fat-reduced data to form a unique control group. We correlated the changes in fat mass to the changes in OC concentrations for the control group and the fat-substituted group. We compared the slopes and intercepts of the regression equations derived from these relationships and found that for all OC, the regression lines tend to parallel each other. However, no significant differences could be found between either intercepts or slopes.
Discussion
The main preoccupation underlying the present two pilot studies was to evaluate the potential of some nutritional approaches (adopting a vegan diet in study 1 and olestra supplementation in study 2) in an attempt to prevent or reduce the body load of OC in humans. This issue is of great interest since the increase in circulating OC has been shown to be associated with metabolic effects whose common feature is a decrease in thermogenesis(Reference Imbeault, Chevrier and Dewailly13, Reference Pelletier, Doucet and Imbeault24, Reference Tremblay, Pelletier and Doucet25). Thus, the metabolic handicap produced by OC pollutants may complicate obesity management. It is, however, important to underline the fact that the present results are the outcome of preliminary work and that due to evident lack of statistical power, they cannot be generalised to the entire population and should be interpreted with caution. Indeed, non-significant results should be interpreted as trends.
In study 1, after analysing OC concentrations expressed in μg/l of plasma, we found that vegans were significantly less polluted than omnivores regarding aroclor 1260 and PCB 99, PCB 138, PCB 153 and PCB 180, with a trend for hexachlorobenzene (P = 0·076) and oxychlordane (P = 0·092), even after adjustment for age and BMI. These findings are striking considering the very low power of the study and are in accordance with previous studies that found a lower OC concentration in breast milk and adipose tissue of vegetarians compared with omnivores(Reference Hergenrather, Hlady and Wallace7–Reference Noren9). Interestingly, when corrected for serum lipid values, OC concentrations tended to be similar between both groups (with the exception of PCB 99; P = 0·023). The latter results are strengthened by Fisher's exact test that showed a difference for PCB 99 only (P = 0·033). In this regard, it is reasonable to hypothesise that significance could be obtained in other OC concentrations with larger sample sizes that provide more statistical power.
A certain number of factors may explain why we did not see a difference in all plasma OC concentrations between vegan and omnivore subjects. First, studies that found a lower OC concentration in breast milk and adipose tissue of vegetarians are all more than 25 years old. In that period, the concentrations of OC in humans and animal products were higher. Now, we are exposed to much lower levels and it might be that we have reached a steady state. For example, in a recent study by Agudo et al. (Reference Agudo, Goni and Etxeandia39), the concentration of PCB in Spanish adults was on average 12 % higher in samples from 1993 than those from 1995. Second, the vegans in the present study may have been breast-fed as infants, and might thus have been exposed to OC accumulated by the mother and which are transferred to her baby at the time of lactation(Reference Rozman, Klaassen, Casarett, Amdur, Klaassen and Doull40, Reference Anderson and Wolff41). Moreover, becoming a vegetarian or a vegan is often a decision that is made in adulthood. Thus, the omnivore diet followed during childhood and adolescence results in a contamination by OC that is still detectable in adults, since these compounds are resistant to degradation. In order to see a significant difference between plasma OC concentration in vegans and omnivores, we should maybe study individuals that have been vegans for more than 10 years. In fact, PCB half-lives have been found to be 5–25 years, depending of the specific congener make-up of the PCB mixture(Reference Wolff, Fischbein and Selikoff42). Another reason that may explain the presence of OC in vegans is that they were 7 years older than the omnivores. In our cohort, age was positively correlated with total plasma OC concentration, suggesting an OC accumulation with age. This observation is concordant with our recently reported data(Reference Hue, Marcotte and Berrigan10, Reference Hue, Marcotte and Berrigan14) and those published by other investigators(Reference Luotamo, Jarvisalo and Aitio43–Reference Kiviranta, Tuomisto and Tuomisto48). In addition, vegans may, on rare occasions, depart from their diet and eat some animal products. In addition, the consumption of imported fruits containing OC may also be a problem(Reference Hall49). Furthermore, it is relevant to emphasise that the contamination might not only come from food sources. Indeed, there are countries that still use OC which can be transported by air and thus contaminate rivers and fields of other countries, particularly in Nordic areas(Reference Barrier50–Reference Ma, Daggupaty and Harner52). In this respect, OC may contaminate the water that vegans drink, the air that they breathe and the vegetables, fruits and cereals that grow in fields. Therefore, even if an individual eats exclusively biologically certified food, exposure to OC is not excluded.
We observed a positive correlation between fat mass and total plasma OC concentration, and this finding agrees with many previously reported studies(Reference Chevrier, Dewailly and Ayotte11, Reference Pelletier, Imbeault and Tremblay53). Indeed, the body load of these lipid-soluble compounds is increased in obese individuals because of their increased dilution space (body fat mass) and slightly increased concentrations in plasma and fat tissues(Reference Pelletier, Doucet and Imbeault24). Finally, the fact that plasma T3 concentration was negatively related with total plasma OC concentration is consistent with the results of Cheek et al. (Reference Cheek, Kow and Chen54) who showed that changes in OC concentrations alter the serum level of some hormones because they have a thyroid hormone-like affinity for the serum transport protein transthyretin. Furthermore, recent results from our laboratory showed that body weight/fat loss is related to a greater than predicted decrease in plasma T3 concentration(Reference Pelletier, Doucet and Imbeault24).
After severe contamination, the ingestion of olestra is the only potential solution that has been shown to accelerate the body clearance of OC(Reference Geusau, Tschachler and Meixner27–Reference Jandacek, Anderson and Liu29). To our knowledge, however, olestra's depolluting effects in human have not been investigated with lower levels of contamination that are generally observed in response to the usual weight-reducing programmes. Thus, study 2 is the first to examine the extent to which olestra could prevent the increase in plasma OC concentrations following a small to moderate decrease in the lipid dilution space for OC. With the exception of β-HCH, which decreased in the fat-substituted group while increasing in the two other groups (P = 0·045), changes in OC concentrations were not significantly different between the groups. Moreover, when the two non-olestra groups are compared with the fat-substituted group by contrast analyses, only mirex shows a significant difference (P = 0·029). However, this result appears to be mainly driven by the standard diet group giving a high mean value and should be interpreted with caution since the one-way ANOVA did not demonstrate a difference between the three groups. These results were reinforced by the fact that no significant differences were seen between the slopes and intercepts of the regression lines correlating changes in fat mass and changes in OC concentrations. Once again, these results are preliminary and would necessitate larger sample sizes to really detect an effect of olestra.
Apart from the small sample sizes, some limitations of the present study could also contribute to explain the apparent inability of olestra to reduce most OC plasma concentrations. First, the range that we have on the body burden of OC (plasma concentrations) reflects a relatively stable depot (adipose concentrations) that is in equilibrium with the plasma(Reference Jandacek, Anderson and Liu29). There is no clear indication about the possible influence of duration of weight loss on the mobilisation of OC from tissues to the blood circulation. Thus, in the present study, a 3-month weight-loss period could have been too short to show a depolluting effect. Another possible explanation could be that the doses of olestra administrated to the participants were too low to prevent the hyperconcentrations of OC. In fact, a previous study showing a potential depolluting role for olestra was based on results derived from experiments in mice, which received relatively high dosages in terms of human levels(Reference Jandacek, Anderson and Liu29). Moreover, human subjects in whom a depolluting effect of olestra was observed were severely contaminated with TCDD(Reference Geusau, Tschachler and Meixner27) and aroclor 1254(Reference Redgrave, Wallace and Jandacek28), which may suggest that the body load of our subjects was too low to detect an effect of olestra. Moreover, we based our analyses on changes in plasma OC concentrations alone but not on changes that could have resulted in other tissues or by way of faecal excretion. In fact, in previous studies, olestra had large effects on reduction of OC concentrations in tissues(Reference Jandacek, Anderson and Liu29, Reference Redgrave, Wallace and Jandacek28) with little effect on plasma levels. Taken together, these observations support the relevance of retesting the effects of olestra on plasma and tissue concentrations and on faecal excretion of OC in obese patients experiencing a larger weight loss, such as massively obese patients subjected to bariatric surgery. For instance, in a recent study, the mean cumulative plasma concentration of OC was found to increase by 388 % at 1 year after a biliopancreatic diversion(Reference Hue, Marcotte and Berrigan14). In such patients, olestra might exert a detectable depolluting effect that could facilitate the control of energy expenditure and eventually help prevent weight regain. Finally, in the present study, there was no weight-stabilisation period before blood samples were taken. Lack of weight stability should have influenced the results because the plasma and adipose tissue compartments would probably not be in equilibrium. Thus, any effect of olestra to drain OC via the stool would not show its full effect in plasma.
In summary, the two pilot studies presented in this paper represent a valuable effort aiming at evaluating the potential of some nutritional approaches to prevent or reduce the body load of OC in humans. Taken together, these observations emphasise the difficulty of preventing body accumulation or promoting clearance of OC compounds in free-living individuals. The first study seems to demonstrate trends in favour of a preventive effect of a vegan diet. In study 2, olestra favourably influenced the plasma concentrations of β-HCH but the data do not yield enough evidence to support an effect on the other OC compounds measured before and after the weight-loss programme. For individuals subjected to weight loss, studies of greater statistical power (sample sizes more than twenty individuals) and longer duration (>3 months) in individuals displaying a greater body load of pollutants (morbidly obese, older, omnivores and/or professionally exposed to OC) and given more pronounced doses of a therapeutic agent, for example, olestra, are necessary before excluding OC clearance as a target of nutritional decontaminating approaches.
Acknowledgements
The authors express their gratitude to the participants for their excellent collaboration and the staff members for their contribution to both studies. They also thank Claude Leblanc for his important contribution to the statistical analyses. Study 1 was supported by the Canada Research Chair in Environment and Energy Balance. The Ole Study (Baton Rouge) was supported in part by grant 96034323-3031 from the United States Department of Agriculture and by the Procter and Gamble Co., Cincinnati. H. A. is supported by the Fonds de la Recherche en Santé du Québec (FRSQ).
H. A. drafted the manuscript and contributed to data analysis. M. S. contributed to the development of the design of study 1, tested subjects and contributed to data analysis. G. A. B. and J. C. L. contributed to the development of the design of study 2 and to its realisation. J. C. P. contributed to the preparation of the manuscript, particularly in regards to the effects of olestra. R. J. J. contributed to the preparation of the manuscript, particularly about the body clearance of OC. J.-P. C. contributed to data analysis and to the preparation of some parts of the manuscript. A. T. contributed to the development of the design of study 1 and to its realisation. He also planned the conceptual integration of the global issue documented in this paper. All authors contributed to the revision of the manuscript.
There are no conflicts of interest to declare.








