During the past few decades, the incidence of thyroid cancer has increased substantially worldwide. In China, the incidence is also obviously rising, from less than 1·78/100 000 in 1988 to nearly 90/100 000 in 2015(Reference Chen, Zheng and Baade1,Reference Kitahara and Sosa2) . For women, thyroid cancer is the most commonly diagnosed cancer before the age of 30 years, and for men, a significant upward trend in age-standardised incidence rates has occurred(Reference Kitahara and Sosa2,Reference La Vecchia, Malvezzi and Bosetti3) . Many researchers attribute this dramatic rise in thyroid cancer to the improved technology for assessing the thyroid gland, including high-resolution imaging and fine-needle aspiration biopsy(Reference Morris, Sikora and Tosteson4,Reference Ahn and Welch5) . However, not only the incidence of thyroid microcarcinoma but also the incidence of tumours with diameters greater than 1 cm has increased, which can be detected using a classic diagnostic methodology, suggesting that the use of better detection techniques on the thyroid does not fully account for the overall increasing incidence of thyroid cancer(Reference Schneider and Chen6). Possible explanations for the rising incidence of thyroid cancer include increased iatrogenic or ‘diagnostic’ radiation exposure; changes in the population’s BMI; fertility drugs; changes in menstrual cycles or iodine(Reference Kitahara and Sosa2,Reference Veiga, Holmberg and Anderson7–Reference Renehan, Tyson and Egger9) . These potential causative factors, especially iodine consumption, require further exploration in the population.
Iodine is an essential trace element required for thyroid function, which plays an indispensable role in development, growth and metabolism throughout human life(Reference Zimmermann and Boelaert10). Both excessive and insufficient iodine intake can cause thyroid disease and even have an effect on thyroid cancer(Reference Sherman11). In recent years, a few epidemiological studies have suggested that iodine nutrition is associated with thyroid cancer risk, but the results remain very controversial. Some studies reported a higher thyroid cancer frequency in areas with insufficient iodine intake than in areas with optimal intake(Reference Vucemilo, Znaor and Kulis12). Others discovered that patients with thyroid cancer were more likely to be distributed in areas with high urine iodine concentration (UIC) than those with benign thyroid nodules(Reference Kim, Park and Byun13). Additionally, animal experiments using rat thyroid models have demonstrated that both iodine deficiency and iodine excess significantly increase thyroid tumourigenesis(Reference Lakshmanan, Scarberry and Shen14,Reference Yamashita, Noguchi and Murakami15) . To explore the possible relationship between iodine nutrition and thyroid cancer, more epidemiological studies are needed.
Iodine nutritional levels in humans can be evaluated by several indicators. Traditionally, the median UIC (MUIC) and goiter prevalence (GP) have been recommended as sensitive indicators for assessing iodine nutritional status in the population(Reference Wong, Sullivan and Perrine16). Other indicators such as salt iodine content, the consumption rate of qualified iodised salt (CRQIS) and water iodine content can also provide references for the evaluation of iodine nutrition(17). In China, large-scale surveys are usually conducted to evaluate the iodine nutritional level in a population(Reference Fan, Su and Shen18). In the present study, a comprehensive analysis combining and matching thyroid cancer incidence rates with iodine nutritional indicators in China was performed to provide more evidences into the relationship between iodine nutrition and thyroid cancer risk.
Materials and methods
A new study, which combined the data of the 2018 National Annual Cancer Report, the 2018 National Iodine Deficiency Disorders (IDD) surveillance and the 2017–2018 Water Iodine Survey to conduct a new ecological synthesis analysis.
National Iodine Deficiency Disorders Surveillance
A multiple sampling technique was used in this cross-sectional survey. The sampling unit was a county (in this article, county-level units such as counties, cities, districts and banners were referred to as counties for short), and each monitoring county was divided into five sample areas: east, west, south, north and middle. Then, one town in a rural area or a district in an urban area (including at least one district) was randomly selected from each sampling area. Forty children aged 8–10 years were randomly selected from the local primary schools in each sampling area. A total of 200 children were recruited in each county. In total, nearly 2800 counties were investigated in China. It was a cross-sectional design, and the unit of analysis was a county in the present study.
Edible salt samples were collected from the sampled children’s households. Urine specimens were collected from the children. The volume of children’s thyroids was detected by ultrasound. Goiter status in children was diagnosed by determining thyroid volume. The standard for a goiter was >4·5 ml for children aged 8 years, >5·0 ml for children aged 9 years and >6·0 ml for children aged 10 years(19). The urinary iodine content of the children was tested by As3-—Ce4+ catalytic spectrophotometry(20). The MUIC of school-aged children, which has been used as the proxy biological indicator for iodine nutrition status in the general population, was analysed to evaluate iodine nutrition status(17). An MUIC less than 100 μg/l for children aged 8–10 years was classified as deficient; between 100 and 199 μg/l was classified as adequate; between 200 and 299 μg/l was classified as more than adequate and greater than or equal to 300 μg/l was classified as excessive(17). The direct iodine titration method was adopted to test the iodine content in potassium iodate salts, and the arbitration method was used for other kinds of salt(21). An iodine content in table salt (SI) < 5 mg/kg indicated non-iodised salt. In China, each province could select iodised salt contained 20, 25 or 30 mg iodine/kg according to the situation of IDD in the province. The qualified range of iodised salt in the standard iodine contained ±30 %, that is, 18–33 mg/kg in fourteen provinces, namely Shaanxi, Hainan, Hubei, Guangxi, Jiangxi, Anhui, Yunnan, Shanxi, Jiangsu, Fujian, Inner Mongolia, Shandong, Zhejiang and Jilin; 21–39 mg/kg in twelve provinces, namely Sichuan, Gansu, Guizhou, Xinjiang, Qinghai, Hunan, Chongqing, Henan, Ningxia, Tibet, Tianjin and Shanghai; and 18–33 mg/kg for general edible salt and 21–39 mg/kg for pregnant women’s salt in five provinces, namely Heilongjiang, Liaoning, Hebei, Beijing and Guangdong. The iodised salt whose iodine content is within the above range in each province is qualified iodised salt. The CRQIS is the percentage of qualified iodised salt in all salt samples, that is:
The resident’s annual income in the previous year and other information including different geographic locations (including plant, hilly and mountainous locations) and coastal status (including inland and coastal) were also collected at the county level(22).
National Survey on Iodine in Drinking Water
The county was taken as the unit to carry out a survey of the iodine content in drinking water. In one county, if the water supply was dispersed, then the drinking water was collected from two households in each of the five directions: east, west, south, north and middle. If the water supply was centralised, two water samples were collected, and the average iodine concentration was calculated. In total, iodine levels in drinking water were surveyed in 3100 counties(23).
National Annual Cancer Report
The National Central Cancer Registry of China is responsible for the collection, evaluation and publication of cancer data in China. Cancer diagnoses are reported to local cancer registries from multiple sources, including local hospitals and community health centres as well as the insurance department responsible for the Urban Resident Basic Medical Insurance programme and the New Rural Cooperative Medical Scheme. Annual thyroid cancer report incorporating one series indicators of thyroid cancer, including the onset constituent ratio (CR), crude incidence rate (CIR), age-standardised incidence rate according to the world standardised population (AIRWSP), cumulative incidence rate in persons aged 0–64 years (CI of 64) and cumulative incidence rate in persons aged 0–74 years (CI of 74). Totally, 339 registries submitted the cancer registration data and meet the quality control standards in China(24). The details have been described by Chen et al. (Reference Chen, Zheng and Baade1).
Statistics analyses
All data were matched at the county level according to the county code, and 281 counties had complete data including population cancer prevalence, iodine nutrition and water iodine content. Rank correlation analysis was first used to describe correlations between different variables. When the indicators were not normal distribution, it was transformed to normality with log transformation before statistical analysis. The incidence of thyroid cancer in different regions was compared by variance analysis, and post hoc comparisons of the means were performed by the SNK-q test. To determine whether the independent factors influenced thyroid cancer incidence, multiple linear regression analysis was adopted. In the present study, median water iodine concentration, GP, MUIC, CRQIS, mean iodine content in table salt, coastal status, geographic region and income were put into the regression models. All statistical analyses were performed using SAS software version 9.1.3. All statistical tests were two-sided using an α level of 0·05.
Results
Correlation analysis of iodine nutrition and thyroid cancer incidence rates
The basic information of the 281 counties was shown in Table 1. Maps of the CRQIS, the GP for children aged 8–10 years and AIRWSP in males and females in China are shown in Fig. 1. The maps show that areas with a low CRQIS and areas with a high GP among children seem to have a higher incidence of thyroid cancer.
Table 1. Basic information of the studied counties (n 281)
(Medians and quartile ranges)
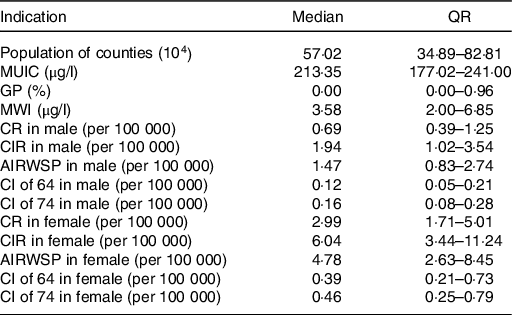
MUIC, median urine iodine concentration; CRQIS, consumption rate of qualified iodised salt; MSI, mean iodine content in table salt; GP, goiter prevalence; MWI, median water iodine concentration; CR, the onset constituent ratio; CIR, crude incidence rate; AIRWSP, age-standardised incidence rate according to the world standardised population; CI of 64, cumulative incidence rate in persons aged 0–64 years; CI of 74, cumulative incidence rate in persons aged 0–74 years.
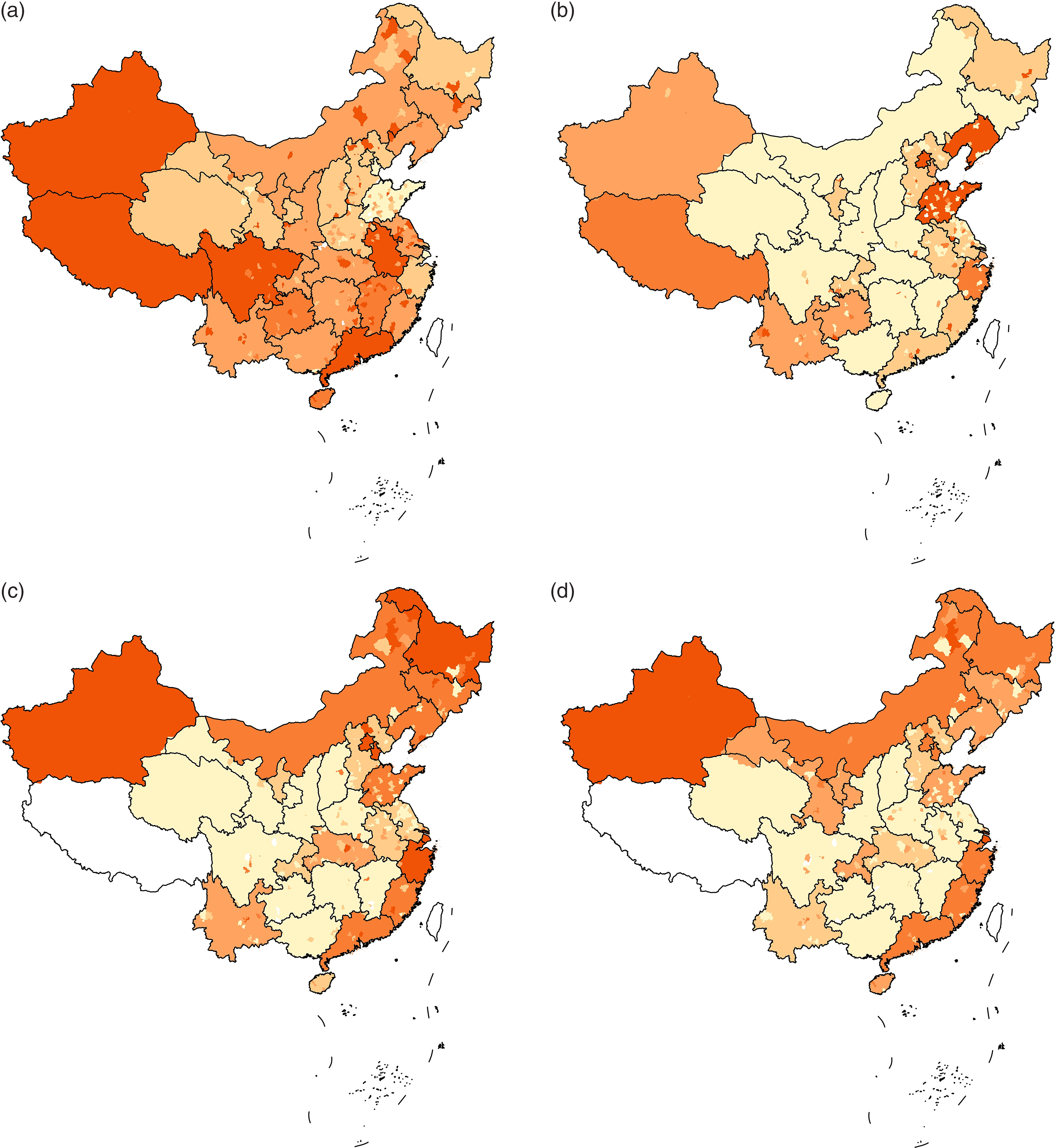
Fig. 1. Maps of Iodine Deficiency Disorders indicators and thyroid cancer incidence. (a) Map of the availability of qualified iodised salt (2800 county). Availability of qualified iodised salt:  , no data;
, no data;  , 2·50 %-;
, 2·50 %-;  , 70·00 %-;
, 70·00 %-;  , 90·00 %-;
, 90·00 %-;  , 95·50 %-;
, 95·50 %-;  , 96·40 %-. (b) Map of goiter prevalence in children aged 8–10 years (2800 county). GP:
, 96·40 %-. (b) Map of goiter prevalence in children aged 8–10 years (2800 county). GP:  , no data;
, no data;  , 0-;
, 0-;  , 0·55 %-;
, 0·55 %-;  , 1·83 %-;
, 1·83 %-;  , 2·20 %-;
, 2·20 %-;  , 3·70 %-. (c) Map of age-standardised incidence rates according to the world standardised population (AIRWSP) in males (339 registries). AIRWSP of thyroid cancer, per 100 000:
, 3·70 %-. (c) Map of age-standardised incidence rates according to the world standardised population (AIRWSP) in males (339 registries). AIRWSP of thyroid cancer, per 100 000:  , no data;
, no data;  , 0·17-;
, 0·17-;  , 5·00-;
, 5·00-;  , 7·87-;
, 7·87-;  , 10·00-;
, 10·00-;  , 15·80-. (d) Map of the AIRWSP-based rates in females (339 registries). AIRWSP of thyroid cancer, per 100 000:
, 15·80-. (d) Map of the AIRWSP-based rates in females (339 registries). AIRWSP of thyroid cancer, per 100 000:  , no data;
, no data;  , 0·20-;
, 0·20-;  , 2·08-;
, 2·08-;  , 2·73-;
, 2·73-;  , 4·23-;
, 4·23-;  , 14·16-.
, 14·16-.
Furthermore, the correlation analysis showed that thyroid cancer incidence rates, including the CR, CIR, AIRWSP, CI of 64 and CI of 74 in female, were statistically negatively correlated with the CRQIS, whereas CR, CIR, AIRWSP, CI of 64 and CI of 74 in both males and females were statistically positively correlated with GP of children aged 8–10 years (Table 2). As AIRWSP could more accurately reflect the incidence of thyroid cancer, the relationship between AIRWSP and iodine nutrition level was further analysed. When the CRQIS was divided into seven groups (<50, 50-, 60-, 70-, 80-, 90- and 100 %), the AIRWSP decreased with a reduction of the CRQIS in both males and females (Fig. 2(a) and (b)). When GP was divided into six groups (0-, 1-, 2-, 3-, 4- and 5 %-), no significant correlation was found between the GP and AIRWSP for males, while for females, AIRWSP appeared to increase with an elevated GP (Fig. 2(c) and (d)). In addition to these two iodine-related indicators, the thyroid cancer incidence rates were higher in the coastal areas and in the areas with higher resident annual incomes in both males and females.
Table 2. Correlation of the thyroid incidence and the iodine nutrition (n 281)
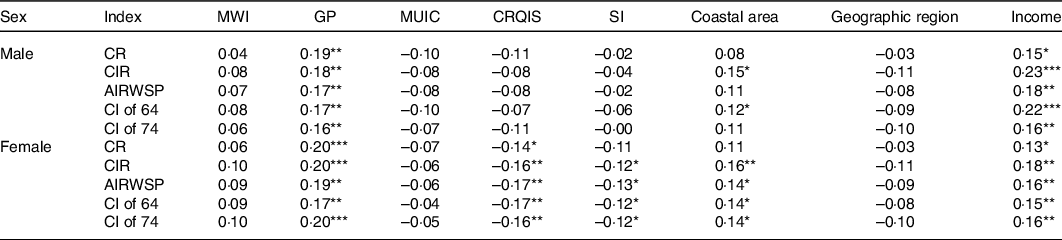
CR, the onset constituent ratio; CIR, crude incidence rate; AIRWSP, age-standardised incidence rate according to the world standardised population; CI of 64, cumulative incidence rate in persons aged 0–64 years; CI of 74, cumulative incidence rate in persons aged 0–74 years; MWI, median water iodine concentration; GP, goiter prevalence; MUIC, median urine iodine concentration; CRQIS, consumption rate of qualified iodised salt; MSI, mean iodine content in table salt.
*P < 0·05, **P < 0·01, ***P < 0·0001.
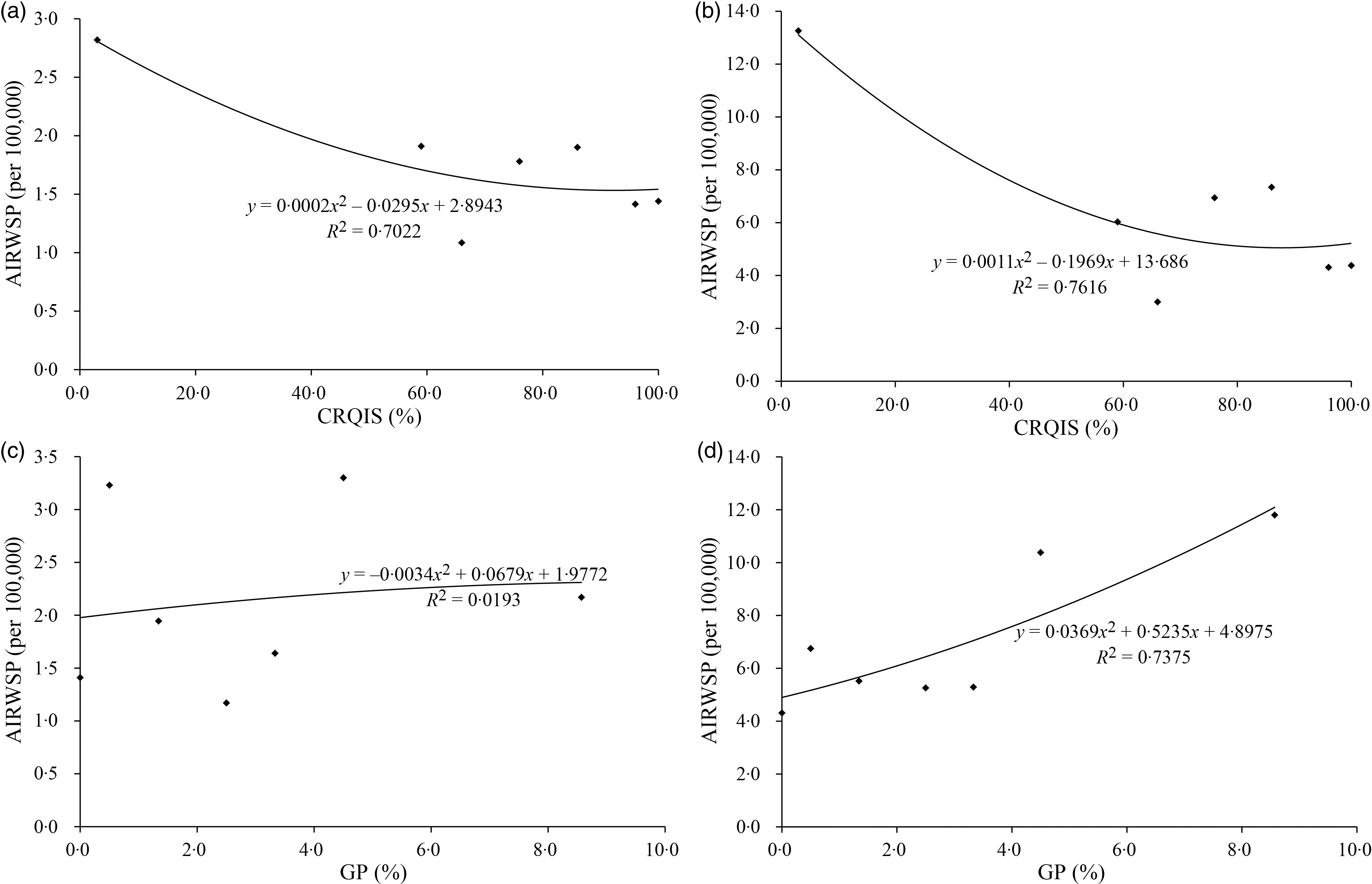
Fig. 2. Relationships of iodine nutrition status and thyroid cancer incidence (n 281). (a) Relationship of the CRQIS and AIRWSP in males; (b) relationships of the CRQIS and AIRWSP in females; (c) relationships of the GP and AIRWSP in males; (d) relationship of GP and the AIRWSP in females. CRQIS, consumption rate of qualified iodised salt; AIRWSP, age-standardised incidence rate according to the world standardised population; GP, goiter prevalence.
Thyroid cancer incidence in different types of regions in China
The thyroid cancer incidence rates were compared between areas with different CRQIS values. In males, CIR, AIRWSP and a CI of 74 were significantly different among CRQIS groups (<70, 70–90 and >90 %). By pairwise comparison, CIR, AIRWSP and CI of 74 in areas with CRQIS in the range of 70–90 % were found to be significantly higher than in areas with CRQIS > 90 %. In females, CR, CIR, AIRWSP, CI of 64 and CI of 74 were also significantly different among areas with different CRQIS values. According to pairwise comparisons, areas with CRQIS < 70 % and CRQIS in the range of 70–90 % had a higher incidence of thyroid cancer than areas with CRQIS > 90 % (Fig. 3). These differences persisted after adjustment for economic and coastal status.
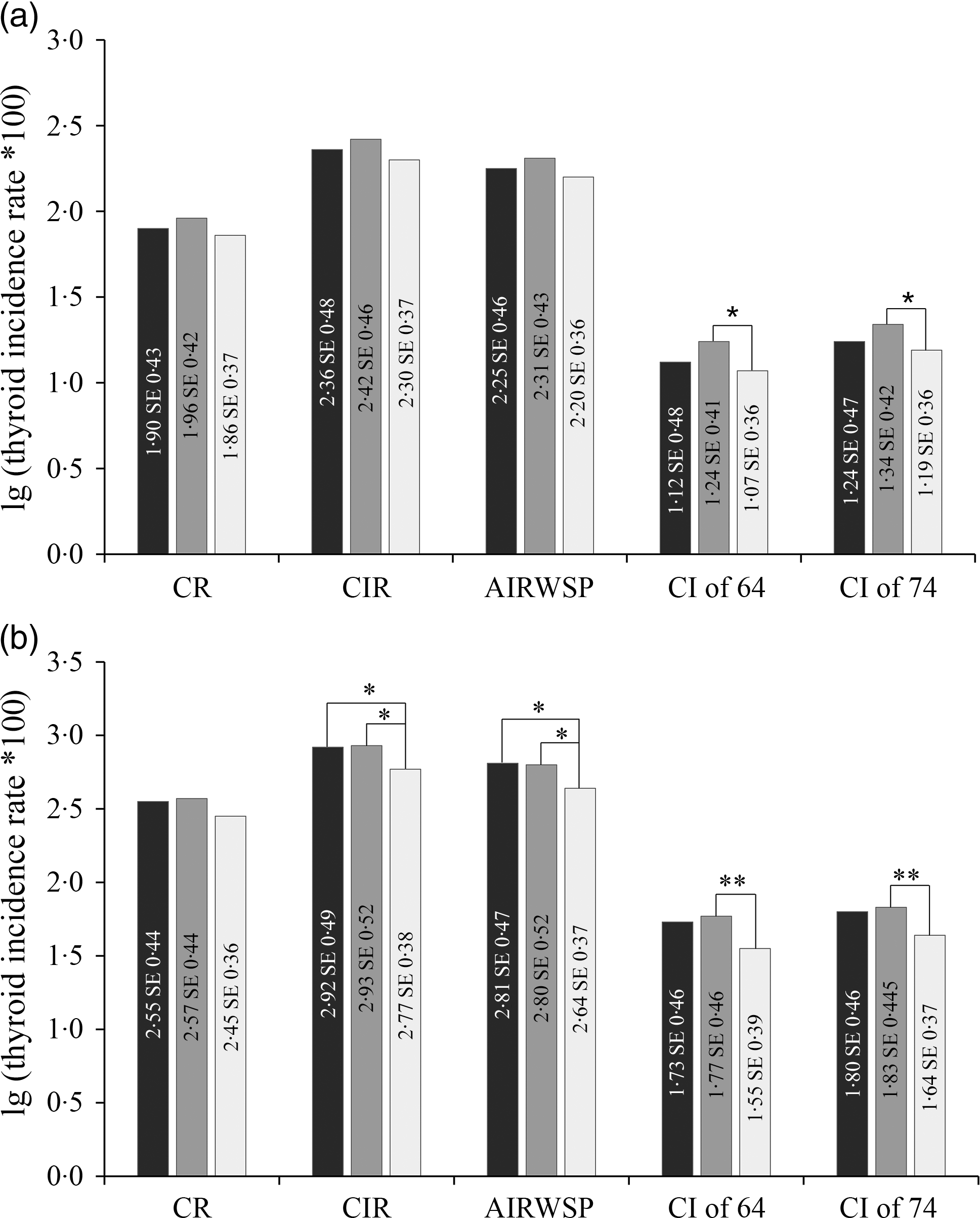
Fig. 3. Thyroid cancer incidence in areas with different CRQIS values (n 281). (a) Males; (b) females. Note: CRQIS ≥ 90 % as the control group; *represents 0·01 < P < 0·05, **represents P < 0·01; CR, onset constituent ratio; CIR, crude incidence rate; AIRWSP, age-standardised incidence rate according to the world standardised population; CI of 64, cumulative incidence rate in persons aged 0–64 years; CI of 74, cumulative incidence rate in persons aged 0–74 years; CRQIS, consumption rate of qualified iodised salt.  , CRQIS < 70 %;
, CRQIS < 70 %; ![]() , 70 % ≤ CRQIS < 90 %;
, 70 % ≤ CRQIS < 90 %; ![]() , CRQIS ≥ 90 %.
, CRQIS ≥ 90 %.
Thyroid cancer incidence rates also varied among areas with different GP values of children aged 8–10 years. Among males, the incidence rates including CIR, AIRWSP, CI of 64 and CI of 74 were higher in areas with GP ≥ 5 % than in areas with GP < 5 %. In females, the incidence rates including CR, CIR, AIRWSP, CI of 64 and CI of 74 were higher in areas with GP ≥ 5 % than in areas with GP < 5 % (Fig. 4). These differences also persisted after adjustment for economic and coastal status.
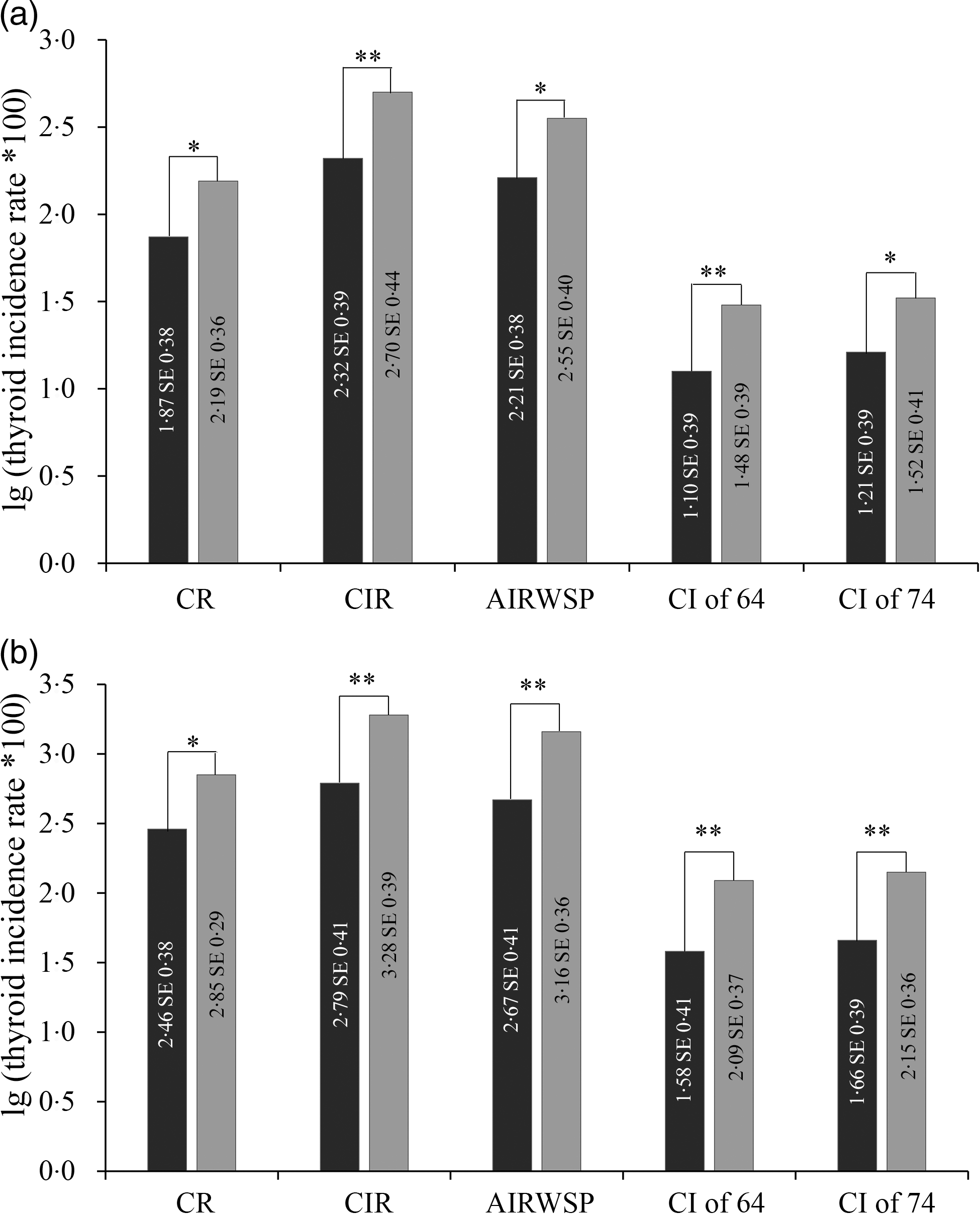
Fig. 4. Thyroid cancer incidence in areas with different goiter prevalence in children aged 8–10 years (n 281). (a) Males; (b) females. Note: GP < 5 % as the control group; *represents P < 0·05, **represents P < 0·01; CR, onset constituent ratio; CIR, crude incidence rate; AIRWSP, age-standardised incidence rate according to the world standardised population; CI of 64, cumulative incidence rate in persons aged 0–64 years; CI of 74, cumulative incidence rate in persons aged 0–74 years. ![]() , GP < 5 %;
, GP < 5 %; ![]() , GP ≥ 5 %.
, GP ≥ 5 %.
No significant difference of thyroid cancer incidence rates was found among areas with different water iodine levels or among areas with different UIC.
Regression analysis
To explore which factors are related to thyroid cancer incidence rates, multiple linear regression analyses were performed. Regression models (CR, CIR, AIRWSP, CI of 64 and CI of 74 as dependent variables) were statistically significant, and the R 2 values of these models were 0·12, 0·17, 0·13, 0·16 and 0·11 in males and 0·11, 0·15, 0·14, 0·13 and 0·13 in females, respectively (Table 3). The GP was also found to usually play a positive role, while the CRQIS usually played a negative role in a high thyroid cancer incidence. Additionally, a high annual income and living in coastal areas also contributed to a high thyroid cancer incidence. Other variables, including the median water iodine concentration, MUIC, mean iodine content in table salt and geographic region, were not significant in the models.
Table 3 Multiple linear regression analyses of thyroid cancer incidence (n 281)
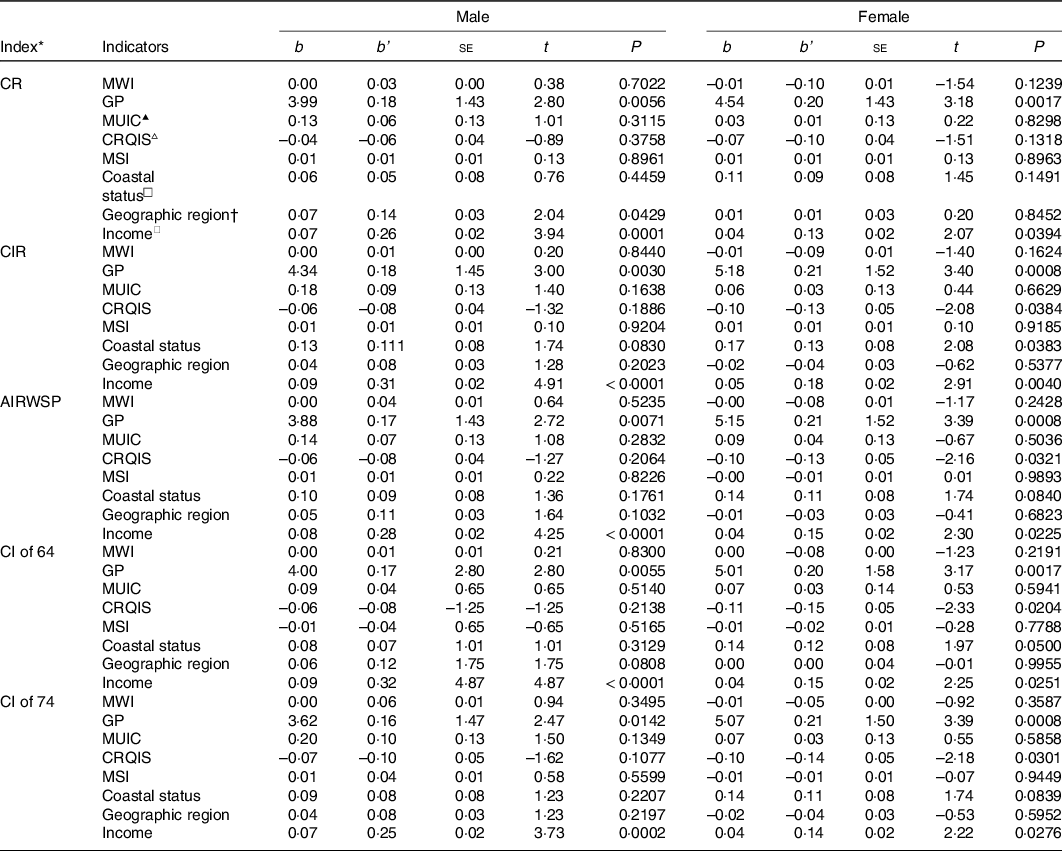
▴MUIC was divided into three levels: < 100, 100–300 and >300 μg/l; ▵CRQIS was divided into three levels: < 70, 70–90 and >90 %; □coastal status included coastal and non-coastal areas.
* The indexes of thyroid incidence were transformed to normality with log transformation; b, regression coefficients; b’, partial regression coefficients; SE, stander error; CR, the onset constituent ratio; CIR, crude incidence rate; AIRWSP, age-standardised incidence rate according to the world standardised population; CI of 64, cumulative incidence rate in persons aged 0–64 years; CI of 74, cumulative incidence rate in persons aged 0–74 years; MWI, median water iodine concentration; GP, goiter prevalence; MUIC, median urine iodine concentration; CRQIS, consumption rate of qualified iodised salt; MSI, mean iodine content in table salt.
† The geographic region was divided into three levels, plain, hilly areas and mountainous areas; ◇per capita disposable income was divided into six levels: < ¥10 000, ¥10 000, ¥20 000, ¥30 000, ¥40 000 and >¥50 000.
Discussion
In the present study, the relationship between iodine intake and thyroid cancer incidence was explored by combining and analysing data from the large national surveys. The results showed that thyroid cancer incidence rates are higher in areas with relatively low iodine nutrition and in areas with a relatively high GP of children aged 8–10 years. A regression analysis was performed and revealed that the GP of children aged 8–10 years and the CRQIS had greater impacts on thyroid cancer incidence than other predictors. The high thyroid cancer incidence in some areas is speculated to be likely a consequence of the relatively low iodine nutritional level in these areas.
Both deficient and excessive iodine intake are associated with the development of thyroid disease, exhibiting a U-shaped curve. However, the relationship between iodine and thyroid cancer is complex, and no definite conclusion has been reached yet. As shown in the present study, a higher GP and a lower CRQIS, which indicated that the iodine nutrition status in the area was inadequate, may increase the incidence of thyroid cancer. Some epidemiological studies also suggested that the incidence of thyroid cancer is high in areas with deficient or mildly deficient of iodine nutrition level. A study in Zagreb compared two areas with different iodine intake levels, and a statistically higher thyroid cancer frequency was observed in areas with insufficient iodine intake than in the control area(Reference Vucemilo, Znaor and Kulis12). Cardis’s study showed that the risk of radiation-related thyroid cancer was 3-fold higher in iodine-deficient areas than elsewhere and that administration of potassium iodide as a dietary supplement reduced the risk of radiation-related thyroid cancer(Reference Cardis, Kesminiene and Ivanov25). A study in Zhoushan, China, also showed that the population with a high thyroid cancer incidence had a lower iodised salt consumption and a lower UIC than populations with a relatively low thyroid cancer incidence(Reference Zhang, Li and Liu26). Similarly, in our study, it is also found that Daishan County, which is located in Zhoushan, had a low CRQIS (20–70 %) in recent years(Reference Sun, Lei and Liu27,Reference Sun, Lei and Liu28) and had a high incidence of thyroid cancer. Iodine deficiency is postulated to be associated with an increased thyroid cancer incidence largely via benign thyroid conditions such as goiters and nodules, which are, in turn, strongly associated with thyroid cancer(Reference Dal Maso, Bosetti and La Vecchia8). The sex differences were also found in our study, as the association between the IDD indicator (GP and CRQIS) and thyroid cancer was more obvious in women than in men. Generally speaking, women are more susceptible to iodine deficiency than men(Reference Zimmermann29). Due to the different physiological characteristics of women, in the same iodine-deficient environment, the goiter rate was higher in women than in men(Reference Malboosbaf, Hosseinpanah and Mojarrad30). Moreover, the sex disparity of the presence of thyroid nodules including thyroid cancer was also found in previous studies(Reference Yao, Chiu and Strugnell31). The incidence of thyroid cancer is two to four times higher in reproductive-age women compared with men(Reference Ron and Schneider32). Oestrogen is a possible risk factor for the sex discrepancy(Reference Magri, Capelli and Rotondi33). Oestrogen receptors have been found in both normal and neoplastic thyroid tissue(Reference Yane, Kitahori and Konishi34). Faria et al. reviewed the literature and found evidence demonstrating that oestrogen-induced cell growth in primary cultures of human thyrocytes obtained from benign and malignant thyroid nodules and in most human thyroid carcinoma cell lines(Reference Faria, Peixoto and Carvalho35,Reference Kumar, Klinge and Goldstein36) . In conclusion, women are more susceptible to iodine deficiency and more likely to develop thyroid cancer, similarly, thus the sex differences were found in the present study.
However, the correlation between MUIC and thyroid cancer incidence was not shown in the present study. First, urine iodine reflects the recent iodine nutritional status, while the goiter rate reflects the long-term iodine nutritional status(17). Thyroid cancer is not caused by a recent iodine deficiency, but is a long-term and complex process; hence, GP, rather than MUIC, is more susceptible to be found correlation with the thyroid cancer incidence. Second, the distribution of MUIC in the county level was relatively concentrated in the present study, and most of them were in the range of 100–300 μg/l. Therefore, the correlation between MUIC and thyroid cancer incidence might be covered. Third, the goiter (rather than low MUIC) and thyroid cancer may share a common pathogenesis. A recent study has reported that both transcript and protein levels of Nuclear Factor (Erythroid-derived 2)-Like 2 (NFE2L2) and Vascular Endothelial Growth Factor A (VEGFA) were elevated in papillary thyroid carcinoma and colloid goiter than normal thyroid tissue(Reference Stuchi, Castanhole-Nunes and Maniezzo-Stuchi37). Studies have reported functional defects and increased expression of NFE2L2 in the thyroid, whose dysregulation may contribute to the development of goiter as well as tumourigenesis of thyroid. Increasing endothelial cells is the first step in goiter formation, which exhibits levels of VEGFA expression similar to those observed in some types of thyroid cancer(Reference Ramsden38,Reference Klein and Catargi39) . Thyroid cancer was associated with goiter and other risk factors.
In addition to iodine nutrition factors, the relationship between residents’ annual income and thyroid cancer in some research remains controversial(Reference Schwartz and Klug40–Reference Choi, Ryu and Han42). In the present study, economic level was positively correlated with thyroid cancer incidence. A higher incidence of thyroid cancer may be mainly due to a greater frequency of medical examinations. Thyroid cancer screening is thought to be provided via opportunistic screening, and opportunistic screening is more commonly used by people with a higher income(Reference Cho, Choi and Seo43).
Coastal status was also found to be related to the incidence of thyroid cancer. Multiple studies have shown that the incidence of thyroid cancer in coastal areas is higher than that in inland areas(Reference Rahib, Smith and Aizenberg44–Reference Mackenzie and Mortimer46). Some studies attributed this difference to lower iodine nutrition in coastal areas than in non-coastal areas(Reference Rahib, Smith and Aizenberg44). Moreover, other studies suggested that improved thyroid cancer diagnosis in coastal areas may also contribute to the higher incidence of thyroid cancer(Reference Poljak, Kontić and Colović47). In the present study, after adjusting for the economic and iodine nutrition-related factors, coastal status was still associated with a high incidence of thyroid cancer, and the reasons need to be further investigated.
In the present study, more than 300 cancer registries located in thirty-one provinces in China that have submitted cancer registration data to the National Cancer Registry Center, and meet the quality control standards, with urban and rural populations accounting for 48 and 52 %, respectively, equivalent to 21·7 % of the national population. Combined with IDD surveillance data, a total of 281 monitoring sites have the complete data (the cancer registration data and the IDD surveillance data). Although tumour registration results in China only covered less than one-third of the population, it covered a wide area of China (including the urban and rural areas, the east, west, middle, north, south areas, and the coastal and inland areas) and the distribution of the registry centres is based on the population density. Therefore, we believed that the results of 281 counties in this analysis basically represent the overall results of China.
According to the results of the present study, China should pay more attention to maintaining optimal iodine nutrition level in the future, especially in coastal area. The results showed that areas with a high GP and a low CRQIS had a relatively high incidence of thyroid cancer, and maintaining appropriate iodine nutrition may help to reduce the occurrence of thyroid cancer. In China, the supervision of iodised salt is not strict as before in some parts of China in these years influenced by ‘the System Reform of Salt Industry’. Therefore, in the future, more attention should be paid to the iodine nutrition of population, and health education should be strengthened, especially in the areas where the consumption of qualified iodised salt is poor.
Limitations exist in this research. First, the incidence of thyroid cancer is general and lacks information on the incidence of various types, so it is impossible to examine the effect of iodine on different types of thyroid cancer in detail. Second, the premise of our analysis is that the UIC of children aged 8–10 years can reflect the iodine nutrition level of the general population, and more research is needed to directly focus on the target population in the future. Third, although it still has representative for the whole country, the number of counties in National Annual Cancer Report was far less than in the IDD surveillance data. Fourth, a possible connection between thyroid cancer and iodine nutrition was found in the present study, but the causal relationship between them still needs to be determined by more cohort studies.
In conclusion, relatively low iodine intake might contribute to a high thyroid cancer risk in China. More epidemiological studies, especially cohort studies, are needed to conduct in areas with different iodine nutrient level to determine the possible impact of inadequate iodine intake on thyroid cancer. Moreover, new molecular studies on the potential underlying mechanisms are still needed to provide additional insights into the aetiology of thyroid cancer and to help identify the safe limit of iodine intake for prevention.
Acknowledgements
All the participants in the present study and the staff were appreciated working for the Iodine Deficiency Disease Surveillance in China.
This work was supported by the Natural Science Foundation of China (NSFC 81602808, NSFC 81773370, NSFC 81830098).
Conceptualisation: P. L.; data curation: L. F. and F. M.; funding acquisition: L. F. and P. L.; investigation: L. F. and F. M.; visualisation: Y. G.; writing – original draft: L. F.; Writing – review and editing: F. M., Y. G. and P. L.
The authors have declared that no competing interests exist.










