Introduction
Haemorrhagic fever with renal syndrome (HFRS) is an acute zoonotic viral disease caused by Hantaviruses with abrupt onset of fever, lower back pain, varying degrees of haemorrhagic manifestations and renal involvement. The disease is characterised by five clinical phases which frequently overlap: febrile, hypotensive, oliguric, diuretic and convalescent. Infection occurs via inhalation, ingestion, or mucous membrane contact with virus-contaminated particles of urine, faeces or saliva from infected rodents. Symptoms appear 1–4 weeks after exposure [Reference Heymann1]. Hantavirus infection is thought to provide a lifelong immunity and antibodies can be found decades after recovery [Reference Vapalahti2].
The main hantaviruses in Europe causing HFRS are Puumala viruses whose reservoir is Myodes glareolus (bank vole), Dobrava viruses; reservoir Apodemus flavicollis (yellow-necked mouse) and Saaremaa viruses; reservoir Apodemus agrarius (striped field mouse) [Reference Vapalahti2–Reference Vaheri4]. Puumala virus, dominant in Europe, causes mild disease with a case-fatality rate <1%, while Hantaan (mainly in Asia) and Dobrava viruses (in the Balkans) cause severe illness [Reference Avšič Županc, Korva and Markotić5].
Sighting of rodents, exposure to rodent droppings and trapping rodents have been reported to increase the risk of acquiring HFRS infection [Reference Van Loock6, Reference Vapalahti7]. Further risk factors include spending time or working in a forest, woodcutting and housewarming with firewood. Increased incidence in professional groups was reported in the army, farmers and forestry workers [Reference Markotic8–Reference Crowcroft10]. Smoking is associated with aggravated Puumala hantavirus-induced HFRS [Reference Tervo11].
In Croatia, the first case of HFRS was reported in 1952 [Reference Radosevic and Mohacek12]. Croatia is considered endemic for HFRS, except in the coastal and island regions [Reference Markotic8, Reference Kuzman13]. HFRS is a notifiable disease according to the ‘Act on the Protection of the Population against Communicable Diseases’. The main rodent reservoirs of hantaviruses in Croatia are the yellow-necked mouse (Apodemus flavicollis), the striped field mouse (Apodemus agrarius) and the bank vole (Myodes glareolus) [Reference Cvetko14]. Several large outbreaks were reported in 1995, 2002, 2012 and 2014 [Reference Markotic8, Reference Kuzman13, Reference Vilibic-Cavlek15, Reference Tadin16]. In the 2012 and 2014 outbreaks, 95% of HFRS cases were caused by Puumala virus and 5% by Dobrava virus [Reference Vilibic-Cavlek15]. During 2011–2016, in Zagreb city, the number of notified cases ranged between 0 and 42 annually. Between January and the end of April 2017, 11 cases of HFRS from Zagreb were notified to the Teaching Institute for Public Health ‘Andrija Stampar’ (TIPH), while during the same period in previous 5 years, 0–4 cases were reported. We investigated in order to estimate the extent of the outbreak and identify specific risk factors for acquiring HFRS infection.
Methods
We conducted a matched case-control study. We defined HFRS cases as residents in Zagreb with laboratory-confirmed IgM positive Puumala and/or Dobrava Hantavirus infection with symptoms onset after 1 January 2017. Cases were identified from notifications from the Croatian surveillance system. Following the first case, all general practitioner (GP) practices in Zagreb were notified about the increased HFRS cases and the need to refer to the infectious disease specialist to the University Hospital for Infectious Diseases, ‘Dr Fran Mihaljevic’. We also alerted hospitals to take serum samples for the laboratory confirmation of suspected HFRS cases and report all HFRS cases. In April, we compared cases with matched controls who were household members or the closest neighbours of the cases. One control was recruited per case.
We interviewed cases and controls using a structured questionnaire. Cases were asked about their symptoms, date of onset of symptoms and information on hospitalisation. Both cases and controls were asked about demographic data and risk exposures for acquiring HFRS infection in the 4 weeks prior to the symptom onset of the cases, including visiting a forest, visiting Mount Medvednica and activities on Mount Medvednica, observing rodents, housing activities such as cleaning a basement or attic (exposure to dust potentially infested with rodent's excreta). All cases were interviewed face-to-face while controls were interviewed either by telephone or face-to-face. Hantavirus infection in HFRS cases was confirmed by detecting IgM antibodies using indirect immunofluorescence assay (IFA) at the University Hospital for Infectious Diseases ‘Dr Fran Mihaljevic’.
We described cases by time, place and person. We calculated matched odds ratios (mOR) for risk exposures adjusted for age, sex and smoking, using conditional logistic regression. All statistical analyses were performed using STATA software version 12 (Stata Corp LP, Texas, USA). All data were treated confidentially and were only available to the researchers.
Results
During 2017, 104 cases were reported to the TIPH that fulfilled the case definition. Cases were between 11 and 81 years old (median 37 years); 71% (73/104) were male. One (1%) case was <18 years old. Cases were reported throughout the year and the onset of illness peaked on week 18 in May (Fig. 1). The distribution of cases according to their place of residence can be found in Figure 2. Cases reported having symptoms of high fever (n = 104, 100%), headache (n = 87, 84%), back pain (n = 79, 76%), decreased urinary output (n = 64, 66%), blurred vision (n = 45, 44%), nausea (n = 38, 37%), diarrhoea (n = 27, 26%), cough (n = 26, 25%) and breathing difficulties (n = 16, 16%). A total of 89 (86%) cases were hospitalised with a median hospital stay of 8 days (range 1–17). Three cases (3%) required haemodialysis and all had Puumala virus infection. Laboratory analysis confirmed that 101 (97%) cases had Puumala virus infection, while three (3%) were positive for Dobrava virus. One case with confirmed Dobrava virus died; a case fatality ratio of 1%.
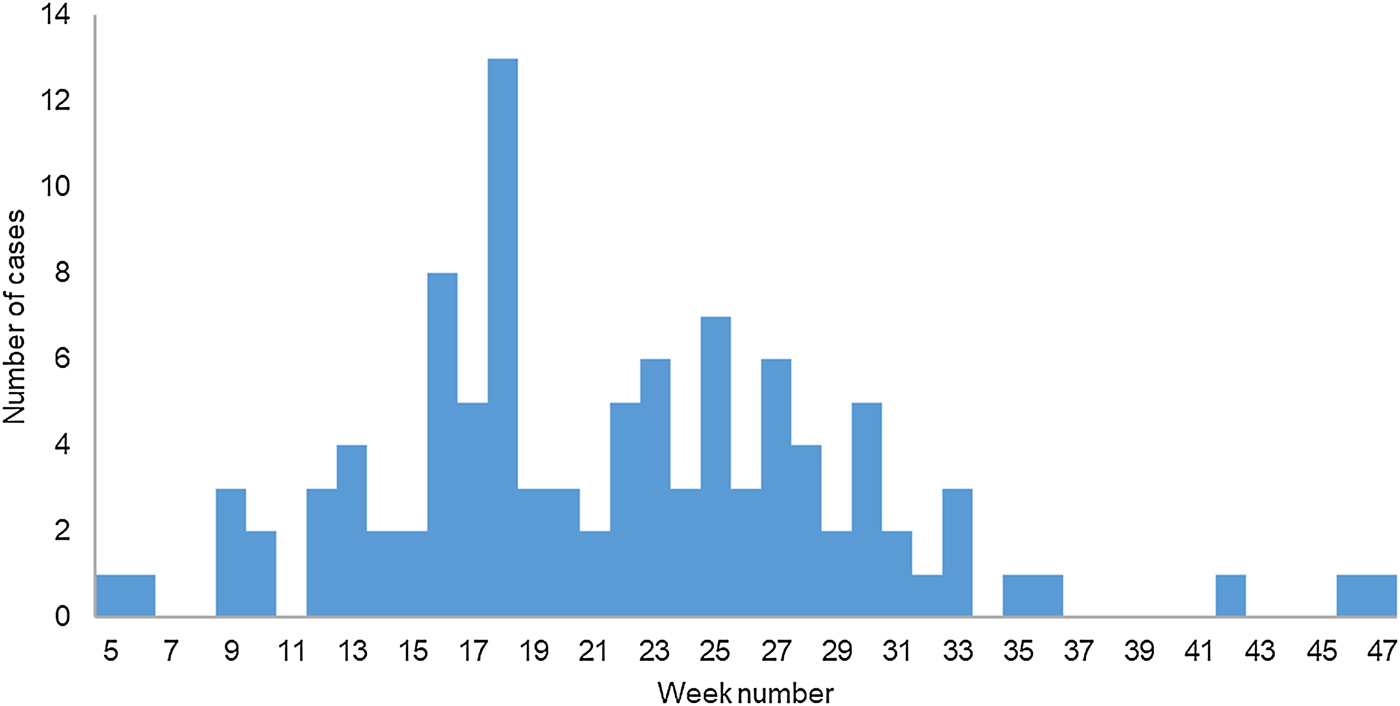
Fig. 1. Cases of haemorrhagic fever with renal syndrome (N = 97) by week of symptom onset, Zagreb, 2017.
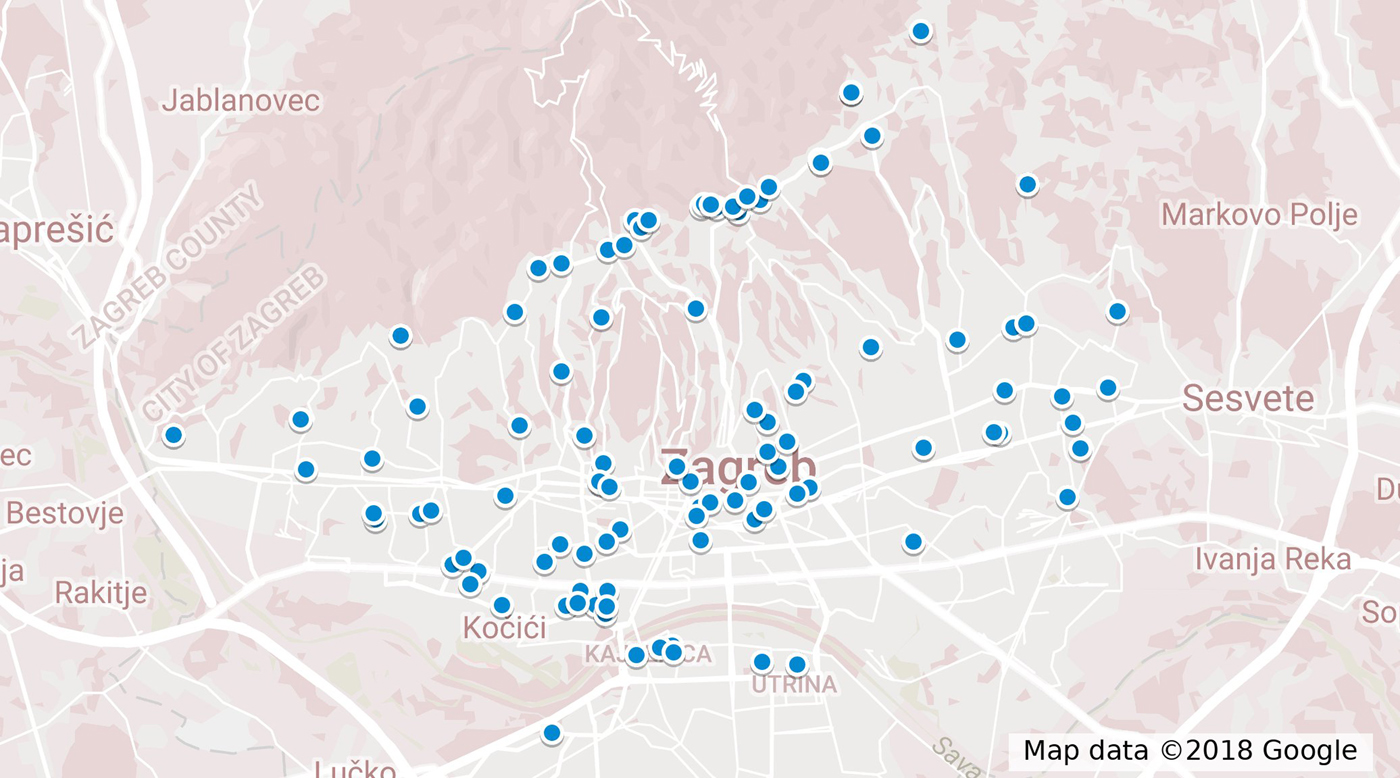
Fig. 2. Map of distribution of cases according to the place of residence.
In total, 104 matched case-control pairs were included in the case-control study. Compared with controls, cases were more likely to report visiting a forest, visiting Mount Medvednica and observing wild rodents (Table 1). In total, 94% of cases reported visiting a forest area during the 4-week recall period, two (2%) of which were for professional activities (forest workers). Seventy per cent (73/104) of cases had visited Mount Medvednica prior to becoming ill and 52% (54/104) had observed wild rodents. Among participants who had visited Mount Medvednica, cases were more likely to have observed wild rodents, drunk water from a spring, cycled or picked flowers from the ground, compared with controls (Table 1).
Table 1. Frequency of exposures among HFRS cases and controls, Zagreb, 2017
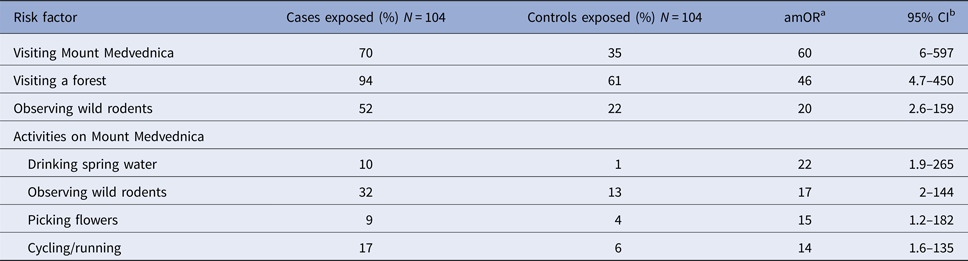
a amOR = adjusted matched odds ratio.
b 95% CI = 95% confidence interval.
Discussion
Although outbreaks of HFRS have been previously reported in Zagreb [Reference Cvetko14, Reference Tadin16], this was the largest HFRS epidemic to date. In previous outbreaks, Mount Medvednica was suspected as natural focus of infection, but this was the first analytical study that indicated a strong association between exposure to Mount Medvednica and HFRS infection. Almost three-quarters of cases visited the area prior to becoming ill. Mount Medvednica is a popular recreational area for Zagreb residents with more than one million visitors per year [Reference Farkaš Topolnik17]. Our findings indicate that running, cycling, picking flowers and drinking spring water on Medvednica were associated with the disease. While performing these activities, dust is raised that could potentially contain particles of urine, faeces or saliva contaminated with virus from infected rodents, or contaminated water is ingested. A 2011–2014 study on rodents in beech forests of Medvednica indicated two dominant rodent species, yellow-necked mouse (Apodemus flavicollis) and bank vole (Myodes glareolus), with 7%–42% of trapped rodents being positive with hantaviruses (Dobrava and Puumala virus) [Reference Bjedov18]. Comparison of Puumala virus sequences obtained from five humans and rodents during a winter outbreak of HFRS in 2012 indicated 98%–100% sequence similarity, suggesting that the patients were probably exposed to Puumala virus on Medvednica mountain [Reference Tadin16].
In accordance with other studies, we identified an association between observing wild rodents and being at risk of hantavirus infection [Reference Van Loock6, Reference Winter9]. This exposure may reflect an increase in the reservoir population due to favourable feeding and climate conditions. In mast years, trees produce high quantities of seeds, an important food source for rodents. Climate conditions such as high seasonal air temperature have a great influence on the yield of beech mast [Reference Övergaard, Gemmel and Karlsson19, Reference Drobyshev20]. Climate monitoring reports and an assessment in 2016 demonstrated that during the autumn and winter months of 2015/2016 seasonal air temperatures around the Medvednica area were higher than average [Reference Pandzic and Likso21]. The yield of beech has an important impact on the dynamics of the small rodent population [Reference Bjedov18, Reference Jensen22]. Abundant production of forest seed allows small rodents to continue to breed during the winter, resulting in a high rise in the number of rodents early in the spring of the next year [Reference Tersago23]. Previous studies during outbreaks reported a positive correlation between the size of rodent population and the number of human infections [Reference Kallio24, Reference Tersago25]. A 2011–2014 study on rodents in beech forests at Medvednica indicated a correlation of beech mast year and rodent abundance in the next year followed by high infection rates of rodents with Hantaviruses [Reference Bjedov18]. In the years with rodent abundance, a high number of human HFRS cases were reported [Reference Bjedov18].
Our results also indicated an association between a visit to a forest and the HFRS infection, as has been reported in other studies [Reference Sin26, Reference Gherasim27]. Almost all cases reported visiting a forest area before symptoms onset. Exposures such as cleaning the basement, the attic or other utility rooms were not identified as risk factors in our study, further suggesting that in this outbreak, forest exposure was the sole risk factor among this urban population. Cleaning utility rooms was identified as risk factor for Puumala virus infection during an outbreak in Germany [Reference Winter9].
Some limitations must be considered in interpreting results of our study. First, some cases may not have been reported through the surveillance system but we expect under-reporting to be low, as we had enhanced contacts with the laboratories and hospital staff in Zagreb during the outbreak. Cases with mild and asymptomatic HFRS infection may not have been included if they had not contacted their doctor. In our study, all cases sought medical attention and most of the cases were hospitalised due to the severity of their medical condition. Second, since many controls lived in the same household as the cases, they may have had similar habits to the cases and may have been more likely to report visiting Medvednica. This may have underestimated the strength of the association between visiting Medvednica and HFRS infection. Third, recall bias might have been present due to the delay between the time of exposure/symptom onset and the time of the interview. Fourth, we did not collect information on smoking habits. Smoking is a known risk factor for hantavirus infection and could potentially confound the reported associations.
During this outbreak, TIPH epidemiologists regularly provided advice to the public, through the internet and the local media, on general hand hygiene and avoiding contact with rodent urine, droppings, saliva and nesting materials and information on the safety measures to be followed when cleaning rodent-infested areas and during outdoor activities. Also on weekly basis, the outbreak trends and epidemiological features of cases were analysed and information has been distributed to hospitals and GP practices. Extensive rodent control measures could not be performed as Mount Medvednica is a nature park with some special legal regulations.
Our findings point out that forest exposures in Mount Medvednica were the main risk factors of HFRS during this outbreak. We recommend enhanced surveillance of the recreational areas when an outbreak is identified. A seroprevalence study of Hantavirus infection among Zagreb residents could provide a better insight into the extent of the infection. Finally, in order to effectively control (by early warning signals, outbreak investigation and preventive measures) the HFRS outbreak there is a need to strengthen inter-sectoral collaboration between veterinarians, biologists, ecologists, forestry specialists and epidemiologists.
Acknowledgements
We would like to thank Natasa Cetinic Balent, MD from the University Hospital for Infectious Diseases ‘Dr Fran Mihaljevic’ and all epidemiologists from TIPH who contributed to the outbreak investigation. In addition, we wish to thank Dr Sooria Balasegram and Anita Lovric Vlah for their assistance in improving the use of English in the manuscript.
Conflict of interest
None.






