Velo-cardio-facial syndrome (VCFS) is associated with intellectual impairments and with IQ-independent deficits in visuoperceptual function and social and abstract reasoning (Reference Henry, van Amelsvoort and MorrisHenry et al, 2002). These deficits may underlie the social impairments of VCFS (Reference Swillen, Devriendt and LegiusSwillen et al, 1997). Facial expressions are important social cues. As explicit processing of facial emotions is positively correlated with IQ and involves higher cognitive processes (Reference Kroeger, Rojahn and NaglieriKroeger et al, 2001), we used functional magnetic resonance imaging (fMRI) to investigate implicit processing, which involves (para)limbic areas in an automatic, attention-independent manner (Reference Critchley, Daly and PhillipsCritchley et al, 2000). We predicted that compared with controls, people with VCFS have less activation of brain regions normally activated during implicit emotional processing (the amygdala, insula and frontal cortex).
METHOD
Our sample included eight right-handed adults – seven women and one man (mean age 34 years, s.d.=9; mean IQ 72, s.d.=10), three of whom had schizophrenia and five had no mental illness – with clinical features of VCFS and a 22q11 deletion confirmed by fluorescence in situ hybridisation (Oncor Inc., Gaithersburg, Maryland, USA). Our control group comprised nine healthy individuals (five women and four men; mean age 37 years, s.d.=11, mean IQ 73, s.d.=16). Details of inclusion criteria, recruitment methods and psychiatric and neuropsychological assessments are described elsewhere (Reference Murphy, Jones and OwenMurphy et al, 1999; Reference Van Amelsvoort, Daly and Henryvan Amelsvoort et al, 2004). The control group did not differ significantly in age, IQ and gender from the VCFS group. The study was approved by the local research ethics committee. Written informed consent was obtained from all participants (or their guardians).
All participants were familiarised with the scanner and the task. Ten blocks of eight facial stimuli from the Ekman series (Reference Ekman and FriesenEkman & Friesen, 1975) were used, as described previously by Critchley et al (Reference Critchley, Daly and Phillips2000). The participants viewed a pseudo-randomised mixture of happy or angry (‘on’ phase) and neutral (‘off’ phase) facial expressions, with instructions to attend to and judge the gender of each face. During this task 100 T 2*-weighted echoplanar images, depicting blood oxygenation level-dependent contrast (14 non-continuous slices, thickness 7 mm (gap 0.7 mm), inplane resolution 3.1 mm, echo time (TE) 40 ms, repetition time (TR) 3000 ms), and one T 1-weighted whole-brain anatomical image (43 continuous slices, thickness 3 mm, in-plane resolution 1.5 mm, TE=73 ms, TR=1600 ms) were acquired using a 1.5 T GE Signa System 7(General Electric, Milwaukee, Wisconsin, USA). Data were analysed using Statistical Parametric Mapping 99 (http://www.fil.ion.ucl.ac.uk/spm). Functional images were movement corrected, normalised into standard space and smoothed with 12 mm full width at half maximum gaussian kernel prior to statistical comparisons. Condition-specific task effects were assessed by comparing the ‘on’ phases of each task with their respective ‘off’ condition. Changes in regional blood flow were determined by applying the general linear model. Between-group comparisons of brain activation patterns were performed using t statistics. The resulting statistical parametric (SPM (t)) maps were transformed to SPM {Z} values.
RESULTS
Participants were task-compliant, and debriefed after scanning. Responses were made in the required time window, and there was no group difference in task performance. Between-group analysis revealed an anterior–posterior dichotomy in activation patterns during emotional processing (on>off). Compared with the control group, the VCFS group showed less activity in the right insula and frontal regions. In contrast, people with VCFS had more activity in bilateral occipital regions (Table 1).
Table 1 Between-group differences in brain activity during emotional processing task in the velo-cardio-facial syndrome group (n=8) compared with IQ-matched controls (n=9)
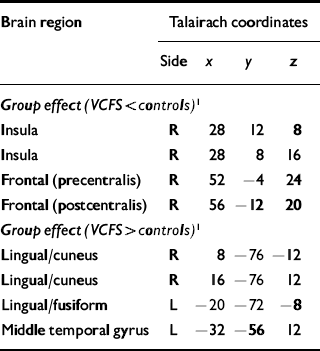
| Brain region | Talairach coordinates | |||
|---|---|---|---|---|
| Side | x | y | z | |
| Group effect (VCFS < controls) 1 | ||||
| Insula | R | 28 | 12 | 8 |
| Insula | R | 28 | 8 | 16 |
| Frontal (precentralis) | R | 52 | -4 | 24 |
| Frontal (postcentralis) | R | 56 | -12 | 20 |
| Group effect (VCFS > controls) 1 | ||||
| Lingual/cuneus | R | 8 | -76 | -12 |
| Lingual/cuneus | R | 16 | -76 | 12 |
| Lingual/fusiform | L | -20 | -72 | -8 |
| Middle temporal gyrus | L | -32 | -56 | 12 |
DISCUSSION
To our knowledge this is the first fMRI study investigating facial emotional processing in VCFS. Compared with controls, people with VCFS had more activation of the bilateral occipital brain regions involved in early visual processing (including face perception) (Reference Haxby, Hoffman and GobbiniHaxby et al, 2002). In contrast, people with VCFS showed less activation in the right insula and premotor cortex – areas implicated in emotional processing (Reference Critchley, Daly and PhillipsCritchley et al, 2000; Reference Haxby, Hoffman and GobbiniHaxby et al, 2002).
The neural network mediating face perception includes a ‘core system’ for visual face analysis, in which occipital gyri provide input into fusiform and superior temporal regions. These then interact with an extended system (e.g. amygdala, insula, limbic system) to process the meaning of information (including emotions) from faces (Reference Haxby, Hoffman and GobbiniHaxby et al, 2002). Ventral stream disruption could be one explanation for social impairments in VCFS (Reference Lajiness-O'Neill, Beaulieu and TitusLajiness-O'Neill et al, 2005). Moreover, adults with VCFS perform poorly on object perception tasks (relying on the ventral ‘what’ pathway), whereas they perform better on space perception tasks (relying on the dorsal ‘where’ pathway) (Reference Henry, van Amelsvoort and MorrisHenry et al, 2002). Our results suggest that in VCFS the pathways between ‘face perception’ areas and the extended ventral ‘what’ pathway for processing emotional expressions may be dysfunctional.
A consistent finding in face expression neuroimaging research is increased extrastriate activation in association with facial emotional expressions. Hence, our results may also reflect greater modulation of visual cortices by emotional faces in VCFS compared with controls, with downstream areas involved in attributing the social significance of faces being hypoactive. Hence, people with VCFS may show normal or enhanced affective responses to facial expressions (intact affective empathy), but an impaired ability to identify the social and contextual significance of socioemotional cues (impaired cognitive empathy). This could partially explain why people with VCFS have high levels of anxiety and affective symptoms.
In our between-group analysis, we observed an anterior–posterior dichotomy in brain activation patterns. This dichotomy has been reported previously in anatomical studies of VCFS (Reference Kates, Burnette and JabsKates et al, 2001) and might be partially explained by dysmaturation of white-matter tracts in this syndrome (Reference Van Amelsvoort, Daly and Henryvan Amelsvoort et al, 2004). White-matter integrity is associated with better performance on cognitive tasks; for example, improvement in working memory is associated with increased frontal fractional anisotropy (Reference Nagy, Westerberg and KlingbergNagy et al, 2004). Thus, people with VCFS may need to activate occipital brain regions more in order to process visual stimuli, owing to reduced white-matter integrity or connectivity. This ‘compensation’ hypothesis is supported by the findings of Eliez et al (Reference Eliez, Blasey and Menon2001), who reported increased activation in parietal regions of children with VCFS compared with controls during mathematical reasoning.
Limitations of our study include the use of a block design including both ‘happy’ and ‘angry’ faces; we are therefore unable to comment on brain activations to separate emotions or neutral faces. Event-related fMRI is needed to evaluate this. Our results did not survive correction for multiple comparisons and should be interpreted as preliminary. Men and women were included in the study, but there was no difference in gender distribution. Inclusion in the VCFS group of three people receiving medication for psychosis could have confounded our results. Given the high prevalence of psychiatric disorders in VCFS, however, a population without any psychiatric problem is rare.
In summary, the results of our preliminary study of emotional processing in VCFS suggest that social impairments in this syndrome may be associated with abnormal connectivity between early visual processing areas and limbic brain regions.




eLetters
No eLetters have been published for this article.