Poor food intake is common among older adults living in long-term care homes (LTC), yet our knowledge of actual food intake and adequacy with respect to Dietary Reference Intake (DRI) recommendations is limited( Reference Engelheart and Akner 1 – Reference Rakicioğlu, Aksoy and Tamer 3 ). Inadequate intake, including micronutrients, can lead to diverse functional changes that are detrimental to health and cognitive function( Reference Porter, Hoey and Hughes 4 , Reference Zhou, Huang and Liu 5 ). Although malnutrition in LTC has been well described( Reference Bell, Lee and Tamura 6 , Reference Volkert 7 ), micronutrient malnutrition is under-investigated. A first step towards improving micronutrient status is determining the micronutrient adequacy of the consumed diet. This evidence is necessary as the foundation for recommendations with respect to menu planning policies, raw food cost and adequate labour to produce nutrient-dense meals in LTC. Specifically, the distribution of resident nutrient intakes is required for menu planning at the home level to meet the DRI( 8 ) and prevent deficiencies.
Prior research on food intake is based on small, convenience samples( Reference Mila, Abellana and Padro 9 – Reference Suominem, Laine and Routasalo 15 ) with relatively inaccurate methods for collecting intake, including relying on staff to record consumption( Reference Engelheart and Akner 1 , Reference Aghdassi, McArthur and Liu 2 , Reference Nowson, Sherwin and McPhee 13 , Reference Rumbak, Satalić and Keser 16 ). Data on nutrient intake is not typically adjusted for intra-individual variation( Reference Engelheart and Akner 1 , Reference Aghdassi, McArthur and Liu 2 , Reference Nowson, Sherwin and McPhee 13 ) nor is it based on a comprehensive panel of micronutrients( Reference Mila, Abellana and Padro 9 , Reference Volkert, Pauly and Stehle 11 , Reference Grieger and Nowson 14 , Reference Strathmann, Lesser and Bai-Habelski 17 , Reference Lopez-Conteras, Zamora-Portero and Lopez 18 ). Further, studies have yet to determine if current micronutrient supplement use mitigates an inadequate diet( Reference Engelheart and Akner 1 – Reference Rakicioğlu, Aksoy and Tamer 3 ) or if differences exist in nutrient consumption because of the requirement for a texture modified diet, despite the known increased vulnerability of these residents( Reference Adolphe, Whiting and Dahl 19 – Reference Bannerman and McDermott 21 ). The Making the Most of Mealtimes (M3) prevalence study was designed to overcome these challenges and provide a better assessment of dietary intake in LTC. The purpose of this study was to determine the prevalence of inadequate micronutrient intakes consumed by LTC residents, comparing adequacy by diet texture and use of micronutrient supplements (e.g. pills).
Methods
Sampling, subjects and recruitment
Four provinces in Canada (Alberta, Manitoba, Ontario, New Brunswick) were included in the M3 study; eight LTC homes were purposively selected within each province to provide diversity in profit status, size, and cultural distinctiveness (e.g. predominately Jewish residents with Kosher meals). To be eligible for inclusion in this cross-sectional study, LTC had to have at least fifty residents who required more than 2 h of nursing care per day for basic activities of daily living, be in operation for more than 6 months and be supportive of the M3 data collection procedures. Within each LTC, two to four units were randomly selected for inclusion and if available, a unit dedicated to the care of residents with dementia was included. From these units, eligible residents were identified by trained home staff, that then used a random number list to approach residents who were interested in hearing more about the study. Up to forty interested, eligible and randomly identified residents were provided as a list to researchers who then completed informed written consent until a quota of twenty residents per home was reached. Inclusion criteria were as follows: medically stable (not recent or impending hospitalisation, not palliative), typically ate in the dining room, consumed food orally (residents with artificial feeding were excluded), were 65 years of age and older, and they or their alternative decision maker (ADM) provided informed consent to participate. Further details about the LTC, the protocol for recruitment and sample representativeness have been previously documented( Reference Keller, Carrier and Slaughter 22 ). Sample size estimation needed to take into account the provincial and home clusters and the intra-class correlation (ICC) of food intake among residents within the same facility (e.g. residents in the same home are more likely to have similar intakes)( Reference Keller, Carrier and Slaughter 22 ). To estimate this ICC, previously published data on energy intake data from two Canadian studies involving several homes was attained and the ICC were calculated (mean 6322 kJ (1511 kcal), ICC 0·17; mean 4937 kJ (1180 kcal) ICC 0·04)( Reference Keller, Carrier and Slaughter 22 ). Using this range of estimated ICC, twenty residents in each of the thirty-two homes in the M3 sample provided sufficient statistical precision to estimate median energy intake with a 95 % CI of ±234–284 kJ (56–68 kcal). Thus, twenty residents per home was considered a sufficient sample to estimate micronutrient intake in the M3 study.
Data collection
Only data collection pertinent to this analysis will be described; details of all procedures can be found in the protocol( Reference Keller, Carrier and Slaughter 22 ). All food and beverage intake for 3 non-consecutive days (2 weekdays and 1 weekend day), at meals and between meals was recorded by trained research staff (two per province). In-person training was completed with each provincial coordinator who subsequently trained research assistants( Reference Keller, Carrier and Slaughter 22 ). Food weigh scales (V22PWE3T; Ohaus) were identical among provinces and calibration was completed daily during data collection. Main plate food products were weighed before and waste weighed after consumption; any additional servings and condiments provided by staff were also recorded. Consumption of side dishes, snacks and beverage intake was estimated and before collection in each home, dishware was measured to determine volume. Use of salt at the table was not estimated. Morning and afternoon snack consumption was reported to researchers by residents, family, or staff and the evening snack and any other food consumed until midnight was recorded by staff. The complete menus with portion sizes and home recipes were provided to the researchers for nutrient analysis using the ESHA Food Processor Nutrition analysis software version 10.14.1 (ESHA Research). The Canadian Nutrient File was also used where appropriate for fortified and other foods specific to the Canadian food supply. Food and beverage intakes were entered into ESHA after each meal for each participant to ensure a thorough and accurate reporting and analysis; if required, LTC staff was asked to clarify ingredients or any substitutions that may have occurred. Where recipes were not available, researchers followed a standardised protocol to identify potential substitutes (e.g. other recipe from a home in the same province; recipe for a similar product from another province; or generic recipe from the internet based on product ingredients). Nutrient profiles of commercial products used were also accessed. Research assistants crosschecked data entry for accuracy and data were reviewed centrally to confirm completeness.
Before each meal, researchers also asked licensed nursing staff dispensing medications if prescribed oral nutritional supplements (ONS; e.g. commercial high protein/energy milkshakes) were provided and taken with medications; amount and type of ONS was recorded. Use of these nutritional supplements was included in the food/beverage estimation of nutrient intake. Micronutrient supplement (e.g. pill) use on the day of data collection was confirmed, with brands and type/dose of nutrient recorded from the medication record. Only values from formulations that could be confirmed with confidence were used in this analysis (10·8 % not included: injectable nutrients, multivitamins where brand was not specified or any nutrient pill where dose was not recorded and/or could not reasonably be inferred (e.g. when unit was clearly recorded incorrectly)).
The medical record was also reviewed for each participant to determine their sex, birthdate, most current body weight, diet prescriptions (e.g. high-fibre diet, diabetic diet), prescribed diet texture (categorised using International Dysphagia Diet Standardisation Initiative (IDDSI) framework( Reference Cichero, Steele and Duivestein 23 ) based on description in medical record and confirmation at meals as: regular, soft and bite sized, minced and moist, pureed, liquidised), diagnoses (total number and any dementia diagnosis), and use of prescription medication (total number). An ulna measurement( Reference Finch and Arumugam 24 ) was taken by the provincial project coordinator and used to estimate BMI. This coordinator also interviewed staff that worked with residents to complete key aspects from the LTC InterRAI, and specifically for this analysis the Cognitive Performance Scale (CPS) and the Activities of Daily Living scale (ADL). The CPS was used to describe resident cognitive status. It is a seven-point scale, where 0 represents no cognitive impairment and 6 represents very severe cognitive impairment with the person unable to make daily decisions, make themselves understood or eat independently( Reference Morris, Fries and Mehr 25 ). The ADL score has a maximum of 28 and higher scores indicate increased dependency for various activities of daily living( Reference Morris, Fires and Morris 26 ). Finally, the Edinburgh Feeding Evaluation in Dementia Questionnaire (Ed-FED-Q), a valid measure of eating challenges at mealtimes, was collected by observation at three meals. Scores range from 10 to 30 and the average across the three observed meals was calculated for each resident, where higher scores indicate more eating challenges( Reference Watson and Deary 27 ).
Statistical analyses
Only participants whose food intake was observed over at least six mealtimes (equivalent to 2 d) were used in this analysis (n 632; 519 (82·1 %) had all nine meals, 98 (15·5 %) had seven to eight meals, and 15 (2·4 %) had six meals). Participant characteristics were summarised (mean values and standard deviations, frequency) and compared by sex group and regular and modified textures using Student’s t test, or the χ 2 test. A P value for statistical significance was set at <0·01 due to the number of comparisons completed (Table 1; test statistics and P values provided in text).
Table 1 Making the Most of Mealtimes sample description and comparisons by sex and food texture (Mean values and standard deviations; percentages; n 632)
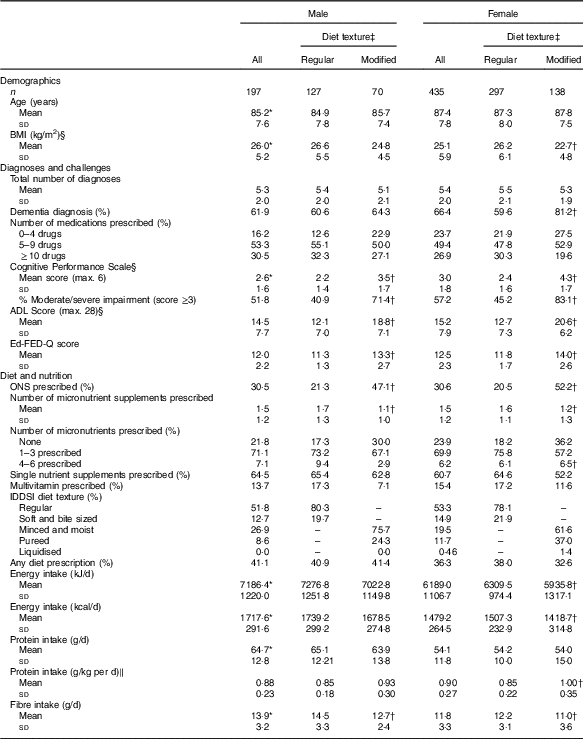
ADL, activities of daily living; CPS, Cognitive Performance Scale; Ed-FED-Q, Edinburgh Feeding Evaluation in Dementia Questionnaire; ONS, oral nutritional supplement; IDDSI, International Dysphagia Diet Standardisation Initiative.
Values are significantly different between males and females: * P<0·01. Values are significantly different between modified and regular texture categories, within sex group: † P<0·01.
‡ Regular textures include ‘regular’ and ‘soft and bite sized’ (IDDSI categories 7 and 6); modified textures include ‘minced and moist’, ‘pureed’ and ‘liquidised’ (IDDSI 5, 4 and 3).
§ Missing values: ADL Score, n 627; BMI, n 620; CPS score, n 627.
‖ kg of body weight.
Food and fluid intake data were stratified by sex and diet texture for comparison with the DRI. Modified texture for this analysis was defined as those consuming a ‘liquidised’, ‘pureed’ or ‘minced and moist’ texture (IDDSI 3–5), whereas the regular category included ‘soft and bite sized’ (IDDSI 6) and ‘regular’ (IDDSI 7) consumers. To enable comparison of nutrient intakes to the DRI( 28 ) usual intake of nutrients was estimated by adjusting 3-d average intakes for intra-individual variation according to the method outlined by the National Research Council( 28 , 29 ). The first comparison to the DRI was based on all food, beverage and ONS consumed. A second comparison to the DRI was completed, with micronutrients from micronutrient pills added to the adjusted food/beverage/ONS intake values for those participants consuming these preparations. Percentiles of the distribution of intake were determined for each sex and for regular and modified texture diet groups to determine prevalence of inadequacy. Median, quartile 1 (Q1) and quartile 3 (Q3) values are reported. The distribution of nutrient intake from food/beverage/ONS was used to determine the proportion of participants with inadequate intake for those nutrients with an Estimated Average Requirement (EAR) using the EAR cut-point method( 28 ). For nutrients with an Adequate Intake (AI; vitamin K, K, Na), the proportion of participants falling below the AI with respect to their intake is reported. The number of participants, whose intake surpassed the EAR/AI with micronutrient supplements, is also reported. Median intake and proportion below the EAR/AI were compared for modified texture and regular texture consumers using Wilcoxon Mann Whitney and chi-square tests. All analyses were performed using SAS® software package version 9.4 (SAS Institute).
Ethics
All study participants or alternative decision makers for residents provided their written consent to participate. This protocol received clearance from ethics boards at the University of Waterloo, University of Alberta (Pro00050002), University of Manitoba (J2014:139), Université de Moncton (1415-022) and University Hospital Network, University of Toronto (16-5051-DE). Some individual LTC homes also required ethics review by a local/regional committee.
Results
Participant characteristics are provided in Table 1. Modified texture consumers were 33 % (208/632) of the sample. Female participants consuming modified texture foods had a lower BMI than women consuming regular texture foods and women had lower average BMI than men (women’s mean: 22·7 (sd 4·8) kg/m2; male mean: 26·2 (sd 6) kg/m2·12; t=6·38, P<0·001). In females, protein intake in g/kg body weight was significantly higher and energy intake significantly lower for those consuming a modified texture as compared with those consuming a regular texture (energy: t=2·95, P=0·004; protein: t=−4·39, P<0·001). The proportion of participants with dementia was significantly higher for females consuming a modified texture (81·2 %) as compared with females consuming regular texture foods (59·6 %; χ 2=19·65, P<0·001; OR 2·92; 95 % CI 1·8, 4·75). CPS scores were higher (greater cognitive impairment) across both sexes for those consuming modified texture foods as compared with those consuming regular texture foods (male: t=−5·53, P<0·001; female: t=−11·26, P<0·001). Similarly, higher ADL (more dependency) and Ed-FED-Q (more eating challenges) were seen in those consuming modified texture foods as compared with those consuming regular texture foods for both sexes. Those consuming modified textures for both sex groups were more likely to receive ONS than those consuming regular textures (male: χ 2=14·27, P<0·001; OR 3·3; 95 % CI 1·75, 6·22; female: χ 2=44·42, P<0·001; OR 4·22; 95 % CI 2·73, 6·53). Micronutrient supplement use was significantly different across texture comparisons for both sexes with those consuming regular textures being prescribed more vitamin/minerals than those consuming modified textures (male: t=3·71, P<0·001; female: t=3·20, P=0·002). The online Supplementary Table S1 provides a summary of the proportion of residents taking various micronutrient supplements and the dosage provided. About three-quarters of participants were consuming at least one formulation, with single nutrients being the most common including: vitamin D, vitamin B12, Ca, Fe and vitamin C.
Tables 2 and 3 provide median (Q1, Q3) micronutrient intakes from food/beverage/ONS intake for males and females, respectively, for those consuming regular or modified texture diets. For males (Table 2), median values for the following nutrients were significantly lower for modified textures v. regular texture participants: folate, Fe, Se and Na. Vitamin C, D, Ca and Zn were consumed in higher amounts for modified texture males as compared with regular texture consumers. High proportions of male residents had intakes from food/beverages/ONS below the EAR/AI for regular and modified textures for: vitamin B6, folate, vitamins D, E, K, Mg, K and Zn. Vitamin A and C (for regular texture only) were consumed at levels below the EAR by >25 % of males. A significantly higher proportion of males on modified texture foods were below the EAR than regular texture consumers for folate. But a significantly higher proportion of males receiving regular texture foods were below the EAR as compared with modified texture consumers for vitamins C, D, Ca and Zn. Intake increased to above the EAR for vitamin D (regular n 83/127; modified texture n 38/70) and Ca (regular texture n 23/127) with micronutrient supplement use.
Table 2 Nutrient analysis of food intake only and food intake with micronutrient supplements added for males on regular (n 127) and modified (n 70) texture diets
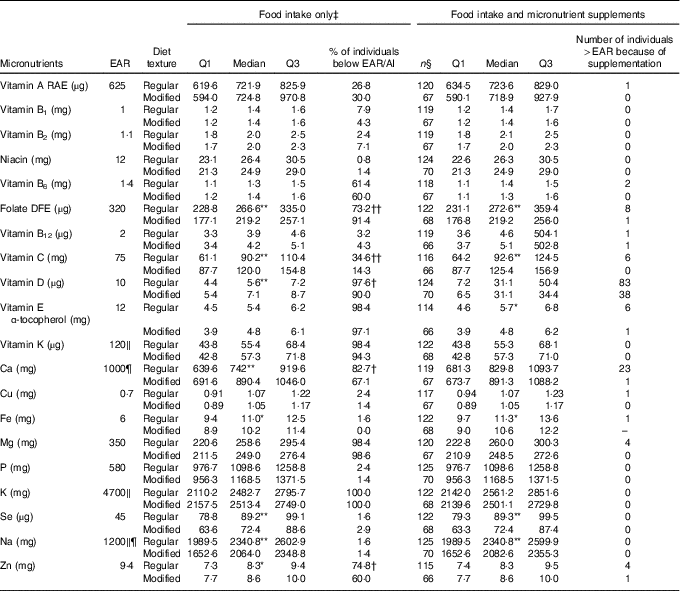
EAR, Estimated Average Requirement; Q, quartiles; AI, Adequate Intake; RAE, Retinol Activity Equivalent; DFE, Dietary Folate Equivalent; DRI, Dietary Reference Intake.
Median of nutrient intake is significantly different between regular and modified texture diets: * P<0·05, ** P<0·01. Proportion of individuals below the EAR/AI is significantly different between regular and modified texture diets: † P<0·05, †† P<0·01.
‡ Food intake only includes food and fluids from meals and snacks, and oral nutritional supplements.
§ Represents sample size with known supplement use where detail on dose was available.
‖ EAR unavailable, value is the AI for specified nutrient.
¶ DRI for males 51–70 years is different from >70 years; Ca, EAR=800 mg; Na, AI=1300 mg.
Table 3 Nutrient analysis of food intake only and food intake with micronutrient supplements added for females on regular (n 297) and modified (n 138) texture diets
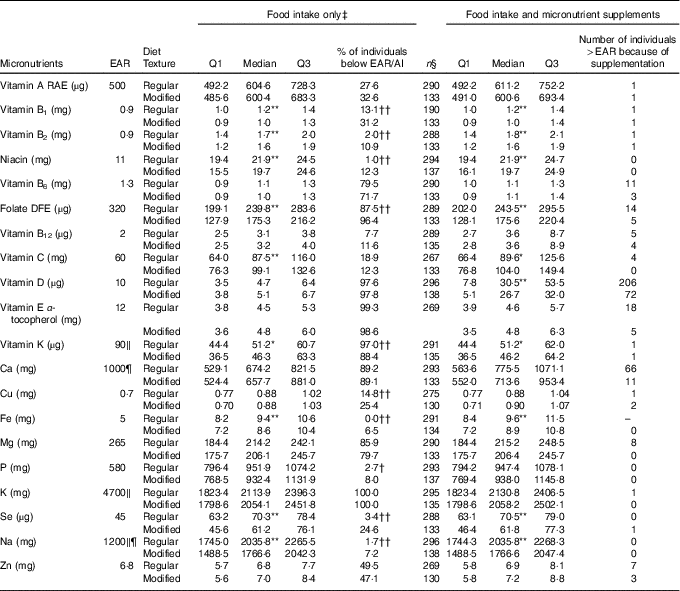
EAR, Estimated Average Requirement; Q, quartiles; AI, Adequate Intake; RAE, Retinol Activity Equivalent; DFE, Dietary Folate Equivalent; DRI, Dietary Reference Intake.
Distribution of nutrient intake is significantly different between regular and modified texture diets: * P<0·05, ** P<0·01. Proportion of individuals below the EAR/AI is significantly different between regular and modified texture diets: † P<0·05, †† P<0·01.
‡ Food intake only includes food and fluids from meals and snacks, and oral nutritional supplements.
§ Represents sample size with known supplement use where detail on dose was available.
‖ EAR unavailable, value is the AI for specified nutrient.
¶ DRI for females 51–70 years is different from >70 years; Na, AI=1300 mg.
For females (Table 3), modified texture consumers had significantly lower intakes as compared with regular texture consumers for the following nutrients: vitamin B1, B2, niacin, folate, vitamin K, Fe, Se and Na. Vitamin C intake was significantly higher for modified texture female consumers as compared with regular texture consumers. High proportions of female residents had intakes from food/beverages/ONS below the EAR/AI for regular and modified textures for: vitamin B6, folate, vitamins D, E, K, Ca, Mg and K. Vitamin A and Zn intakes were below the EAR for more than 25 % of female participants for both textures. Vitamin B1, Cu and Se intakes were below the EAR for >25 % of modified texture consumers only. Significantly higher proportions of females on modified texture diets were below the EAR/AI than regular texture consumers for vitamins B1, B2, niacin, folate, Cu, Fe, P, Se and Na. Females consuming a regular texture had a higher proportion below the AI as compared with modified texture consumers for vitamin K. Intake increased to above the EAR for vitamin D (regular n 206/297; modified texture n 72/138) and Ca (regular n 66/297; modified texture n 11/138) with micronutrient supplement use.
Discussion
The M3 study provided an opportunity to examine adequacy of nutrient intake in LTC residents and for the first time, how this is affected by food texture modifications and use of vitamin/mineral supplements. Poor food intake in LTC is well known, but until now, rigorous evidence to demonstrate the extent of nutrient inadequacy has been limited. Differences between consumption of modified and regular textures were identified; males had fewer differences and four nutrients higher in modified texture consumers, whereas females had several nutrients (8/20) where modified texture consumers ate significantly less than regular texture participants. Similarly, there were more statistically significant differences in the proportion below the EAR/AI for modified texture consumers than regular food consumers for females, with modified textures having higher proportions below the EAR/AI for all but one nutrient. Males had few differences, with modified texture consumers having lower proportions below the EAR/AI for four nutrients, as compared with regular texture food consumers. This difference by sex and texture can be largely explained by the significantly lower energy intake of females consuming a modified texture diet as compared with regular texture female participants. Several nutrients were inadequate (<EAR) or below the AI for both textures for at least 50 % of the participants for both sexes: vitamins D, E, K, B6, folate, Ca, Mg and K. Of greatest concern were nutrients where 90 % consumed less than the EAR/AI: vitamin D, E, K, Mg (males only) and K. Vitamin D micronutrient supplement use improved inadequate intakes for 50–70 % of residents and Ca supplementation improved intake for approximately 20 % of regular texture consumers, but other micronutrient inadequacies were not resolved with micronutrient formulations, due to their infrequent use (online Supplementary Table S1).
A Canadian study (n 48) conducted in one province compared 3 d of food record intake of residents in five LTC homes to the EAR( Reference Lengyel, Whiting and Zello 30 ). Proportions of males with intakes below the EAR/AI were very similar to the current study with a few exceptions; fewer men in this smaller study were at risk for inadequate intakes of vitamin A, E and Mg, whereas more were at risk for folate, B1, B2 and Zn when compared with the M3 sample. Females in this smaller study had the same or higher proportions below the EAR/AI as compared with the M3 sample, potentially due to the low energy intake of these residents. Noted differences may also be due to more complete nutrient databases used in the M3 study, including foods fortified with folate( Reference Lengyel, Whiting and Zello 30 ), improvements in menu planning since 2008 and a larger, more diverse sample, where modified textures were analysed separately. In a single Swedish nursing home study (n 52)( Reference Lammes and Akner 10 ), although the EAR cut-point was not used, the authors note that mean intakes for vitamins D, E, folate and Se were <60 % of Swedish recommendations at baseline before an intervention, which is in line with the M3 findings on inadequacy for these nutrients. Further, absolute intake values for nine nutrients were consistent with the current study; however, intakes of vitamin A, B12, Na and Ca (females only) were higher in this Swedish sample, whereas mean intakes of vitamin D, E (males only), C, folate (as folic acid), Fe and Se were lower than in the M3 sample( Reference Lammes and Akner 10 ). Finally, a Finnish study that collected 3 d of weighed food intake data in twenty-three female residents from the same home identified that food intake generally met recommendations, with vitamin D, E and folic acid the only nutrients potentially inadequate, when compared with the Finnish requirements( Reference Suominem, Laine and Routasalo 15 ). As energy intake was on average lower (5042 kJ (1205 kcal)) than the M3 sample, differences are not due to increased intake overall, but may be due to the use of the Finnish requirements which were lower than the DRI for several nutrients. Actual values of nutrients consumed were not provided to make direct comparisons to the M3 sample. Differences in these international studies may also be due to use of different nutrient databases, which are known to vary in quality( Reference Finglas, Berrry and Stley 31 ) as well as differences in food supply, micronutrient content of the soil (e.g. Se), fortification practices and menu planning strategies, including use of nutrient-dense foods. As all of these smaller studies are almost a decade old, M3 provides updated data on the intake of residents in LTC.
A prior analysis of menus from the M3 study homes, comparing regular and pureed texture menus( Reference Vucea, Keller and Morrison 32 ) identified that several nutrients were provided at levels below DRI recommendations for both texture groups (vitamins B6, vitamin D, vitamin E, vitamin K, folate, Ca, Mg, K and Zn). Thus, it is not surprising that intake of participants was largely inadequate for many of these nutrients in this study. However, this analysis of M3 data identified that some nutrients were consumed at higher levels for modified texture consumers as compared with regular texture consumers. Although this may seem counterintuitive, it may be explained in two ways. First, as demonstrated in this analysis, modified texture consumers were more likely to consume ONS, which provides not only energy and protein, but also micronutrients. Second, the analysis of menus( Reference Vucea, Keller and Morrison 32 ) found that pureed menus generally provided lower amounts of nutrients than regular menus, except for vitamin D, Ca and K. This was attributed to use of pureed recipes that were enhanced with milk, skimmed milk powder or cheese to promote nutrient density( Reference Vucea, Keller and Morrison 32 ), explaining the intake differences noted in this analysis. Higher vitamin C levels for modified textures may be due to the standard of care in some provinces that ensures that final portion size for modified textures are the same as the regular texture (e.g. ½ cup of peas)( Reference Vucea, Keller and Morrison 32 ); more product needs to be pureed (e.g. ¾ cup) to result in the same final portion size as the regular texture. Well planned menus that meet the recommended daily allowance are essential to promoting AI of residents in LTC( Reference Lam, Keller and Duizer 33 ). The lower intake for some nutrients by modified texture consumers in this analysis can also be attributed to eating challenges and greater disability and cognitive impairment (Table 1). Even if a menu met the DRI, these challenges would still result in potentially inadequate diets. A prior M3 analysis demonstrated that eating challenges and assistance are important predictors of energy and protein intake when site, staff, dining room and resident-level factors are considered( Reference Keller, Carrier and Slaughter 34 ). Person-centred care practices, being on a dementia-focused unit, malnutrition and dietitian involvement were also predictive of intake( Reference Keller, Carrier and Slaughter 34 ). Other research has also noted the multifactorial nature of poor food intake in LTC( Reference Bell, Lee and Tamura 6 , Reference Iuliano, Olden and Woods 35 ) and thus the need for multifactorial strategies beyond quality and nutrient density of the food provided.
Micronutrients consumed at levels below the EAR may not advance to micronutrient deficiency and the implications of low intake in this population are unclear. Deficiency of a nutrient is dependent on absorption and utilisation, disease states and interaction with medications, and for vitamin D, exposure to the sun( Reference Ter Borg, Verlaan and Hemsworth 36 ). Deficiency is best identified through functional changes due to inadequate intake or utilisation of a nutrient, especially as some nutrients have insufficient biomarkers( Reference Ter Borg, Verlaan and Hemsworth 36 ). Further, the DRI were designed for healthy individuals to prevent deficiency or diseases associated with deficiency( 8 , 28 ). For this population of typically frail older adults with various disease conditions, the DRI may not apply. Interventions that demonstrate improved health and functional measures with increased nutrient intake are needed to further understand the implications of low intake in this population.
In addition to use of ONS, there are typically three ways in which nutrient intake can be improved: nutrient-dense food, micronutrient supplement use, or fortification of the nutrient in staple foods. Prior work suggests that use of nutrient-dense ingredients can increase micronutrient content of LTC menus( Reference Lam, Keller and Duizer 33 ), meaning that iatrogenic micronutrient malnutrition due to inadequate intake is not inevitable for most nutrients in this population. In addition to careful menu planning, recipes can be further enhanced with nutrient-dense ingredients to promote intake. For example, energy and protein enhancements have been documented to improve intake( Reference Castellanos, Marra and Johnson 37 , Reference Abbott, Whear and Thompson-Coon 38 ). Micronutrient supplement use for most nutrients occurred in <15 % of participants in this study, excepting for vitamin D, Ca and B12. With two-thirds to three-quarters of the sample being prescribed vitamin D, it is apparent that guidelines( Reference Papaioannou, Santesso and Morin 39 ) for supplementing this nutrient for LTC residents are resulting in practice change. Vitamin D inadequacy was resolved for a good portion of participants because of this provision of micronutrient pills, whereas relatively few residents consumed B12 at levels less than the EAR without vitamin pill use. Micronutrient supplements may be underutilised in LTC due to concerns for toxicity( Reference Andrews, Roseland and Gusev 40 ); residents bearing the cost of these formulations as they are not covered by drug benefit programs; persons with dysphagia having challenges with pill consumption; and increased burden placed on care staff during medication administration. Injectable forms of nutrients provided on a monthly or less frequent basis may be a strategy to overcome some of these challenges. Fortification of selected nutrients like vitamin D and Ca in key food products has been demonstrated to be effective ( Reference Lam, Pfisterer and Duizer 41 ). However, there are several potential barriers to fortification of food in LTC( Reference Lam, Keller and Duizer 42 , Reference Field, Duncan and Keller 43 ).
Limitations
Sites were purposively selected and cannot be considered representative of all homes in the four provinces included in the M3 study. Nutrient analysis of food intake is complex and fraught with challenges( Reference Church 44 , Reference Church 45 ) and despite the use of rigorous methods, there are still limitations to this work. To promote efficiency, only estimation was required of side dishes and beverages as well as snacks; the evening snack was noted by LTC staff and the reporting was less accurate. Not all LTC had detailed recipes for modified texture products and some commercial products did not have a full nutrient analysis for all micronutrients considered. Also, LTC staff may not have followed standardised recipes. Nutrient analysis is only as accurate as the database used for foods and ingredients, and errors can occur when converting units and household measures to weights, and assigning weight change factors of raw ingredients due to cooking the food( Reference Church 44 , Reference Church 45 ). Further, databases are known to be inaccurate for some nutrients( Reference Luby, Vernon and Maeda 46 ). Finally, micronutrient adjustments for supplement usage may be inaccurate due to poor recording of type of formulation and dosage, as well as known overages and shortages in these products( Reference Andrews, Roseland and Gusev 40 ). The exclusion of approximately 10 % of vitamin/mineral use as it was injectable or because dose was unavailable is another limitation of this work.
Future work
This analysis is consistent with prior research that has identified that some but not all nutrients are consumed below recommendations by residents living in LTC. Future work should examine if products that are enhanced with use of nutrient-dense ingredients are appealing to LTC consumers and can promote micronutrient intake sufficiently to minimise the proportion of residents with intakes below the EAR/AI. Further, research demonstrating that adequate consumption of micronutrients delays progression or improves conditions linked to key nutrients (e.g. B vitamins and neurological problems), is also needed. This research will also help to fully understand the implications of inadequate intake in LTC residents.
Conclusions
This analysis of the M3 data from 632 residents living in thirty-two LTC using weighed and estimated food records for 3 non-consecutive days, demonstrated that 9/20 micronutrients examined were consumed in amounts below recommendations by a high proportion of participants regardless of sex or texture. Except for vitamin D, micronutrient supplements failed to improve this inadequacy. Consumers of modified texture foods (minced and moist, pureed or liquidised) had lower median intake for some (males=4; females=8) nutrients, potentially due to eating challenges and low energy intake (for women), as compared with regular food texture consumers. Interventions to target micronutrients that are consumed below recommendations, especially those that are low for almost all of the sample (vitamin D, E, K, Mg and K), are required.
Acknowledgements
The authors thank the research assistants, provincial coordinators, dental hygienists and project managers for their significant contributions to the M3 project. The authors would also like to express the gratitude to the long-term care homes, staff, residents, and families who participated in the M3 study.
The M3 project was funded by the Canadian Institutes of Health Research (Operating Grant 2014 no. 326892). Funders had no involvement in the design, analysis or writing of this article.
The primary author, H. H. K. is the primary investigator for M3 and developed the first draft of the manuscript. J. M. conducted all data analyses and provided tables of results. Co-authors are co-investigators on the M3 project and reviewed and provided input for this manuscript based on their specific research expertise.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518000107






