Depression and dementia are the most prevalent and disabling mental disorders among older populations. It has been predicted that the number of people with dementia in the world will double every 20 years to 42 million by 2020 as the aged population grows. Reference Ferri, Prince, Brayne, Brodaty, Fratiglioni, Ganguli, Hall, Hasegawa, Hendrie, Huang, Jorm, Mathers, Menezes, Rimmer and Scazufca1 Evidence that depression and dementia often exist concurrently has stimulated speculation that there are complex associations between these two conditions. Reference Emery and Oxman2 Many studies, Reference Jorm3,Reference Andersen, Lolk, Kragh-Sorensen, Petersen and Green4 but not all, 5 have reported that a history of depression is a risk factor for dementia. However, recent data Reference Vinkers, Gussekloo, Stek, Westendorp and van der Mast6,Reference Ganguli, Du, Dodge, Ratcliff and Chang7 have shown that depressive symptoms are cross-sectionally associated with cognitive impairment but not with subsequent cognitive decline, suggesting that the presence of depression alone does not increase the risk of cognitive decline. This may raise questions about the risk of dementia in relation to depression. Particularly, we could find no investigation linking depressive syndromes, as opposed to depressive symptoms with increased risk of dementia.
Previous studies on the association of depression with dementia have mainly been carried out in the Western world. Also, few investigators have examined the effects of the severity of depression on dementia. Reference Jorm3,Reference Ownby, Crocco, Acevedo, John and Loewenstein8 In many Western populations, low socio-economic status, low social support, high cardiovascular risk factors and depression tend to co-occur, Reference Woodward, Oliphant, Lowe and Tunstall-Pedoe9–Reference Almeida, Flicker, Norman, Hankey, Vasikaran, van Bockxmeer and Jamrozik11 making a relationship between depression and dementia difficult to unravel. By contrast with Western populations, older people in China exhibit different patterns of risk factors with extremes of absolute deprivation combined with high levels of social supports, Reference Chen, Wei, Hu, Qin, Copeland and Hemingway12 low levels of depression Reference Chen, Copeland and Wei13,Reference Chen, Hu, Qin, Xu and Copeland14 and low levels of some cardiovascular risk factors (e.g. serum cholesterol, body mass index). Reference Hu, Wang, Chen, Jin, Yang, Stampfer and Xu15,Reference Chen, Peto, Collins, MacMahon, Lu and Li16 Studying such a population may therefore, offer internationally applicable insights into the aetiological role of depression in dementia.
Using a semi-standardised method, the Geriatric Mental State (GMS), Reference Copeland, Prince, Wilson, Dewey, Payne and Gurland17 we examined the mental status of older residents in China, Reference Chen, Hu, Qin, Xu and Copeland14 following our Medical Research Council – Ageing in Liverpool Project Health Aspects (MRC–ALPHA) study in the UK. Reference Wilson, Chen, Taylor, McCracken and Copeland10 The participants were diagnosed as having both different levels of syndromes and cases of depression and dementia. In this study we analysed data from two cohorts, one in China and the other in the UK, to investigate whether there were: any ‘dose–response’ associations for depressive syndromes and cases, with incident dementia; and possible variations with age, gender, ethnicity, cardiovascular disease (CVD) comorbidity, and type of depression.
Method
Participants
Chinese participants were those from our Hefei cohort study in Anhui Province, China. The methods used in the study have been fully described elsewhere. Reference Chen, Hu, Qin, Xu and Copeland14 Briefly, in 2001 we randomly selected 1810 people aged ⩾65 years from the residency committee lists who had lived for at least 5 years in Yiming district of Hefei city, Anhui. Permission for interview and informed consent were obtained from each perosn but if that was not possible, from the closest responsible adult. Refusals were respected. Ethical approval was obtained from Anhui Medical University and the district government.
There were 1736 people who agreed to participate in the study (a response rate of 95.9%). Participants were interviewed at home by a trained survey team from the School of Health Administration, Anhui Medical University. The main interview materials were the GMS – a comprehensive semi-structured mental state interview Reference Copeland, Prince, Wilson, Dewey, Payne and Gurland17 and a general health record that included risk factors. Reference Chen, Hu, Qin, Xu and Copeland14 Blood pressure and physical measurements were taken. The validation study of depression cases was carried out by two consultant psychiatrists. A year after the baseline interview, 1293 participants (74.5%) were successfully re-investigated, using the same protocol.
British participants were those from the MRC–ALPHA study, Reference Wilson, Chen, Taylor, McCracken and Copeland10 part of the MRC Cognitive Function and Ageing Study (www.cfas.ac.uk). The study methods have been fully described elsewhere. Reference Copeland, McCracken, Dewey, Wilson, Doran, Gilmore, Scott and Larkin18 In brief, a sample of 6035 older people aged ⩾65 were randomly selected using the Liverpool Family Practitioner Committee central computerised list of general practice patients in 1989. Of these, 5222 participants were interviewed with a response rate of 86.5%. The main interview materials included the GMS and the Minimum Data Set. Reference Saunders, Copeland, Dewey, Davidson, McWilliam, Sharma and Sullivan19 Two years later, 3519 (67.4%) participants were successfully re-interviewed, and 4 years later 2238 (42.9%) participants were re-investigated. Ethical approval was obtained from the multicentre and local ethical committee.
Assessment of syndromes and cases of depression and dementia
A computer program assisted diagnosis – the Automated Geriatric Examination for Computer Assisted Taxonomy (AGECAT) – and was used to analyse the information from the GMS to identify the principal mental disorders in participants. Reference Copeland, Prince, Wilson, Dewey, Payne and Gurland17,Reference Copeland, Dewey and Griffiths-Jones20 It was developed using a theoretical model and tested against its success at replicating diagnoses on samples diagnosed by psychiatrists. It attempts to replicate the process by which a psychiatrist achieves a syndromal diagnosis followed by a differential diagnosis. Geriatric Mental State symptoms are coalesced into a 150 ‘symptoms components’. In stage I the symptom components are brought together into groups which typify the major symptom areas of each diagnostic syndrome. The scores on these individual groups determine the final syndromal level of ‘confidence of diagnosis’. Thus, the system uses both quantitative and qualitative measures when allocating participants to the levels of confidence; required for its construction are many hundreds of clinical decisions on the placement of groups of symptom components on the syndrome levels. Individual participants are allocated to levels of confidence of diagnosis (0–5) on each of the eight diagnostic syndromes: organic disorder, depression, mania, schizophrenia and paranoid, obsessional, phobic, hypochondriacal, and general anxiety. In stage II the various syndrome levels are compared to derive a final differential diagnosis, a level of confidence of diagnosis from 0–5. A level of ⩾3 in most circumstances designates a ‘case level’ that has been shown to correspond with what psychiatrists usually recognise as a case for intervention. Levels 1 and 2 are designated as ‘sub-cases’, and level 0 (no confidence level on any syndrome) is classified as ‘well’. Reference Copeland, Chen, Dewey, McCracken, Gilmore, Larkin and Wilson21 Although the levels are intended to be levels of confidence of diagnosis, it has been our observation that, in those conditions where diagnosis tends to be on a continuum with normality, for example in cognitive decline and depression, the different levels tend also to reflect levels of severity in terms of type and numbers of symptoms. Here we use ‘severity’ in terms of the symptoms themselves not in terms of their impact on daily living. In community-based samples the proportion of organic cases not resulting from dementia is small; acute confusional states have been estimated in about 0.5% of the community sample and the proportion of participants with depression sufficiently severe to be diagnosed as an organic state at case level also appear to be small. It has been the practice in community studies to ignore them.
A number of studies comparing AGECAT diagnoses for depression and dementia with psychiatrists' diagnoses using DSM–III and DSM–IV or ICD–10 criteria achieved a good level of agreement, Reference Copeland, Dewey and Griffiths-Jones20 including in older Chinese people. Reference Liu, Li, Zhang and Chen22,Reference Kua23 The AGECAT also divides depression into psychotic and neurotic types that appear to equate well with DSM–III 24 /DSM–IV 25 major affective disorder and dysthymia without the 2-year rule. ‘Type of depression’ refers to diagnostic cases which emerge after stage II where syndrome diagnoses have been converted into a single differential diagnosis. The symptoms defining depression and dementia are not similar. The GMS–AGECAT diagnosis has been the most widely used international community-based study method for investigations of depression and dementia in older people. Reference Copeland, Prince, Wilson, Dewey, Payne and Gurland17
Statistical analysis
Data were analysed for those who did not have dementia at baseline and were followed up (1254 Chinese and 3341 British). The associations of baseline depressive syndromes with subsequent dementia at follow-up were explored using a Cox regression model with adjustment for age, gender, educational level and CVD. In examining the risk of dementia in relation to depression, baseline sub-cases of dementia were further allowed in the multiple regression model for analysis. Differences in the relationship between subgroups of age, gender, ethnicity, CVD comorbidity and type of depression were tested. Reference Altman and Bland26 All analyses were performed in SPSS version 13.0 for Windows.
Results
Table 1 shows number and incidence of dementia across baseline depressive syndromes. There were no level 5 depressive syndromes (nor cases) in our community-based samples. The risk of dementia was not associated with level 1–3 depressive syndromes, but significantly increased with level 4. Differences in the increased hazard ratio (HR) between Chinese and British participants were not significant (P>0.20). This relationship was further observed with baseline depression cases at level 4 only, but not with less severe cases at level 3 and sub-cases at levels 1–2. These findings remained significant even after further consideration of baseline dementia subcases, which were associated with incident dementia (Fig. 1).
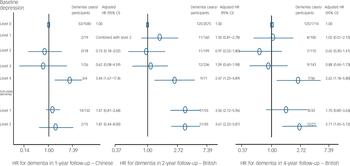
Fig. 1 Associations of baseline depression and sub-cases of dementia and incident dementia. HR, hazard ratio.
Table 1 Number, percentage and relative risk of incident dementia across baseline depressive syndromes
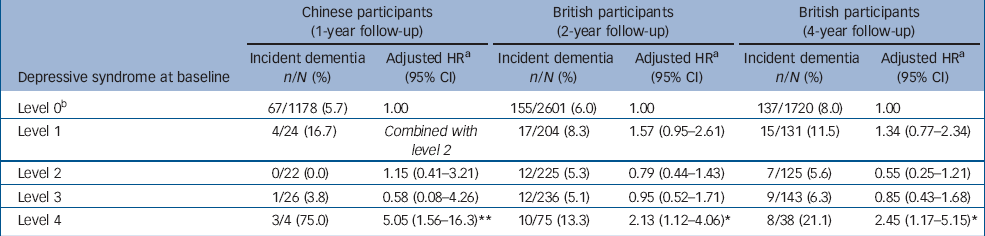
| Chinese participants (1-year follow-up) | British participants (2-year follow-up) | British participants (4-year follow-up) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Depressive syndrome at baseline | Incident dementia n/N (%) | Adjusted HR a (95% CI) | Incident dementia n/N (%) | Adjusted HR a (95% CI) | Incident dementia n/N (%) | Adjusted HR a (95% CI) | |||
| Level 0 b | 67/1178 (5.7) | 1.00 | 155/2601 (6.0) | 1.00 | 137/1720 (8.0) | 1.00 | |||
| Level 1 | 4/24 (16.7) | Combined with level 2 | 17/204 (8.3) | 1.57 (0.95–2.61) | 15/131 (11.5) | 1.34 (0.77–2.34) | |||
| Level 2 | 0/22 (0.0) | 1.15 (0.41–3.21) | 12/225 (5.3) | 0.79 (0.44–1.43) | 7/125 (5.6) | 0.55 (0.25–1.21) | |||
| Level 3 | 1/26 (3.8) | 0.58 (0.08–4.26) | 12/236 (5.1) | 0.95 (0.52–1.71) | 9/143 (6.3) | 0.85 (0.43–1.68) | |||
| Level 4 | 3/4 (75.0) | 5.05 (1.56–16.3) ** | 10/75 (13.3) | 2.13 (1.12–4.06) * | 8/38 (21.1) | 2.45 (1.17–5.15) * | |||
a. Adjusted for age, gender, educational level, and cardiovascular diseases (hypertension, angina, coronary or other heart diseases and stroke)
b. All participants without any depressive syndromes
* P⩽0.05
** P⩽0.01
We investigated differences in baseline covariables between MRC–ALPHA participants with depression at level 4 (n=108) and those at level 3 (n=375). There were no significant differences with age (77.9 v. 77.6), gender (male 40.7% v. 38.9%), education years (9.0 v. 9.2), Townsend score – an index for socio-economic deprivation Reference Townsend, Phillimore and Beattie27 – (6.14 v. 5.56), treated hypertension (26.9% v. 30.7%), heart attack (9.3% v. 13.6%), angina/chest pain (15.7% v. 17.9%), and stroke/parts of body paralysed (11.1% v. 11.7%). However, individuals with level 4 depression had more organic syndromes at level ⩾3 (8.3% v. 0.8%, P<0.001), more depressive neurosis (62.0% v. 21.9%, P<0.001) and more antidepressant drug use Reference Wilson, Copeland, Taylor, Donoghue and McCracken28 (benzodiazepines 37.0% v. 21.6%, P=0.001; tricyclic antidepressants, selective serotonin reuptake inhibitors, lofepramine and others 17.6% v. 8.0%, P=0.004). After further adjustment for these medications, the results were not substantially changed; multiple adjusted HR for incident dementia was 2.41 (95% CI 1.20–4.85) and 2.54 (1.13–5.69) in the 2- and 4-year follow-up respectively.
We examined differences in the effects of level 4 depression on incident dementia between the subgroups in the MRC–ALPHA combined data of incident dementia in the 4-year follow-up with a 2-year follow-up (i.e. adding the Wave III incident dementia into Wave II). There were no significant differences in the adjusted HRs between men (HR=1.73, 95% CI 0.61–4.91) v. women (HR=2.07, 95% CI 1.03–4.15) (P=0.78); people with CVD comorbidities (HR=1.47, 95% CI 0.44–4.86) v. without CVD (HR=2.17, 95% CI 1.12–4.22) (P=0.58); and depressive neurosis (HR=2.77, 95% CI 1.22–6.26) v. depressive psychosis (HR=1.66, 95% CI 0.78–3.53) (P=0.37). However, the effect increased with younger age; adjusted HRs in those aged 65–74, 75–84 and 85+ years were 6.10 (95% CI 1.92–19.4), 2.16 (95% CI 0.92–5.08) and 1.05 (95% CI 0.45–1.94) respectively; P=0.012 in the youngest v. the oldest.
We carried out two sensitivity analyses to investigate further the effects of baseline depression on incident dementia using the above combined data. First, after excluding all participants with baseline organic syndrome at level ⩾3, adjusted HRs in level 4 depression (n=10, 15.4% incident dementia) was 1.89 (95% CI 1.00–3.57), while those for depression at levels 1, 2 and 3 were 1.36 (95% CI 0.84–2.21), 0.85 (95% CI 0.52–1.40) and 0.88 (95% CI 0.53–1.44) respectively. Second, excluding 73 participants with incident dementia who at the same time had depressive syndrome at level ⩾3 from 330 individuals with incident dementia, the matched figures were 1.46 (95% CI 0.68–3.10), 0.53 (95% CI 0.26–1.08), 0.55 (95% CI 0.28–1.07) and 1.18 (95% CI 0.66–2.13).
Discussion
In two large population-based cohorts in China and the UK, we found that an increased risk of dementia was associated with only the most severe syndromes and cases of depression, but not with less severe syndromes and cases. The increased risk was not statistically different with ethnicity, gender, cardiovascular disease comorbidity or type of depression, but was higher in younger participants.
Our findings may weigh more towards particular causal pathways. Depression may be associated with increased risk of dementia because depression is a reaction to perceived cognitive deficits, because depression is an early symptom of dementia (occurring before the syndrome becomes clinically definable), or because depression is actually a risk factor for dementia. The fact that subcase and less severe case syndrome levels were not associated with dementia in this study may support the third explanation more than the first two (which would be less likely to show specificity to a particular syndrome level).
Strengths and limitations
The strengths of the study were that we employed two markedly different populations in terms of levels of socio-economic status, social support, CVD and depression; the two cohorts had high response rates and the number of participants was relatively large, which gave sufficient power to test the relationship, including that in the subgroups; and the GMS–AGECAT, potentially measuring syndrome and diagnosis at 5 levels, offered a unique opportunity to examine dementia in relation to depressive severity (rather than simply as a dichotomous variable of depression).
The limitations of the study were that in the Chinese cohort, the incident dementia may be over-diagnosed because it included some illiterate participants Reference Chen, Hu, Qin, Xu and Copeland14 (although the GMS–AGECAT was satisfactory for high-income countries with literate populations). Reference Prince, Acosta, Chiu, Scazufca and Varghese29,Reference Prince, Acosta, Chiu, Copeland, Dewey, Scazufca and Varghese30 The follow-up in the Chinese cohort was relatively short (1 year), and the numbers with depression and dementia were small (and thus it was not possible to examine differences in the baseline covariables between people with depression levels 3 and 4, and differences in the effect of level 4 depression on dementia between the subgroups of participants). However, the findings were consistent and supported by the large British data with the long-term 4-year follow-up. Another limitation was that we could not separate dementia into Alzheimer's disease, vascular dementia, or both in order to examine their associations with depression. Thus, we could not directly link level 4 depression cases to Alzheimer's disease or vascular dementia. However, our data showed that depression at level 4 increased the risk of dementia in both participants with and without CVD, suggesting that the most severe cases of depression may be related to both vascular dementia and Alzheimer's disease. The finding that depression increased the risk of Alzheimer's disease has been shown in a recent meta-analysis paper, which did not examine the effects of the severity of depression on Alzheimer's disease. Reference Ownby, Crocco, Acevedo, John and Loewenstein8
Effects of depression on incident dementia
To our knowledge, our study is the first to examine the relationship between depressive syndromes and the risk of dementia, and report that within clinical depressions, the less severe cases of depression did not increase any risk for developing dementia, but the most severe cases did so significantly. The MRC–ALPHA data showed that individuals with level 4 depression at baseline were more likely to have level 4 depression within 2 years than those with baseline level 3 (data on request), suggesting depression lasted longer at level 4 (although we cannot exclude some participants who may have recovered and then relapsed in the meantime). This longer duration may be a reflection of the level of depression severity. Several epidemiological studies have examined depression as a risk factor for dementia with conflicting results. Reference Andersen, Lolk, Kragh-Sorensen, Petersen and Green4 In a meta-analysis, Jorm concluded that a history of depression increased the risk for developing dementia by 87%. Reference Jorm3 Our data suggest that the most severe level of depression increased the incidence of dementia to a greater degree than those in previous studies. Reference Jorm3,Reference Ownby, Crocco, Acevedo, John and Loewenstein8 We believe this is because we separated severe from less severe depression. This may explain the variable conclusions in those studies which grouped severity levels of depression together. Failure to confirm this finding may have been partly due to a lower proportion of severe depression in their samples.
Why does the GMS–AGECAT level 4, not case level 3 depression appear to increase the risk of incident dementia? Such a pattern even existed in our sensitivity analyses. It seems not to arise from differences in those covariables at baseline. It could not be entirely explained by the lower proportion of depressive neurosis (or higher depressive psychosis) in participants with level 3 depression at baseline compared with those with level 4, since level 4 depressive psychosis itself increased the risk of dementia. We considered that some symptoms or other unmeasured variables might have been included in level 4, but not in level 3, depression and/or simply the severity of depression could play a role. Perturbations of the hypothalamic–pituitary–adrenal axis in depression can lead to hypercortisolemia-associated hippocampal atrophy which may also explain why the most severe cases of depression are those more likely to increase the risk of dementia. Nevertheless, the GMS–AGECAT diagnosis, adapted for clinical use, could be used to help identify those at higher risk in older populations in an attempt to prevent dementia. The strong effect found in younger elderly people and the long-term effect may imply that depression at a younger age is more important for developing dementia, suggesting that early treatment of depression and prevention of depression in the younger population may effectively reduce dementia in later life.
The increased risk of incident dementia in relation to only the most severe syndromes or cases of depression is unlikely to result from chance or bias. Our study urges that the aetiological roles of the severity of depressive symptoms, syndromes and clinical cases on dementia need to be addressed. Reference Chen, Hu, Wei, Qin and Copeland31 Early treatment and prevention of depression and relieving its severity could reduce the burden of dementia in both high-income and low- and middle-income countries.
Acknowledgements
We thank the many older Chinese and British residents who participated in the surveys, and the survey interviewers. Critical comments from two anonymous reviewers have substantially improved this paper. The Chinese cohort was funded by The Royal Society, UK (Grant No. 574006.G603/22085) and the MRC–ALPHA cohort by the Medical Research Council (MRC) and later by MRC and the Department of Health, UK, as part of the MRC–CFA Study. R.C. is supported by the BUPA Foundation. The opinions expressed in this report are not necessarily those of the funders.





eLetters
No eLetters have been published for this article.