When pregnancy coincides with the continued growth of the mother, the normal hierarchy of nutrient partitioning is altered at the expense of the gravid uterus and mammary gland (Wallace et al. Reference Wallace, Bourke, Da Silva and Aitken2001). In the human up to 50 % of adolescents continue to grow while pregnant and, in spite of larger pregnancy weight gains and increased fat stores, these girls deliver smaller babies compared with non-growing adolescent mothers of equivalent age (Scholl et al. Reference Scholl, Hediger and Schall1997). Similarly, we have consistently shown that overnourishing the singleton-bearing adolescent sheep throughout pregnancy to promote rapid maternal growth results in major placental restriction and leads to the premature delivery of low birth weight lambs (Wallace et al. Reference Wallace, Aitken and Cheyne1996, Reference Wallace, Bourke, Da Silva and Aitken2001, Reference Wallace, Aitken, Milne and Hay2004). Rapid maternal growth is also associated with higher rates of spontaneous abortion in late gestation (Wallace et al. Reference Wallace, Bourke, Da Silva and Aitken2001) and thus our sheep model mimics several of the key features of adverse pregnancy outcome in young human adolescents, namely an increased risk of abortion, preterm delivery and low birth weight (McAnarney et al. Reference McAnarney1987; Scottish Needs Assessment Programme, 1994; Olausson et al. Reference Olausson, Cnattingius and Haglund1999).
The balance between energy and protein intake may be an important factor influencing the extent to which placental and fetal growth is perturbed in adolescents who continue to grow during pregnancy. However, studies assessing nutritional status in human adolescents are poorly controlled and the delivery of low birth weight babies has been associated with both the consumption of high sugar diets (Lenders et al. Reference Lenders, Hediger, Scholl, Khoo, Slap and Stallings1997) and with protein supplementation during late gestation (Rush et al. Reference Rush, Chamberlain and Lumley1986). Neither of these conflicting studies involved concurrent determination of maternal growth status. Similar controversy exists in clinical studies involving mature adult women. Historically, protein deficiency was implicated in depressed fetal growth and impaired infant development (as reviewed by Sloan et al. Reference Sloan, Lederman, Leighton, Himes and Rush2001). However, both high protein supplements in low-income women and high protein intakes in women consuming a self-selected diet (n 2163 pregnancies) are associated with a modest but significant decrease in birth weight (Sloan et al. Reference Sloan, Lederman, Leighton, Himes and Rush2001). Unfortunately, none of these adolescent or adult studies reported placental data. In contrast, a much smaller trial (n 538 pregnancies) has suggested that high carbohydrate intakes in early pregnancy (first trimester) suppress placental and fetal growth, especially if combined with low dairy protein intakes in late pregnancy (Godfrey et al. Reference Godfrey, Robinson, Barker, Osmond and Cox1996).
To date, the adolescent sheep paradigm has involved feeding two levels of the same complete diet throughout gestation. Thus, the overnourished animals who are fed ad libitum to promote maternal growth, receive a diet high in both energy and protein whilst control animals are fed a moderate quantity of the same diet and hence receive adequate energy and protein. To determine whether excessive protein intakes are the cause of adverse pregnancy outcome in rapidly growing adolescents, two isoenergetic diets fed ad libitum and containing 12 % v. 17 % crude protein (CP) have been compared and contrasted with pregnancy outcome in our normal moderate-intake control group (14 % CP). The hypothesis was that if exposure to extra protein is detrimental to pregnancy outcome, then dams receiving the diet supplying the highest amount of protein would have a worse pregnancy outcome than those receiving similar quantities of a diet with lower protein supply. A control moderate-intake group was included as a reference point for optimum pregnancy outcome. Placental proliferation occurs primarily in the first half of gestation (Ehrhardt & Bell, Reference Ehrhardt and Bell1995), while the fetus achieves more than 90 % of its final birth weight during the second half of gestation (Robinson et al. Reference Robinson, McDonald, Fraser and Crofts1977). Thus two further groups fed ad libitum had the protein composition of their diet switched at day 75 of pregnancy to allow comparison of extra protein supply during the main placental versus the main fetal growth phase.
Materials and methods
Animals and experimental design
All procedures were licensed under the UK Animals (Scientific Procedures) Act of 1986 and by the Rowett Research Institute's Ethical Review Committee.
Embryos from superovulated adult ewes (Border Leicester × Scottish Blackface), inseminated by a single sire, were recovered on day 4 after oestrus and transferred synchronously in singleton into the uterus of recipient ewe lambs (Dorset Horn × Mule) exactly as described previously by Wallace et al. (Reference Wallace, Da Silva, Aitken and Cheyne1997). Donor ewes (n 10) were multiparous, between 3 and 4 years of age and had a body condition score of 2·3 (sem 0·03) units at the time of embryo recovery. This protocol ensured that placental and/or fetal growth was not influenced by varying fetal number or partial embryo loss. In addition, the use of a single sire and a limited number of embryos donors maximised the homogeneity of the resulting fetuses. Embryo transfer was carried out during the mid-breeding season and the animals were housed in individual pens under natural lighting conditions at the Rowett Research Institute (57°N, 2°W). At the time of embryo transfer, the recipient ewe lambs (n 71) were pubertal (about 8·5 months old), with a mean live weight of 43·6 (sem 0·25) kg and a body condition score of 2·3 (sem 0·01) units. Immediately following embryo transfer, recipients were initially allocated to one of three nutritional treatments on the basis of live weight, body condition score and ovulation rate at the time of transfer. Where possible, care was also taken to randomise for the maternity of the embryo. Recipients were initially individually offered ad libitum an isoenergetic diet containing either 12 % (basic, B, n 28) or 17 % (extra, E, n 28) CP (Table 1). At day 75 of gestation, half the pregnant ewes on each protein level were switched to yield four groups, BB, EE, BE and EB protein. At this time-point allocation to the subsequent treatment group was on the basis of average maternal live weight gain and change in adiposity score during the first half of gestation. These high or ad libitum intakes were predicted to promote rapid maternal growth throughout gestation (Wallace et al. Reference Wallace, Aitken, Milne and Hay2004). A further control group (C, n 15) was offered a moderate quantity of a ration containing 14 % CP. The protein content and estimated protein supply of this ration was intermediate between the 12 and 17 % rations offered to the ad libitum intake groups. It was important to include this moderate-intake control group for two reasons. Firstly, we needed a reference point for optimum placental and fetal growth and colostrum production in adolescent dams. This ration has been extensively used previously and was originally designed, at the moderate level of intake offered, to maintain maternal adiposity throughout gestation and to provide 100 % of the estimated energy and protein requirement of the adolescent sheep carrying a singleton fetus according to stage of pregnancy. Indeed, previous studies have shown that this ration optimises placental and fetal growth in this genotype (Wallace et al. Reference Wallace, Aitken, Milne and Hay2004). Secondly, we needed to ensure that if the amount of diet consumed (energy intake) rather than its protein composition was responsible for our previously documented adverse pregnancy outcomes in ewes fed ad libitum, then the design of the study would allow us to check this by providing reference data for dams receiving moderate but adequate intakes of a balanced complete diet during pregnancy. In practical terms maximum placental and fetal growth is achieved in the control group by allowing a moderate maternal weight gain (about 65 g/d) during the first two-thirds of gestation, followed by step-wise increases in maternal intake during the final third of gestation to meet the increasing demands of the developing fetus. The composition of the three complete diets is presented in Table 1 together with estimates of protein supply calculated on the basis of average fresh feed intakes and maternal weight and according to AFRC (1993). Diets were mixed on site approximately once weekly and each batch was sub-sampled, ground and analysed to confirm the desired protein content had been achieved (Davidson et al. Reference Davidson, Mathieson and Boyne1970). The diets were offered in two equal feeds at 08.00 and 16.00 hours daily. Animals offered moderate (control, C) intakes received their entire ration immediately after embryo transfer on day 4 of pregnancy, while those offered high intakes had the level of feed gradually increased over a 2-week period until the level of daily feed refusal was approximately 15 % of the total offered (equivalent to ad libitum intakes). For those ewes switching protein level at day 75 of gestation the change in diet composition was immediate. The level of feed offered was reviewed three times weekly and adjusted, on an individual basis as and when appropriate, on the basis of body weight change data (recorded weekly) and the level of feed refused (recorded daily). Maternal body condition or adiposity score was subjectively assessed on a five-point scale (1 = emaciated, 5 = obese; Russel et al. Reference Russel, Doney and Gunn1969) by one member of the team at approximately fortnightly intervals.
Table 1 Diet composition (kg/tonne), metabolisable energy, crude protein content and estimated protein supply

Pregnancy rate was initially measured on about day 45 of gestation by transabdominal ultrasound when twenty-two, seventeen and eleven ewes in the B, E and C groups, respectively, had viable fetuses.
Pregnancy outcome
Pregnancy outcome was determined after spontaneous delivery at term. All ewes were supervised throughout the delivery period. Lambs were dried and weighed after delivery and the sex recorded. Ewe colostrum yield was measured before lamb suckling and within 30 min of parturition. After intravenous injection of oxytocin (10 IU Oxytocin-S® per ewe; Intervet Ltd, Cambridge, UK), ewes were milked by hand until all the colostrum had been removed from the udder. The colostrum was weighed, sampled for IgG and nutrient composition analyses and then fed to the lamb by bottle at a rate of approximately 50 ml/kg body weight. In cases where the dam had insufficient colostrum, frozen ewe colostrum was substituted to ensure lamb survival. After the placenta (fetal cotyledons and membranes) was delivered, it was weighed and cotyledons dissected, counted and weighed.
Blood sampling and biochemical analysis
Fortnightly blood samples were collected from all pregnant ewes from day 14 of gestation onwards by jugular venepuncture, approximately 3 h after the morning feed. These samples were analysed for plasma insulin, glucose, urea and NEFA. Samples collected on days 14, 56, 98 and 140 of gestation from ewes in the BB, EE and C groups were also analysed for homocysteine (Hcy). Insulin concentrations were measured in duplicate by RIA as described previously (McRae et al. Reference MacRae, Bruce, Hovell, Hart, Inkster and Atkinson1991). The limit of detection was 4 μU insulin/ml and the intra- and inter-assay CV were 5·2 and 6·7 %, respectively. Plasma glucose, urea and NEFA concentrations were determined as originally described (Talke & Shubert, Reference Talke and Shubert1965; Peterson & Young, Reference Peterson and Young1968; Matsubara et al. Reference Matsubara, Nishikawa, Yoshida and Takamura1983). Hcy concentrations were determined on 0·9 g plasma using isotope dilution (Calder et al. Reference Calder, Garden, Anderson and Lobley1999) with derivatisation and ions monitored as described by Lobley et al. (Reference Lobley, Shen, Le, Bremner, Milne, Calder, Anderson and Dennison2003).
Colostrum IgG content was determined using an ovine-specific ELISA. Colostrum samples were diluted (1:100 000) and assayed in duplicate as follows. Ninety-six-well high-protein binding flat-bottomed microtitre plates (Immunolon 4 HBX; Dynex Technologies, Chantilly, VA, USA) were coated overnight with purified ovine IgG (I-5131, 0·5 μg/ml in 0·05 m-bicarbonate buffer; Sigma Chemical Co., St Louis, MO, USA). Unbound antibody was removed by washing three times with a 0·05 % solution of Tween-20 in PBS. In a separate round-bottomed ninety-six-well microtitre plate, 120 μl unknown sample or standard (in the range 12·5–0·05 μg IgG/ml) were mixed with 120 μl anti-sheep IgG (S-1265; Sigma). The mix (200 μl) was then transferred to the coated plate and incubated at room temperature for 2 h. Wells were then emptied and washed as described earlier. Anti-rabbit peroxidase conjugate (200 μl, A-0545; Sigma) was added to each well and incubated for 1 h at room temperature. Plates were then emptied and washed as previously and 200 μl 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS)(A-1888; Sigma)–hydrogen peroxide (H-6520; Sigma) enzyme substrate were added. After 15 min, the reaction was stopped by addition of 100 μl 0·4 m-sodium fluoride, and the optical density was read immediately at 405 nm using a Titretek multiscan plate-reading spectrophotometer. The optical density in each well was inversely proportional to the concentration of IgG in the standard or sample. The intra- and inter-assay CV for two pooled quality control samples included in each of two plates were 2·9 and 3·2 %, respectively. Colostral fat, protein and lactose concentrations were determined in duplicate by previously described methods (Davidson et al. Reference Davidson, Mathieson and Boyne1970; Stookey & Zehnder, Reference Stookey and Zehnder1970).
Data analysis
Initial conception rates and the number of male fetuses per group were compared by Fisher's exact test. Pregnancy outcome data were analysed by one-way ANOVA (Minitab 14; Minitab Inc., State College, PA, USA). Where significant differences were indicated (P < 0·05) this was followed post hoc by Fisher's LSD procedure to determine which treatments differed. For the maternal hormone and metabolite concentrations, individual means were calculated for the period spanning the first and second half of gestation. The resulting data were then analysed in Genstat 7th edition (VSN International Ltd, Hemel Hempstead, UK) using split-plot ANOVA with factors for diet between sheep (main plots) and period of gestation within sheep (subplots). P values are reported for the main effects of diet and period of gestation, and the diet × period of gestation interaction. As earlier, where significant differences were indicated, nutritional treatments were further compared within period of gestation by Fisher's LSD. Correlation coefficient analysis was by Pearson's product moment test where appropriate.
Results
Maternal dietary intake, weight and adiposity score
Conception rate following embryo transfer was 78·6, 60·7 and 73·3 % in the B, E and C groups, respectively, and was not significantly influenced by maternal dietary treatment. Weekly feed intakes were independent of protein level in the four groups of ewes fed ad libitum and were significantly higher (P < 0·001) than in the control group throughout (Fig. 1). On average in the groups fed ad libitum this represented a 2·25- and 1·87-fold increase in metabolisable energy intake relative to the control group during the first versus the second half of gestation, respectively. Changes in maternal live weight and adiposity at key points during gestation are presented in Table 2. Maternal adiposity score was successfully maintained at the initial level throughout gestation in the control group. As a result of the high intakes in the four groups fed ad libitum both maternal live weight and adiposity score diverged from that of the control group by the end of the first month of gestation (data not shown) and were profoundly different by mid-pregnancy (day 75; Table 2). Within the groups fed ad libitum, maternal weight was independent of protein level at both mid and late (day 140) gestation time-points. Daily live weight gain from embryo transfer to day 75 of gestation was higher (P < 0·05) in EE than in BE groups, and at day 140 of gestation, adiposity score was higher (P < 0·05) in the BE than the EB group. At approximately 24 h postpartum, mean maternal live weight was 73·6, 76·8, 75·1 and 75·3 kg in the BB, EE, BE and EB groups compared with 54·3 kg in the control group (P < 0·001).

Fig. 1 Weekly food intakes throughout gestation in ewes offered ad libitum a diet containing 12 % (basic, B) or 17 % (extra, E) crude protein. Diets were either offered continuously throughout gestation (BB, □; EE, ■) or switched at day 75 of gestation (BE, Δ; EB, ▲). The control group (○) received a moderate quantity of a diet containing 14 % crude protein which was designed to provide 100 % of the estimated energy and protein requirement of the adolescent sheep according to stage of pregnancy. For details of procedures, see p. 1061. Values are means with their standard errors depicted by vertical bars.
Table 2 Changes in maternal live weight and adiposity score in relation to diet composition and maternal intake* (Mean values with their standard errors)
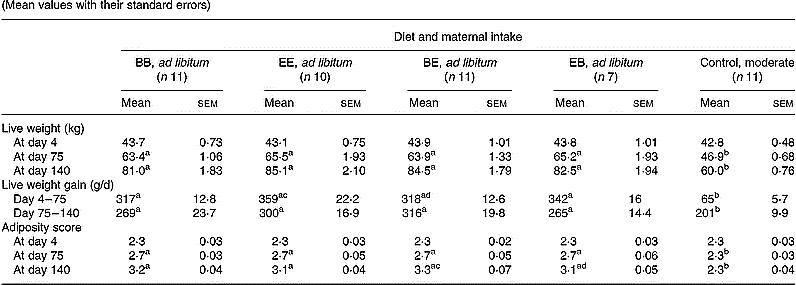
B, basic diet; E, extra diet; at day 75 of gestation, half the pregnant ewes on each protein level were switched to yield four groups: BB, EE, BE and EB.
* For details of procedures and diets, see p. 1061 and Table 1.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Pregnancy outcome
Pregnancy outcome data following spontaneous vaginal delivery at term are presented in Table 3. Gestation length, fetal placental mass, total fetal cotyledon mass and lamb birth weight were reduced (P < 0·05) in all groups fed ad libitum relative to the optimally nourished control group. However, within ad libitum groups, total placental mass, fetal cotyledon mass and lamb birth weight were unaffected by level or timing of exposure to the different protein diets. Mean gestation length was shortest in the group receiving extra protein throughout gestation (EE) and this reached statistical significance (P < 0·05) when compared with the group receiving a basic level of protein throughout (BB). The lamb birth weight to placental weight ratio was similar in all five groups. Placental and fetal weights were positively correlated within all five groups (r 0·686–0·944, P < 0·05 to P < 0·001). Within individual groups there was no statistically significant relationship between maternal live weight gain during the first half of gestation and placental mass. However, when all five groups were combined, maternal live weight gain during the first half of gestation was negatively correlated with placental mass (r − 0·539, n 50, P < 0·001), total cotyledon mass (r − 0·639, P < 0·001), lamb birth weight (r − 0·537, P < 0·001) and gestation length (r − 0·612, P < 0·001). There were no consistent relationships between maternal live weight gain during the second half of gestation and any parameter of pregnancy outcome assessed, either within individual groups or when treatments were combined.
Table 3 Pregnancy outcome in relation to diet composition and maternal intake* (Mean values with their standard errors)
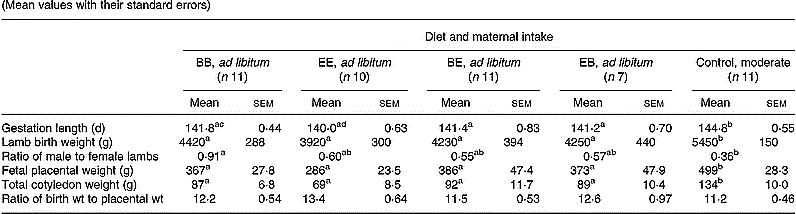
B, basic diet; E, extra diet; at day 75 of gestation, half the pregnant ewes on each protein level were switched to yield four groups: BB, EE, BE and EB.
* For details of procedures and diets, see pp. 1061, 1062 and Table 1.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Colostrum yield, IgG content and nutrient composition
Mean colostrum yield immediately after parturition was suppressed (P < 0·001) in all four groups fed ad libitum when compared with the optimally nourished control group (Table 4). Colostrum production at parturition exceeded the initial minimum requirement of 50 ml/kg birth weight in all animals in the control group, whereas 50 % of lambs born to mothers fed ad libitum (equivalent to six, four, five and three animals in the BB, EE, BE and EB groups, respectively) would have had insufficient colostrum intake had they not been supplemented at birth. Within ad libitum groups, colostrum yield was highly variable but was unaffected by level or timing of exposure to the different protein diets. Colostrum from BB and EE dams had a higher IgG concentration (P < 0·05) than the other three groups including the controls, however, the large differences in yield meant that the total available IgG content at parturition was at least 2-fold higher (P < 0·001) in the control group relative to the four ad libitum groups. Irrespective of treatment group there was a negative correlation between colostrum yield and IgG concentration (r − 0·621, n 47, P < 0·001). On a concentration basis, colostrum from all five dietary groups contained similar amounts of butterfat and CP (Table 4). In contrast, colostral lactose concentrations were significantly higher (P < 0·01) in the control compared with all four groups fed ad libitum. When expressed relative to individual colostrum yield, the total butterfat, lactose and CP available to the newborn lamb was lower (P < 0·001) in the four groups fed ad libitum compared with the control groups. Similarly, the mean total energy content of the colostrum was 2–4-fold lower in the ad libitum v. control groups (P < 0·001). Within groups fed ad libitum, colostrum nutrient composition was unaffected by level or timing of exposure to different protein diets.
Table 4 Colostrum yield, IgG content and nutrient composition in relation to diet composition and maternal intake (Mean values with their standard errors)
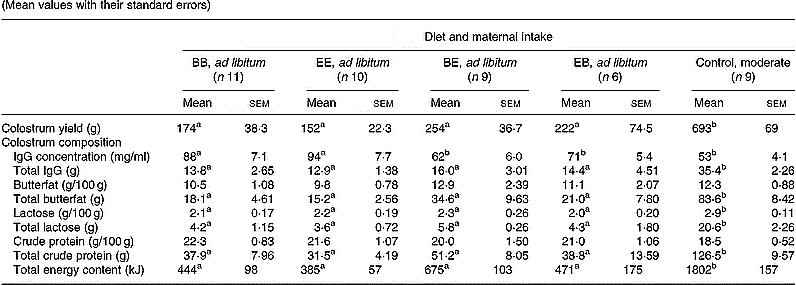
B, basic diet; E, extra diet; at day 75 of gestation, half the pregnant ewes on each protein level were switched to yield four groups: BB, EE, BE and EB.
*For details of procedures and diets, see pp. 1061, 1062 and Table 1.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Maternal insulin and metabolite status throughout gestation
The effect of diet composition and stage of pregnancy on maternal insulin and metabolite status is summarised in Table 5. Maternal plasma insulin concentrations were lower in control than in all groups fed ad libitum during both the first and second half of gestation (P < 0·001). In addition, within groups fed ad libitum plasma insulin levels were higher (P < 0·05) in ewes receiving 17 v. 12 % protein during the second half of gestation. Within groups fed ad libitum maternal plasma urea concentrations reflected the current CP content of the diet offered and were elevated (P < 0·001) in the 17 % (extra) compared with the 12 % (basic) groups (Fig. 2). Similarly, plasma urea concentrations in the optimally nourished control group (14 % CP) were intermediate (P < 0·05) between the extra and basic ad libitum groups during both periods of gestation. During the first half of gestation, maternal glucose concentrations were independent of both dietary intake and protein composition. Thereafter glucose concentrations were generally lower (P < 0·001) in the second half of gestation, with the exception of the EE group whose glucose levels did not change. Maternal plasma NEFA concentrations were higher (P < 0·05) in the control compared with all four groups fed ad libitum during the first half of gestation. Plasma NEFA concentrations increased in all five groups during the second half of gestation (P < 0·001) but were independent of both dietary intake and protein composition during this time.
Table 5 Mean maternal insulin, urea, glucose and NEFA concentrations during the first and second half of gestation in relation to diet composition and maternal intake (Mean values with their standard errors)
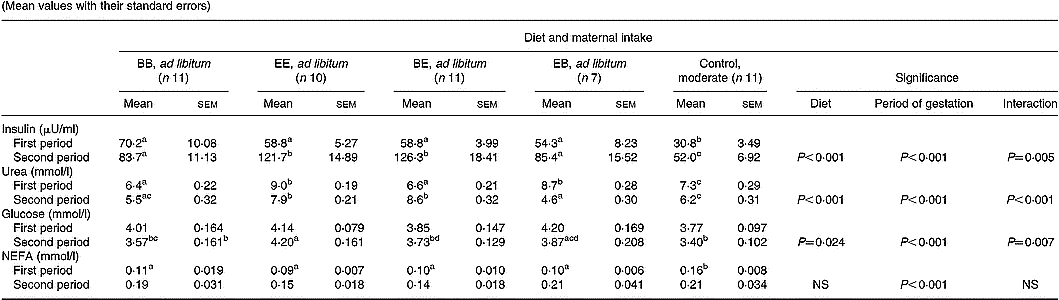
B, basic diet; E, extra diet; at day 75 of gestation, half the pregnant ewes on each protein level were switched to yield four groups: BB, EE, BE and EB.
*For details of procedures and diets, see pp. 1061, 1062 and Table 1.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).

Fig. 2 Changes in maternal plasma urea concentrations determined at about 14 d intervals from day 14 of gestation onwards. Ewes were offered ad libitum a diet containing 12 % (basic, B) or 17 % (extra, E) crude protein. Diets were either offered continuously throughout gestation (BB, □; EE, ■) or switched at day 75 of gestation (BE, Δ; EB, ▲). The control group (○) received a moderate quantity of a diet containing 14 % crude protein which was designed to provide 100 % of the estimated energy and protein requirement of the adolescent sheep according to stage of pregnancy. For details of procedures, see pp. 1061 and 1062.
Maternal homocysteine concentrations
Maternal Hcy concentrations were equivalent throughout gestation at all time-points measured in the BB and EE groups. On days 14, 56 and 98 of gestation Hcy concentrations in both these groups fed ad libitum were lower (P < 0·01 or less) than in the control group (Fig. 3). In contrast, at day 140 of gestation, Hcy concentrations were higher in the ad libitum-fed relative to the control group, reaching significance for the basic versus control group comparisons only (P < 0·05). There were no significant relationships between maternal Hcy concentrations at any stage of gestation and any parameter of pregnancy outcome assessed, either within individual groups or when treatments were combined. However, average live weight gain during the first two-thirds of gestation was negatively correlated with the mean Hcy concentrations during the same period in the BB plus EE groups (r − 0·473, n 21, P < 0·05) groups.

Fig. 3 Maternal plasma homocysteine concentrations at days 14, 56, 98 and 140 of gestation. Ewes were offered ad libitum a diet containing 12 % (basic, BB, □) or 17 % (extra, EE, ■) crude protein throughout gestation. The control group (○) received a moderate quantity of a diet containing 14 % crude protein and designed to provide 100 % of the estimated energy and protein requirement of the adolescent sheep according to stage of pregnancy. On days 14, 56 and 98 of gestation, homocysteine concentrations in both BB and EE groups were lower (P < 0·01) than in the control group. At day 140 of gestation homocysteine concentrations were higher (P < 0·05) in the BB compared with the control group. Values are means with their standard errors depicted by vertical bars. For details of procedures, see p. 1062.
Discussion
The present study confirms our previous observations that overnourishing the pregnant adolescent sheep to promote rapid maternal growth rates and increased adiposity results in placental growth restriction and the premature delivery of reduced birth weight lambs relative to moderate-intake, control-fed adolescents of equivalent age (Wallace et al. Reference Wallace, Aitken and Cheyne1996, Reference Wallace, Aitken, Milne and Hay2004). The present study further demonstrates that it is high metabolisable energy intakes rather than the protein composition of the diet which is the primary cause of adverse pregnancy outcome in these rapidly growing adolescent animals. While there is no directly comparable evidence of a similar relationship between high dietary energy intakes and pregnancy outcome in human adolescents, relationships between maternal growth status and birth weight have been reported. Thus adolescent girls who continue to grow during pregnancy (as defined by measuring knee height) have larger pregnancy weight gains and increased fat stores but deliver smaller babies than non-growing mothers of equivalent age (Scholl et al. Reference Scholl, Hediger and Schall1997). Furthermore, low-income adolescent girls who were categorised as high sugar consumers (top 10th percentile) using a multiple 24 h food recall approach are at increased risk of delivering a small for gestational age infant if of Black, White or Puerto Rican ethnicity and for delivering prematurely if Puerto Rican (Lenders et al. Reference Lenders, Hediger, Scholl, Khoo, Slap and Stallings1997). In the latter study, high sugar consumers also had a higher total energy intake but protein intake was relatively reduced. Unfortunately neither of these adolescent studies reported placental weight data.
This robust alteration in nutrient partitioning during pregnancy in response to high metabolisable energy intakes appears to be unique to the young adolescent in that it does not occur in overnourished primiparous adult sheep studied under identical experimental conditions (Wallace et al. Reference Wallace, Milne and Aitken2005). Similarly, in adult humans living in wealthier sections of developed societies there is a paucity of robust relationships between maternal nutrition and birth weight, at least in women gestating a single fetus (Mathews et al. Reference Mathews, Yudkin and Neil1999, Reference Mathews, Youngman and Neil2004; Robinson et al. Reference Robinson, Moore, Owens and McMillan2000). Thus the competition for nutrients between the maternal body and her gravid uterus in the gynaecologically immature and still growing adolescent makes her particularly vulnerable to variations in maternal dietary intake.
The design of the present study allowed us to compare extra protein supply during the main placental versus the main fetal growth phase of pregnancy. The maternal plasma urea concentrations reflected the current protein composition of the diet in all groups and verify that the desired changes in protein supply had been achieved during the required period of pregnancy. There was no evidence of an inhibitory effect of high protein on appetite in that dietary intakes of the isoenergetic diets were equivalent between ad libitum groups during both periods of gestation. Thus any effects within the groups fed ad libitum could be attributed to differences in protein rather than energy intake. Furthermore, the inclusion of a moderate-intake control group, receiving a diet which met 100 % of the nutrient requirements of a singleton pregnancy, provided an essential reference group for optimum outcome of the various pregnancy parameters measured. In the present study, the animals receiving the highest level of protein continuously throughout gestation had the lowest mean placental and fetal weight but this was not statistically different when compared with the other groups fed ad libitum. Intriguingly, gestation length was also shortest in the former group and reached statistical significance when compared with the animals who were offered the basic protein diet throughout gestation. During the second half of gestation, both groups of ewes receiving the highest level of protein also had higher maternal insulin concentrations and in the EE group maternal glucose concentrations remained elevated, suggesting a differential degree of insulin resistance in this cohort relative to the other three groups fed ad libitum. Larger study cohorts may be required to determine if there is an interaction between high energy intake and the protein composition of the diet. Negative effects of protein supplementation of adolescent and adult women have been reported (Rush, Reference Rush, Chamberlain and Lumley1986; Sloan et al. Reference Sloan, Lederman, Leighton, Himes and Rush2001) but these women were generally from low-income groups with inadequate or sufficient energy intakes, rather than the 2-fold higher energy intakes studied here. Indeed, all the adolescent animals in the present study had good nutrient reserves at conception and were well nourished throughout gestation as indicated by the general similarities in maternal glucose and NEFA concentrations. The relatively higher NEFA concentrations in the control group during the first half of gestation, and the gestational increase in NEFA levels in all five groups, indicate increased turnover of lipid stores to maintain the maternal glucose pool as pregnancy progresses and fetal demand for glucose increases. In all cases both maternal glucose and NEFA concentrations were within the range characteristic of optimally nourished adult sheep (Russel et al. Reference Russel, Doney and Reid1967; Mellor, Reference Mellor1983). It remains to be established whether similar pregnancy outcomes would result if the adolescent recipients had poor nutrient reserves at conception. Data demonstrate that adult women with short inter-pregnancy intervals ( < 18 months) and hence at risk of nutritional depletion at the outset of pregnancy have a higher incidence of preterm delivery and low birth weight (King, Reference King2003).
In women, high Hcy concentrations have been variously associated with a reduction in birth weight and an increased incidence of preterm delivery (Murphy et al. Reference Murphy, Scott, Arija, Molloy and Fernandez-Ballart2004; Ronnenberg et al. Reference Ronnenberg, Goldman, Chen, Aitken, Willett, Selhub and Xu2002; Yajnik et al. Reference Yajnik, Deshpande, Panchanadikar, Naik, Deshpande, Coyaji, Fall and Refsum2005). Similarly, in rodent models of fetal programming, protein restriction during early pregnancy is associated with elevated maternal serum Hcy (Petrie et al. Reference Petrie, Duthie, Rees and McConnell2002), and defects in the tetrahydrofolate cycle and hence DNA methylation have been postulated as a causative agent in aberrant prenatal programming. The design of the present ovine study allowed us to examine maternal Hcy concentrations in a model characterised by both premature delivery and reduced birth weight and additionally facilitated comparison at two contrasting protein but equivalent and high metabolisable energy intakes. The low maternal Hcy concentrations during the first two-thirds of gestation in both ad libitum versus the control intake groups and the failure to detect a relationship between Hcy concentrations and any of the pregnancy outcome measures assessed suggests that the marked differences in maternal Hcy concentrations in these animals largely reflects the 2-fold difference in dietary supply of sulphur-containing amino acids. The conclusion is further supported by the negative relationship between mean Hcy concentrations and maternal live weight gain within the animals fed ad libitum. Thus in this ovine model of maternal overnutrition, changes in sulphur amino acid metabolism are unlikely to be a cause of placental or fetal growth restriction.
The 53–78 % decrease in colostrum yield at parturition in the four groups fed ad libitum compared with the control groups is similar to that reported previously (Wallace et al. Reference Wallace, Aitken and Cheyne1996, Reference Wallace, Bourke, Da Silva and Aitken2001). This major reduction in colostrum yield and the associated delay in the establishment of lactation is closely associated with the reduction in placental mass and has clear implications for human pregnancies similarly compromised by placental growth restriction. Although not measured in the present study, previously reported reductions in placental lactogenic hormone concentrations (growth hormone, placental lactogen and progesterone) in the ad libitum-fed and rapidly growing dams are likely to be the root cause of attenuated colostrum production (Wallace et al. Reference Wallace, Da Silva, Aitken and Cheyne1997, Reference Wallace, Bourke, Da Silva and Aitken2001). The present study further demonstrates that when metabolisable energy intakes are high, the quantity and quality of the colostrum available to the newborn lamb is largely unaffected by the CP content of the diet. In adult ewes, recent data on the influence of dietary protein supplementation on colostrum yield and composition are somewhat equivocal. In triplet-bearing adult ewes offered high-quality grass diets, protein supplementation for the final 6 weeks of pregnancy modestly increased colostrum yield but decreased its IgG concentration. However, these effects were independent of the degradability of the protein supplement (Annett et al. Reference Annett, Carson and Dawson2005). In contrast, in singleton-bearing adult ewes, high CP intakes (1·4 times control intake) for the final 8 weeks of pregnancy were associated with a 30 % reduction in colostrum yield and a 20 % reduction in lamb survival to weaning (Ocak et al. Reference Ocak, Cam and Kuran2005). Although in the present study colostrum IgG concentration was negatively associated with colostrum volume obtained at parturition, the reduction in yield in the groups fed ad libitum resulted in a major decrease in the total IgG available to the newborn lamb. As the absolute amount of IgG consumed, rather than its concentration, is the most important determinant of immune status in the newborn (Hunter et al. Reference Hunter, Renaeu and Williams1977), the slightly premature and smaller lambs born to the dams fed ad libitum may not receive adequate antibody from their mother to confer passive immunity, leaving them susceptible to both systemic and enteric infection. Indeed when the overnourished adolescent model was first established (Wallace et al. Reference Wallace, Aitken and Cheyne1996), five of eight lambs born to the overnourished dams died within 72 h of parturition following enteric infections. In this initial study, when the dam's colostrum yield was insufficient to meet the lamb's requirements, a synthetic colostrum replacer supplement was used and these are known to be a poor source of IgG. Reduced birth weight lambs have a relatively large surface area relative to their body weight and reduced insulation and thus an increased reliance upon colostrum as a source of energy for heat production after birth. The reduction in total lactose, butterfat, CP and hence metabolisable energy available to the newborns in the groups fed ad libitum in the present study may fail to meet the initial energy requirements of the lamb during the critical neonatal period. Without intervention this would result in hypothermia, starvation and death in a proportion of lambs.
In conclusion, the present study demonstrates that it is excessive energy intakes rather than the high protein composition of the diet which are primarily responsible for the alteration in the hierarchy of nutrient partitioning at the level of the gravid uterus and mammary gland in overnourished and rapidly growing adolescent sheep.
Acknowledgements
We thank David Brown, Graham Calder and Christine Horrocks for technical assistance, Graham Horgan (Bioss) for statistical advice and Dr Linda Mitchell, Dr John Rooke and Professor John Robinson (Scottish Agricultural College) for assistance with diet formulation and estimated nutrient supply. The study was funded by the Scottish Executive Environment and Rural Affairs Department.










