Acute upper respiratory tract viral infection, also referred to as the common cold, is the most frequent infectious disease in human beings. The majority of adults are affected two to four times, and children even six to eight times per year, resulting in economic losses due to absence at work or school. The mean duration of a common cold is 7 to 10 d whereas the peak level of symptom severity is reached 2 to 3 d after incubation(Reference Heikkinen and Järvin1). Symptoms often start with headache, sneezing, chilliness and sore throat followed by nasal discharge, nasal obstruction, cough and general feeling of sickness(Reference Eccles2). Due to the diversity of the viral causes and the fact that the common cold is usually self-limiting, treatment has mainly focused on reducing the duration and severity of symptoms. Besides conventional treatment with over-the-counter analgesics, nasal and/or oral decongestants and antipyretics, self-medication with botanicals is popular(Reference Craig3, Reference Roxas and Jurenka4). Particularly, Echinacea and elder are used based on long experience in folk medicine as well as scientific investigations including human studies(Reference Zakay-Rones, Varsano and Zlotnik5–9). The health-promoting potential is mainly attributed to the immunostimulative, antiviral, anti-inflammatory and antioxidative activity of low- and high-molecular-weight compounds such as flavonoids and polysaccharides(Reference Kaul, Middleton and Ogra10–Reference Middleton12).
Flavonoids, particularly catechins, are also the active principle of green tea and green tea extracts(Reference Song and Seong13). In several in vitro studies the immunomodulatory effects of green tea catechins through immunostimulatory(Reference Hu, Toda and Okuba14, Reference Kamath, Wang and Das15) as well as anti-inflammatory(Reference Clarke and Mullin16), antiviral(Reference Nakayama, Suzuki and Toda17, Reference Imanishi, Tuji and Katada18) and antibacterial(Reference Ikigai, Nakae and Hara19) activities were proven. Furthermore, these findings were affirmed in human studies. In a prospective clinical study, gargling with tea catechin extract significantly reduced the incidence of influenza infection compared with gargling without tea catechin extract in elderly subjects(Reference Yamada, Takuma and Daimon20). Stimulation of immune cell function after consumption of a high-polyphenol juice containing green tea catechins was confirmed by increased lymphocyte proliferative responsiveness, IL-2 secretion by activated lymphocytes as well as enhanced lytic activity of natural killer cells in a further randomised study involving twenty-seven healthy men. Additionally, reduced oxidative DNA damage in lymphocytes due to antioxidative activities of phenolic compounds was observed(Reference Bub, Watzl and Blockhaus21). The capability to increase the antioxidative capacity in healthy subjects is probably the most investigated pharmacological effect of polyphenols from grapes(Reference Whitehead, Robinson and Allaway22–Reference Netzel, Netzel and Maier26). Moreover, as for green tea polyphenols, several in vitro findings and animal experiments support the immunomodulatory effects of phenolic compounds from grapes. Intragastrically applied grape seed proanthocyanidins significantly inhibited tumour growth and affected immune functions through increased natural killer cell cytotoxicity, lymphocyte proliferation, CD4+:CD8+ ratio, IL-2 and interferon-γ (IFN-γ) production in tumour-bearing mice(Reference Zhang, Li and Wu27). Elevated IFN-γ production together with enhanced IFN-γ gene expression and increased regulated number of IFN-γ-positive cells have already been observed in an earlier study investigating the influence of grape seed extract on peripheral blood mononuclear cells from healthy donors(Reference Nair, Mahajan and Chawda28). In addition to such direct effects on the immune system, antiviral as well as antibacterial properties have been described for various grape products and grape seed extracts(Reference Konowalchuk and Speirs29–Reference Jayaprakasha, Selvi and Sakaria31).
Immune-stimulating activity is not only attributed to polyphenols but also to polysaccharides. Particularly, the property to trigger both the innate and adaptive immune system of lentinan, a β-1,6;β-1,3-glucan from shiitake mushrooms, is well documented by numerous in vitro tests. The mechanism is postulated to involve β-glucan binding to lymphocyte surfaces or serum-specific proteins, which activates macrophages, T-helper cells, natural killer cells, and other effector cells. Consequently, the production of antibodies as well as interleukins (IL-1, IL-2) and IFN-γ is increased(Reference Wasser, Coates, Blackman, Cragg, Levine, Moss and White32). This stimulation of the immune system by fungal glucans is accounted to be the active principle in treating cancer and infectious diseases(Reference Chen and Seviour33).
In view of these findings, a polyphenol-rich beverage containing green tea (Camellia sinensis), grape seed and grape skin (Vitis vinifera L.) as well as shiitake mushroom (Lentinus edodes) extract was created and its immune-stimulating activity was investigated in patients suffering from the common cold. Since no single immune marker that accurately reflects an individual's resistance to infection exists, the recommendation of the PASSCLAIM publication on immunity was followed and the severity and duration of the disease were assessed(Reference Cummings, Antoine and Azpiroz34, Reference Albers, Antoine and Bourdet-Sicar35).
Materials and methods
Subjects
Volunteers with a common cold were recruited via advertisements, in practices of the clinical investigators, and from pharmacies that had previously received information material from the contract research organisation. Included were 100 patients aged between 20 and 65 years scoring in total at least 5 points on the severity of five cold symptoms (maximum 15 points; see below) and having present at least one cold-related local finding (throat). Exclusion criteria consisted of the following: active allergic rhinitis or asthma, fever (body temperature>39°C), clinically relevant laboratory value deviations indicating severe organ or system disease, bacterial tonsillitis, pyorrhoea on the back of the throat, cancer or AIDS (HIV positive), pregnancy or nursing, alcohol, medication or drug dependence, participation in a clinical study within the previous 30 d, intake of immune suppressants, immune stimulants, analgesics/anti-rheumatics, anti-inflammatories, antitussives, expectorants, influenza remedies, mouth and throat therapeutics as well as inability to comply due to language difficulties.
The patients gave their informed written consent to participate in the study. The study was approved by the ethical committee of the University Hospital Charité of the Humboldt-University Berlin, Germany, and performed according to the principles of the World Medical Association (Declaration of Helsinki/Hong Kong/Somerset), the German Drug Law (Arzneimittelgesetz; AMG) and the European Union recommendations (CPMP/ICH/135/95).
Study design
The study was performed between February and August 2004, to coincide with the normal season for the common cold. Investigation was conducted as a randomised, prospective, placebo-controlled, multi-centric clinical, double-blind study. A randomisation diagram with a block size of four, and in a parallel-group design with two independent treatment groups was created using EDGAR (Experimental Design Generator and Randomiser; James KM Brown, John Innes Centre, Norwich, UK; http://www.edgarweb.org.uk). Patients were instructed to consume 2 × 250 ml of the verum or the placebo beverage daily. The trial was accomplished within 10 d. Examinations through clinical investigators were scheduled immediately before the start of treatment (first examination, baseline), after 3 to 6 d (second examination) and finally after 7 to 10 d (third examination).
Compliance control
Compliance control was performed on the basis of the empty packaging and unused investigational product, which was documented for each patient. Furthermore, a patient was rated as compliant when he/she carefully filled out the patient diary and followed the assigned examination appointments.
Efficacy evaluation
The primary efficacy criterion was defined as the decrease of the total score of the five cold symptoms (headache and/or joint pains, sore throat and/or difficulty swallowing, hoarseness and/or cough, nasal congestion/sniffle) in comparison with placebo, displaying an improvement of the disease. It was assessed by the physician by questioning.
‘Complaint free by the end of the study’ was specified as the secondary efficacy criterion. For this criterion, the appraisal of the physician and the patient diary were evaluated.
During the three examinations, the total score of the five common cold symptoms was determined by summation of the single scores. The severity of the symptoms was rated on a scale from 0 to 3 points (0 = not present; 1 = mild; 2 = moderate; 3 = strong), giving a theoretical sum score range from 0 to 15. Furthermore, the severity of the concurrent variables, such as the local findings ‘reddening of the Waldeyer's ring or tonsils’, ‘granulation and/or myxorrhoea on the pharyngeal wall’ and ‘herpes labialis’ was categorised as ‘not present’ (0 points), ‘mild’ (1 point) or ‘strong’ (2 points) by the physician. In addition to the medical examinations, the patients were required to record daily, mornings and evenings, the changes of the above-mentioned cold symptoms as well as the concurrent variables ‘disturbance of daily activities’, ‘sleep disorders’, ‘additional concurrent medication’ and ‘the number of tissues used’ for the duration of the study or until an early complaints-free condition could be documented.
Safety evaluation
The safety of the investigational product was assessed by clinical data (weighing, measurement of pulse, blood pressure, and body temperature), safety parameters (Hb, packed cell volume, erythrocytes, blood platelets, alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase, total bilirubin, creatinine, uric acid, and total protein), the occurrence of adverse events, as well as the global evaluations of the patients and clinical investigators.
Test material
The placebo drink was a red-coloured soft drink containing water, sugar, citric acid and flavour. Compared with the placebo drink, the verum drink was standardised with respect to the content of polyphenols (1400 mg/l measured according to Folin–Ciocalteu and expressed as gallic acid equivalents (GAE)). The verum drink contained green tea extract (3 g/l), grape peel extract (12 g/l), grape seed extract (0·5 g/l), shiitake mushroom extract (0·05 g/l) as well as vitamin C (0·3 g/l). The verum and placebo drinks were identical in visual appearance, flavour, as well as brix and titre values, and were packaged in 250 ml cans. Product identity was not revealed to the study investigators and patients until after completion of the study. Sample size was adapted to the normal serving size of beverages.
Analytical parameters
Antioxidative activity in both verum and placebo was determined as Trolox-equivalent antioxidant capacity (TEAC)(Reference Miller, Rice-Evans and Davies36), ferric-reducing ability of plasma (FRAP)(Reference Benzie and Strain37) and photochemiluminescence(Reference Popov and Lewin38). Furthermore, individual polyphenols were quantified by HPLC, and total polyphenols, expressed as GAE, were measured according to the Folin–Ciocalteu method in both the verum and placebo as well as the raw materials green tea, grape skin and seed extracts(Reference Singleton and Rossi39). Ascorbic acid analysis was carried out as previously reported(Reference Ross40).
Statistical analysis
All analyses were carried out in concordance with current good clinical practice guidelines and were given for all variables. Evaluation was performed using the t test and χ2 test according to the intention-to-treat principle. For each comparison, P < 0·05 was considered to be statistically significant.
Results
Study group characteristics
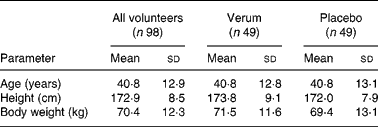
Since no case report forms were received for two participants, the patient number was reduced to ninety-eight patients. Therefore, the verum and placebo groups comprised forty-nine patients each. For both groups there was a uniform baseline in reference to age, height, body weight and temperature (Table 1). Moreover, two other patients, both in the placebo group, prematurely terminated the study. They did not appear for the second and third examination, and hence remained without final examination. Data from the patient missing only the final examination were included, whereas those from the other patient were not considered for analysis.
Table 1 Sociodemographic data of all volunteers, the verum group and placebo group
(Mean values and standard deviations)
Compliance control
All patients remained in the study for the required duration, returned the empty packaging as well as unused investigational product completely and filled out the diary carefully. The only exceptions were the two patients who did not appear for the second and third examination, and one further patient, who did not return the patient diary. All three patients were in the placebo group.
Safety evaluation
No significant changes for any safety parameters and clinical data neither in the verum nor in the placebo group were observed during the course of the study, except for body temperature in the verum group which showed a highly significant reduction (P = 0·003) but remained in the normal temperature range. In respect of adverse events, only one patient mentioned ‘sour burps’ and nausea immediately after taking the placebo product on the first day. The complaints occurred repeatedly for 2 d but were of mild intensity and did not lead to premature termination of the study. Global safety evaluation revealed ‘very good’ and ‘good’ tolerability for the verum and placebo drinks, respectively, as rated by the patients as well as the clinical investigators.
Composition of verum and placebo as well as total phenolic contents of raw material extracts
Table 2 shows the concentration of individual polyphenols, total phenols, and ascorbic acid content in the verum and placebo products. Furthermore, the results of antioxidative activity measurements are summarised. The total phenol content of green tea, grape skin and grape seed extract was 164 185 GAE mg/kg, 45 120 GAE mg/kg and 39 286 GAE mg/kg, respectively.
Table 2 Analytical parameters of placebo and verum beverages
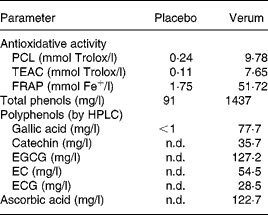
PCL, photochemiluminescence; TEAC, Trolox-equivalent antioxidant capacity; FRAP, ferric-reducing ability of plasma; n.d., not detectable; EGCG, epigallocatechine gallate; EC, epicatechin; ECG, epicatechin gallate.
Improvement of cold symptoms (primary efficacy criterion)
Total score was obtained by summarising the score of the five cold symptoms headache and/or joint pains, sore throat and/or difficulty swallowing, hoarseness and/or cough, and nasal congestion/sniffle, which were evaluated by the clinical investigator during the three examinations. The improvement is reflected in the reduction of the total score between the first and the second as well as the first and the third examination (Table 3), whereby the comparison of the mean group difference in the latter case revealed the primary efficacy criterion. At the beginning of the study, the mean total score was 10 points for both the verum and placebo group, displaying a homogeneous baseline situation. Already at the second examination a highly significant difference (P < 0·001) between the mean total score of both groups was observed, resulting in a reduction of the total score of 8·1 and 4·2 points in the verum and placebo group, respectively, at the end of the study (Table 4).
Table 3 Total score of cold symptoms during the course of the study
(Mean values and standard deviations)

* Verum group v. placebo group (t test).
Table 4 Decrease of total score of cold symptoms during the course of the study (primary efficacy criterion)
(Mean differences and standard deviations)

In addition to the evaluation of the individual symptoms by medical investigators, the documentation in the patient diaries allowed an exact record of the dynamics and the recognition of an early complaints-free condition (Fig. 1). Up to day 7 of the study the evaluation of the five cold symptoms was almost completely documented twice daily in the diary by ninety-five patients (96·8 %). Highly significant differences (P < 0·01) between the groups in favour of the verum group were already shown after 3 to 4 d, whereby the baseline situation for each cold symptom was not different in both groups. The sole exception was the highly significant improvement (P < 0·01) of the general feeling of sickness only on the fifth day. In summary, the improvement of cold symptoms was confirmed by the patients' self-rating.

Fig. 1 Evaluation of the five cold symptoms comparing verum (○ morning, ● evening) and placebo (△ morning, ▲ evening) according to the patient diary in points. (a) General feeling of sickness, (b) headache and/or joint paints, (c) throat complaints, (d) hoarseness and/or cough, (e) nasal congestion/sniffle.
Besides the documentation of the severity of cold symptoms, the medical investigators evaluated the existence of local findings. At the first examination all patients showed reddening of the Waldeyer's ring and/or the tonsils. By the end of the study a significant (P < 0·05) difference between the verum and the placebo groups was documented. While sixteen patients (32·7 %) receiving the verum drink were complaint free, only six patients (12·8 %) in the placebo group revealed no more reddening. Even a highly significant improvement (P < 0·01) could be observed concerning granulation and myxorrhoea on the back of the throat. Granulation on the back of the throat was only absent in six patients, three in each group, at the beginning of the study. Finally, forty (81·6 %) and twenty (42·6 %) patients in the verum and placebo group, respectively, exhibited no granulation. Comparable with the amelioration of granulation, forty-one patients of the verum group (83·7 %) and twenty-three patients of the placebo group (48·9 %) had no myxorrhoea on the back of the throat. At the beginning of the study, all participants revealed this finding with the exception of four patients in the verum group. Last, the percentage of patients without herpes labialis was significantly higher (P < 0·05) in the verum group (93 %) compared with in the placebo group (79 %).
Relief of complaints at the end of the study (secondary efficacy criterion)
The number of complaints-free individuals, determined by the physicians as well as by the patients themselves, was defined as the secondary efficacy criterion. At the third examination nineteen out of forty-nine patients (38·8 %) of the verum group and only four patients out of forty-seven (8·3 %) in the placebo group were without complaints (P < 0·001; χ2 test) (Table 5). This significant difference was even more obvious in the case of the patients' evaluation. Hence, 41·9 % of the patients taking verum reported to be complaint free contrary to 5·0 % of patients of the placebo group on the evening of study day 7. Again, the superiority of the verum compared with the placebo could be proven (verum group 41·9 % v. placebo group 5·0 %; P < 0·001; χ2 test).
Table 5 Relief of complaints at the end of the study (secondary efficacy criterion) (%)
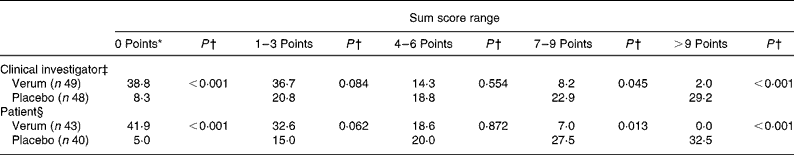
* 0 Points means free of complaints.
† Verum group v. placebo group (χ2 test).
‡ According to the evaluation on the third examination.
§ According to the patient diary on the evening of day 7.
Concurrent variables
Sleep disorders and disturbances of daily activities are frequently accompanying symptoms of colds. In the present study more than 60 and 70 % of the patients, respectively, suffered from these ailments. Starting on the third day of the study, a highly significant improvement (P < 0·01) in sleep disorders was recorded in the verum group, resulting in a reduction from 64·6 to 8·9 % of affected patients on study day 7 (Fig. 2). Similarly, a highly significant improvement (P < 0·01) regarding disturbance of daily activities was observed on the fourth study day. On day 7, a decrease from 87·5 to 9·1 % of affected patients was documented in the verum group. On the contrary, 18·2 and 60·5 % of patients treated with placebo still suffered from sleep disorders and disturbances of daily activities, respectively. Furthermore, the difference in average daily tissue use between both groups was highly significant (P < 0·01) on the seventh day. According to the patient diaries the utilisation in the verum group was only half of that in the placebo group.

Fig. 2 Change of the concurrent variables comparing verum (●) and placebo (▲) ‘sleep disturbance’ (a), ‘disturbance of daily activity’ (b), ‘additional concurrent medication’ (c) and ‘number of tissues used’ (d) during the study.
No significant difference was noted between both groups with reference to additional cold therapy. The application of additional cold therapy was reduced from 39·6 % at the beginning to 18·2 % by the seventh day in the verum group and from 34·1 to 28·6 % in the placebo group.
Global efficacy rating by the physician and patient
The physician rated the efficacy of the verum drink to treat symptoms of the common cold as ‘good’ or ‘very good’ in 42·9 and 34·7 %, respectively (Table 6). Similarly, 42·9 % of the patients assessed the effectiveness as ‘very good’ and 30·6 % as ‘good’. In contrast to the verum treatment, the efficacy in the placebo group was considered as ‘moderate’ (42·6 %) and ‘bad’ (31·9 %) by the investigators. Even 40·4 % of patients evaluated the effectiveness as ‘bad’. Thus, both investigator and patient ratings showed the superiority of the verum treatment in comparison with placebo.
Table 6 Global efficacy rating (%) by the physician and patient

Discussion
The present study clearly indicates that patients suffering from common cold symptoms benefit from the consumption of the investigated polyphenol-rich and shitake extract-containing beverage. Hence, patients receiving the verum drink experienced both a faster decline of symptoms and were complaint free sooner compared with those taking the placebo. Since evaluation of the improvement was established both by patients and clinical investigators, not only self-reported values, which are often considered as ‘soft’ criteria, but also several local findings of a physical examination were significantly improved. At the end of the study, a reduction of the total score of the five cold symptoms from 10·2 (sd 3·1) to 2·1 (sd 2·7) points in the verum group and from 10·5 (sd 3·0) to only 6·3 (sd 3·9) points in the placebo group was observed. Due to the fact that the investigated beverage did not improve some specific symptoms but rather improved virtually all symptoms and constraints, there is no reason to assume that it provided a specific therapeutic option. Therefore, it is much more likely that the polyphenol-rich drink stimulated and supported general defence and immunity. This assumption is further supported by the observation that subjects in the verum group showed a reduced susceptibility for herpes labialis. The percentage of subjects without herpes labialis (93·9 %) in the verum group was significantly higher compared with that in the placebo group (79·2 %) by the end of the study. Since herpes labialis symptoms reflect reactivation of existing viruses due to impaired immune defence it can be concluded that subjects in the verum group were less susceptible due to improved immune function.
This immune-modulating efficacy could be ascribed to the bioactive constituents of the investigated drink, particularly polyphenols from green tea and grapes. Thus, a positive influence of green tea catechins and l-theanin on the incidence and the duration of cold and flu symptoms were observed in a recent randomised, double-blind, placebo-controlled study involving 124 healthy participants. The results showed that treatment with green tea capsules reduced by about one-third the number of subjects with symptoms, and decreased the number of days that test individuals had symptoms as well as the number of patients requiring medication. Similarly, a significant improvement of cold symptoms in the verum group was already observed at the second examination (Table 4). Apart from these in vivo findings, the daily supplementation enhanced γδ T cell function ex vivo, i.e. increased cell proliferation and IFN-γ production, in response to antigen challenge. Taking into account that this subset of T cells plays a role as a first line of defence against infection, these data may explain the health benefits of green tea(Reference Rowe, Nantz and Bukowski41). Moreover, from several non-human studies it can be concluded that green tea compounds modulate the body's defence by inducing anti-inflammatory responses as well as by exerting antiviral and antibacterial effects(Reference Song and Seong13, Reference Clarke and Mullin16).
In contrast to these findings for green tea, human intervention trials reporting a direct immune-modulatory effect of grape polyphenols are still lacking. Until now, several studies have proven the ability of grape polyphenols to alter antioxidative capacity in vivo (Reference Whitehead, Robinson and Allaway22, Reference O'Byrne, Devaraj and Grundy24). For example, in a study including haemodialysis patients as well as healthy subjects, daily supplementation with 100 ml red grape juice, corresponding to 640 mg total phenols (GAE), for 2 weeks significantly increased the antioxidant capacity of plasma measured as Trolox-equivalent antioxidative capacity (TEAC) in both groups(Reference Castilla, Echarri and Dávalos25). Since this polyphenol intake was comparable with the amount of grape-derived polyphenols provided by the 500 ml verum, similar results regarding antioxidative protection could be expected. Furthermore, on the basis of recent findings demonstrating a protective effect of grape seed-derived proanthocyanidin on oxidative DNA damage in rat leucocytes, an indirect way of supporting immune functions as an antioxidant could be assumed(Reference Morin, Narbonne and Ribera42). Furthermore, various studies have demonstrated the health-promoting potential of anthocyanins including anti-inflammatory activity(Reference Ghosh43). Cyanidin 3-O-glucoside, also commonly found in grapes(Reference Kammerer, Claus and Carle44), inhibited cyclo-oxygenase enzymes COX-1 and COX-2 comparable with non-steroidal anti-inflammatory and analgesic drugs such as aspirin and ibuprofen, explaining the pronounced effect on headache and joint pains seen in the verum group (Fig. 1(b))(Reference Reddy, Alexander-Lindo and Nair45, Reference Adhikari, Francis and Schutzki46).
Regarding shiitake mushrooms and lentinan, clinical data investigating their immune-modulating activity after oral application are still lacking. So far, lentinan has been used intravenously, intraperitoneally or intramuscularly as an adjuvant in the treatment of diseases involving depressed immune function(Reference Wasser, Coates, Blackman, Cragg, Levine, Moss and White32). Only two studies examining the oral application of yeast-derived β-glucans are available. In a prospective clinical trial, the in vivo effects on peripheral blood monocytes and their expression of activation markers in twenty-three patients with advanced breast cancer were assessed. After 2 weeks of daily 20 mg 1-3,1-6 β-glucan application, the mean monocyte count as well as the expression of markers identifying activated monocytes were significantly increased(Reference Demir, Klein and Mandel-Molinas47). Further, the immunomodulating activity of a highly purified, soluble yeast β-glucan was studied in eighteen healthy participants assigned to three groups receiving 100, 200 or 400 mg β-glucan per d, respectively. The dosage format was a mouthwash, which was consequently swallowed. At the end of the study, a significant increase of IgA in the saliva of the group receiving the highest dose of β-glucan solution was determined. Since IgA is released into the mucus of the nose, mouth, throat, lung or intestine and serves as a first barrier towards pathogens, the authors stated that any increase is appealing with regard to an oral colonisation by micro-organsims such as Candida albicans (Reference Lehne, Haneberg and Gaustad48). Both studies have in common that higher concentrations of β-glucan compared with the amount of shiitake extract used in the present study were applied. Nevertheless, a higher concentration than 0·05 g/l was not recommendable due to sensory acceptance, and hence, an immunomodulative activity appeared improbable. However, for an unambiguous clarification of whether shiitake extract in the present concentration exhibit any immune-supportive activity or not, further studies are required.
Besides extracts from green tea, grape skin and seeds, as well as shiitake, the verum drink contained vitamin C, whose immunomodulatory effects, particularly by means of reduced susceptibility to infectious diseases and/or its efficacy on symptoms and duration of common colds, has been studied extensively(Reference Hemila49, Reference Moreira, Kekkonen and Delgado50). However, in general, the results give no evidence that vitamin C supplementation is useful in common cold therapy, whereas it may have some effects to prevent infection in specific groups of individuals, such as subjects with low dietary vitamin C intake or exposed to physical or physiological stress(Reference Hemilä, Chalker and Douglas51). Since the study population can be characterised as having impaired immune defence, similar to subjects eating an unbalanced diet or subjects exposed to physical stress, a pharmacological impact might be possible, even if the applied concentration of 61·4 mg vitamin C/500 ml was less compared with the dosages normally used in human studies. Last, only by means of a further study including a placebo containing vitamin C could a possible effect be excluded. In fact, the addition of vitamin C was present due to some technological reasons, to protect the polyphenols against oxidation, rather than for a therapeutic effect. As can be seen from Table 2, a reduction from initially 300 mg ascorbic acid/l to 122·7 mg/l occurred as consequence of oxidative processes during production. Further degradation of vitamin C as well as a change in polyphenol content during storage could be excluded through stability test over a time period of 9 months (data not shown).
In conclusion, the polyphenol-rich beverage exhibited a clear, positive impact on symptoms associated with the common cold through immunomodulative efficacy. Due to the exclusion criteria, the ailment might be considered as rather mild and the physiological functions of these patients had not been modulated by medication or severe organ or system disease. In fact, the study population can be characterised as having impaired immune defence, similar to subjects eating an unbalanced diet or exposed to physical stress, and thus, being sensitive for nutritional benefits. Therefore, the study population can be further considered as an appropriate target to demonstrate the clinical benefits of an immune stimulation and there is no reason to assume that the observed improvement cannot be extrapolated to the general population. Moreover, these benefits may be most obvious during periods of stress or illness. Hence, the results of the present study suggest that consumption of the investigated polyphenol-rich beverage strengthens the body's defence and improves immune function in the general population, which may translate into clinically measurable effects in individuals having impaired immune defence due to physical stress or already existing infections.
Acknowledgements
The authors thank Mrs Natalie Kernich for technical assistance.
The present study was funded by Rudolf Wild GmbH & Co. KG and carried out by analyze & realize ag.
Authors' contributions are acknowledged as follows: K. S. was the main writer of the manuscript; M. S. had the original idea, developed the test beverage and managed the study for Rudolf Wild GmbH & Co. KG; A. de W. proofread the manuscript; H.-J. G. and J. G. conducted the experimental work and performed the statistical analyses. All authors saw the final draft.
None of the authors has any conflicts of interest in the writing and publication of the present study.










