Device closure of perimembranous ventricular septal defects is an interesting catheter procedure with encouraging follow-up results, even in small infants. Reference Pillai, Rangasamy and Balasubramonian1–Reference Yildiz, Narin and Ozdemir5 However, technical and anatomical challenges exist and procedural complications can occur. Reference Holzer, de Giovanni and Walsh6–Reference Santhanam, Yang, Chen, Tai, Rajgor and Quek8 Device embolisation is a rare complication and is usually diagnosed and treated in the early post-operative hours. Reference Santhanam, Yang, Chen, Tai, Rajgor and Quek8,Reference Tai, Tang and Zhu9 Herein, we report the first case of an unusual silent embolisation of a device occluder in a small infant, detected 6 months after its use for the closure of a large perimembranous ventricular septal defect.
Case presentation
A 6-month-old infant (6 Kg/ 64 cm) with a birth weight of 3600 g was referred to our institution for a systolic heart murmur, shortness of breath, and failure to gain weight. An echocardiogram was performed and identified a haemodynamically significant, left-to-right restrictive shunting (Vmax 4.2 m/s), aneurysmal-type, single perimembranous ventricular septal defect. The left ventricle end-diastolic diameter was 31 mm (Z-score 5.6), and the pulmonary pressures were normal. The aneurysm was not deep, and the upper edge of the defect was at the level of the aortic valve annulus (Fig. 1). The left ventricular entry diameter was 9–10 mm, and the largest right ventricular exit diameter was 5–6 mm. There was also a tear-drop-type mild aortic cusp prolapse with a trivial central aortic valve regurgitation. We discussed the case during a multidisciplinary team meeting and decided to attempt a retrograde transcatheter device closure. We informed the parents of the procedural risk and obtained a signed informed consent.
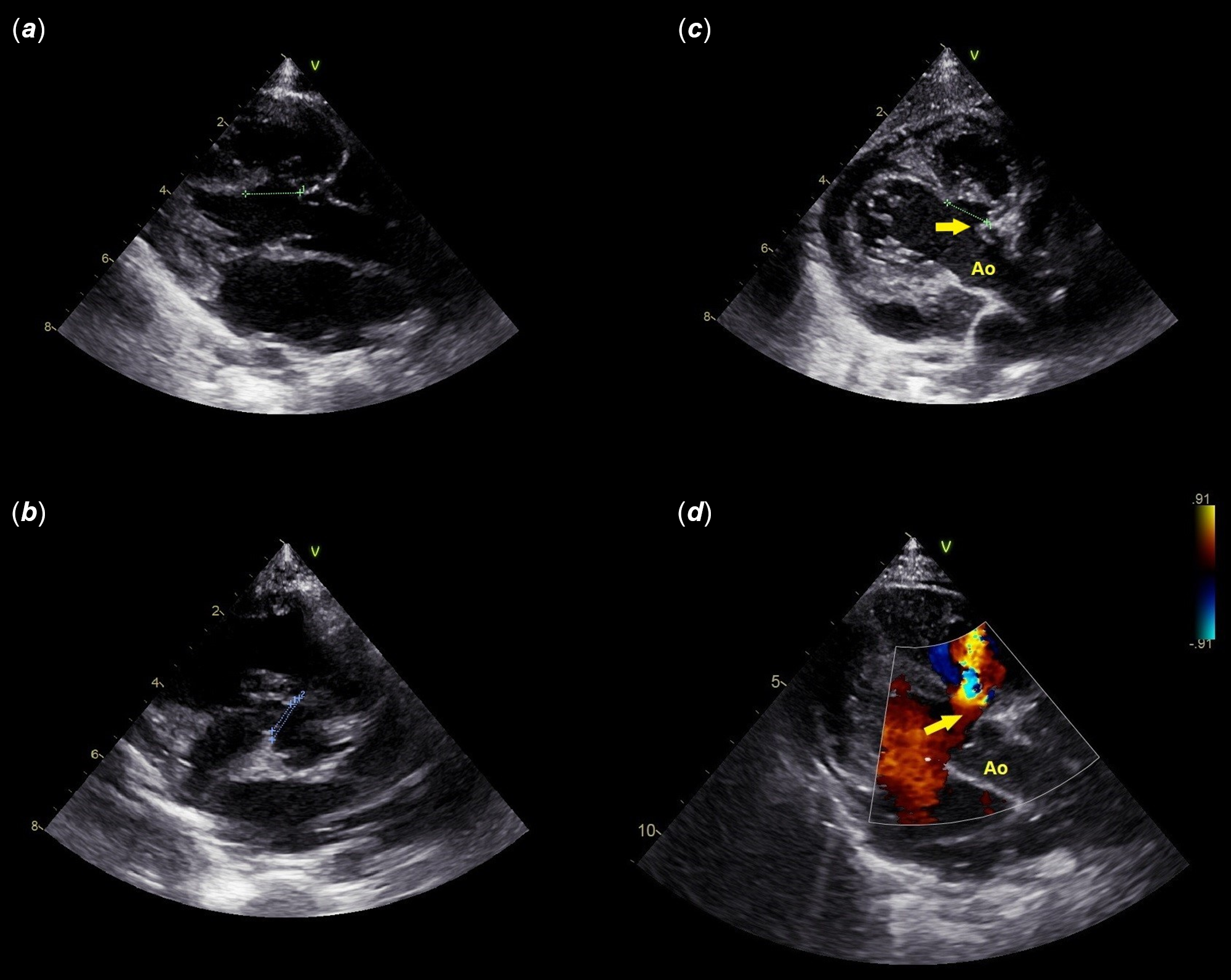
Figure 1. Multiple views of baseline echocardiogram (a–d).
The procedure was performed in the catheterisation laboratory under general anaesthesia, fluoroscopy, and transthoracic ultrasound guidance. A 6-Fr venous and 5-Fr arterial femoral access were obtained. Intravenous heparin and antibioprophylaxis were given. A standard right cardiac catheterisation was performed for baseline haemodynamic evaluation. A left ventricular angiography was performed using a marked pigtail catheter in a 20° cranially tilted 60° left anterior oblique projection to delineate the defect anatomy (Vid. 1). The left ventricular entry diameter was measured at 10 mm, and there were two right ventricular exits with diameters of 4 and 6 mm. The defect was crossed from the left ventricular side and a 5-Fr SteerEaseTM introducer (Lifetech, China) was positionned in the right ventricle. An 8x6 mm KONAR-MF™ VSD occluder (Lifetech, China) was implanted inside the aneurysmal sac to minimise friction between the device and the aortic valve. Before device release, ultrasound showed the device in the desired position, a mild residual shunting with trivial aortic and tricuspid valve regurgitations. A hand-dye injection was performed in the ascending aorta and confirmed the non-interference of the device with aortic valve function, the device inside the aneurysm with the right retention disc sitting nicely in the right ventricle (Vid. 2). After cable release, the device twisted downward and took its expected position completely inside the aneurysm (Vid. 3). The fluoroscopy time was 3.6 minutes. We did not perform an exit left ventricle angiogram, as routine. At the end of the procedure, ultrasound showed a perfectly sitting device inside the defect with two jets of moderate residual shunting, mainly intra-prosthetic (Vid. 4A, B). The echocardiogram performed 48 hours post-procedure was identical (Fig. 2, Vid. 4C-F), and the patient was discharged under oral aspirin. The patient was examined 2 weeks post-operative. The echocardiogram showed that the device did not change position, and the shunt was stable and intra-prosthetic (Fig. 3, Vid. 4G, H). Therefore, we were reassured and scheduled the next follow-up at 3 months post-operative. The next visit was skipped for familial reasons, and the patient came back for the regular 6-month follow-up. The echocardiogram showed surprisingly the device embolised and stuck in the proximal left pulmonary artery. The parents denied any alarming symptoms in between. Two days later, the patient had open-heart surgery to remove the device and close the defect with a pericardial patch. The surgeon described the presence of three tricuspid valve chordae converging into one and attaching to the interventricular septum without straddling into the left ventricle, partially closing the defect and separating it into two exits. The device was endothelialized partially on one edge and stuck to the pulmonary wall (Fig. 4). The surgeon managed to extract the device without patch repair of the left pulmonary artery. The patient was discharged after 5 days with excellent outcomes. The patient is alive and asymptomatic at 3 months of follow-up. There was no residual shunt or pulmonary artery injury.
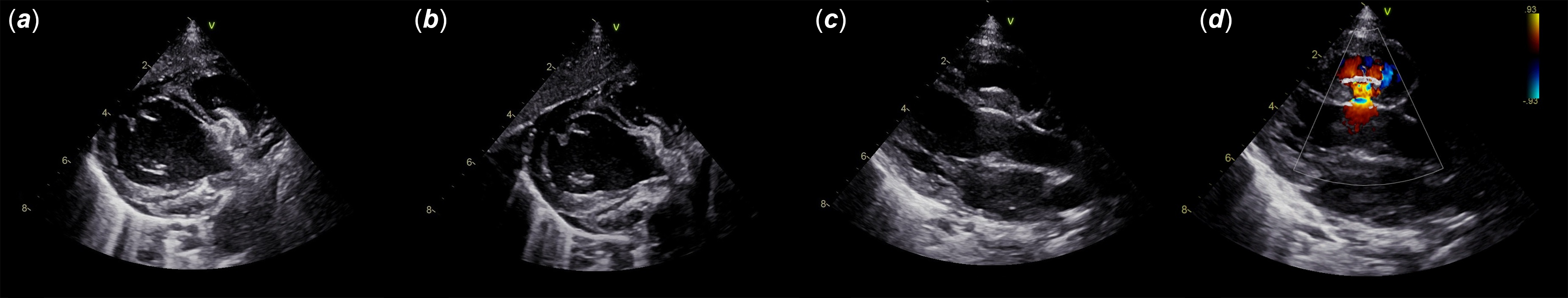
Figure 2. Discharge ultrasound showing the device sitting perfectly within the defect with a moderate residual shunt, mainly intra-prosthetic (a–d).

Figure 3. Two views of ultrasound follow-up at 2 weeks post-operative: device in place (a, b) with two jets of intra-prosthetic residual shunts (c, d).

Figure 4. Surgical removal of the embolised device from the left pulmonary artery 6 months post-operative (a, b). Note the device covered partially by neo-endothelialization (white arrow) (b).
Discussion
Despite encouraging patients cohorts with various device occluders and approaches, Reference Pillai, Rangasamy and Balasubramonian1–Reference Yildiz, Narin and Ozdemir5 transcatheter closure of medium- and large-sized perimembranous ventricular septal defects in infants weighing less than 10 kg is risky because of the technical difficulty, smaller femoral vessels, and higher risk of complications. Reference Holzer, de Giovanni and Walsh6,Reference Butera, Carminati and Chessa7 We report the first case of silent embolisation of a device occluder in a small infant, detected 6 months after its use for the closure of a large perimembranous ventricular septal defect. Device embolisation is usually diagnosed in the early hours after the procedure and is one of the most dreaded complications of transcatheter closure of perimembranous ventricular septal defects. In a recent meta-analysis of transcatheter closure of perimembranous ventricular septal defects in over 6,762 patients, device embolisation was reported in 29 patients, giving an incidence rate of 0.4%, with a minority requiring surgical removal. Reference Santhanam, Yang, Chen, Tai, Rajgor and Quek8 All of these embolizations occurred during hospitalisation or before patient discharge. The most common site of embolisation is the pulmonary artery, and the most common reason for embolisation is an undersized device. Reference Tai, Tang and Zhu9 The second most frequent cause of embolisation is an inadequate sub-aortic rim. Other reasons include defective cable release and operator-related technical issues.
In this patient, the sub-aortic rim was absent and the diameter ratio (right-side exit/left-side entry) was greater than 0.5. Both of these anatomical aspects are together an important limitation for a safe retrograde closure approach. Reference Haddad, Sawan and Saliba10,Reference Haddad and Saliba11 The most challenging procedural aspect in small infants is to avoid device oversizing to avoid the high risk of rhythm disturbances while pre-determining the device interaction with the tricuspid or aortic valves before release. One could argue that we could have delayed closure. However, the patient was symptomatic and presented an early stage of aortic valve prolapse and regurgitation, justifying shunt closure. Reference Sadiq, Qureshi, Younas, Arshad and Hyder12
Despite the limitations, the retrograde approach was chosen for this patient. The presence of an absent aortic rim and a diameter ratio (right-side exit/left-side entry) greater than 0.5 means that selecting a device 1–2 mm larger than the 6 mm right ventricular exit will lead to the 9 x 7 mm or 10x8 mm device. Both of these devices have a 14-mm left retention disc that is 4 mm larger than the 10-mm left ventricular entry. This device selection will have increased significantly the likelihood of impingement on the aortic valve, resulting in significant aortic valve regurgitation. This risk was mitigated by choosing the 8x6 mm device with a 12-mm left retention disc, therefore fitting the device completely inside the aneurysm and keeping the upper edge of the left retention disc away from the aortic valve edge.
Before release, we expected and noticed on fluoroscopy that the device was not squeezed in place; however, we felt secure to release the device with the absence of a severe residual shunting. The device was confirmed in place 48 hours and 14 days post-procedure without any change in the configuration or increase in the degree of residual shunting. Instead, the two jets of residual shunting became less severe and were intra-prosthetic. We were reassured by these findings and scheduled the next visit in months as routinely done. During surgical removal of the device at 6 months post-procedure, the surgeon noticed that the device was endothelialized partially on one edge. This might suggest that the embolisation event occurred somewhere between 3 months and 6 months post-operative. Reference Amin, Berry, Foker, Rocchini and Bass13 Although the exact timing of the embolisation is unknown, this relatively poor endothelialization of the device despite being 3–6 months in situ is noteworthy. Poor endothelialization can lead to device instability, milking, erosion of surrounding structures, clot-forming nidus, and potential raw surface for infective endocarditis.
Post-release, the device showed a considerable change in position, meaning a size mismatch between the device and the defect. Reference Singhi, Mukherji and De14 An exit left ventricle angiogram would have provided greater clarity. The device was undersized, and the left retention disc was just plugging the aneurysmal left ventricular entry without being compressed and held stably in place. During cardiac contraction, this configuration allowed the device to migrate progressively inside the aneurysm towards the right ventricle. The shorter distance between the septal leaflet of the tricuspid valve and the lower edge of the defect is what might have delayed the embolisation event. Baseline echocardiogram and angiography showed that the aneurysm was not deep. The surgical view showed an aneurysmal tissue involving the tricuspid valve chordae splitting defect into two components, that is, pseudoaneurysm. Reference Wu, Qin and Zhao15 However, there was not a true distinct aneurysm leading to the clamping of the device’s right retention disc on the tricuspid valve chordae without creating an alarming tricuspid regurgitation and holding the device in place longer than expected. Retrospectively, the surgeon’s findings could have been noticed on transoesophageal ultrasound and the decision of catheter closure could have been influenced.
Putting all this together, closing this defect with the transvenous approach and a larger device would have led to aortic or tricuspid valve insufficiency and increased the odds of rhythm disturbances and haemodynamical compromise that are usually associated with oversized devices and the creation of arteriovenous circuits. Reference Pillai, Rangasamy and Balasubramonian1,Reference Alshahrani, Linnane and McCrossan4,Reference Yildiz, Narin and Ozdemir5 Similar defects should be closed surgically.
Conclusion
Silent late device embolisation after perimembranous ventricular septal defect closure can occur. Absent sub-aortic rim and diameter ratio (right-side exit/left-side entry) greater than 0.5 and pseudoaneurysm involving the tricuspid valve chordae are important risk factors, particularly in small infants where device oversizing, permissible delivery sheath size, and transvenous approach are serious technical challenges. Defects with these anatomical limitations should be closed surgically, especially in small infants.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S104795112300433X.
Financial support
None.
Competing interests
RH has no conflict of interest to declare. ZS is a proctor and consultant for Abbott Vascular and Lifetech.







