Introduction
Puberty can be defined as the first ovulation and/or first oestrous behaviour in females and the first mount and/or ejaculation with the release of sperm in males. Puberty is influenced by several environmental factors (Foster, Reference Foster1994). There is an interbreed difference in the onset of sexual activity in does and bucks that are well fed. In does of the Shiba breed and in local female goats from the Caribbean Guadeloupe Island, puberty starts from 5.6 to 6.7 months of age, whereas the Saanen does attain puberty at 7.8 months of age (Amoah and Bryant, Reference Amoah and Bryant1984; Chemineau, Reference Chemineau1993; Sakurai et al., Reference Sakurai, Ohkura, Matsuyama, Katoh, Obara and Okamura2004). Tokara and Damascus bucks attain puberty at approximately 4 and 17 months of age, respectively (Elwishy and Elsawaf, Reference Elwishy and Elsawaf1971; Nishimura et al., Reference Nishimura, Okano, Yasukouchi, Gotoh, Tabata and Iwamoto2000). In goats and sheep breeds displaying seasonal reproduction, the onset of puberty in does occur only during the breeding season (Ricordeau et al., Reference Ricordeau, Bouillon, Gaillard, Lajous and Lajous1984; Papachristoforou et al., Reference Papachristoforou, Koumas and Photiou2000). The onset of puberty is influenced by the month of birth and also by photoperiodic changes (Greyling and Van Niekerk, Reference Greyling and Van Niekerk1990; Foster, Reference Foster1994). Spring-born Suffolk ewes and Alpine does attain puberty at approximately 30 weeks of age, but autumn-born females attain puberty at approximately 1 year of age (Ricordeau et al., Reference Ricordeau, Bouillon, Gaillard, Lajous and Lajous1984; Foster, Reference Foster1994). In contrast, in lambs of seasonal breeds, the season of birth does not appear significantly to affect the onset of puberty (Wood et al., Reference Wood, Ebling, I’Anson and Foster1991; Herbosa et al., Reference Herbosa, Wood and Foster1995). Testicular growth, an index of spermatogenesis, was not modified in Suffolk rams subjected to an increasing or decreasing photoperiod from birth (Delgadillo et al., Reference Delgadillo, Hochereau-de Reviers, Daveau and Chemineau1995; Herbosa et al., Reference Herbosa, Wood and Foster1995). In spring-born gonadectomised oestradiol-treated rams raised in natural-simulated photoperiod or reversed-simulated photoperiod, the rise in the level of pubertal LH was similar in both groups (Herbosa et al., Reference Herbosa, Wood and Foster1995) or delayed only for approximately 3 weeks in reverse natural-simulated photoperiod (Wood et al., Reference Wood, Ebling, I’Anson and Foster1991). These results strongly suggest that female sheep and goats of seasonal breeds appear to be more affected by the season of birth compared with males. The local female and male goats from subtropical Mexico display marked seasonal variations in their sexual activity. The breeding season of females lasts from September to March, whereas the sexual activity of males lasts from May to December (Delgadillo et al., Reference Delgadillo, Canedo, Chemineau, Guillaume and Malpaux1999; Delgadillo-Sánchez et al., Reference Delgadillo-Sánchez, Flores-Cabrera, Véliz-Deras, Duarte-Moreno, Vielma-Sifuentes, Poindron-Massot and Malpaux2003). Therefore, this study was carried out to determine the effect of the season on puberty in female and male goats from subtropical Mexico.
Material and methods
Study site and management of experimental groups
This study was performed in Torreón, State of Coahuila, Mexico (latitude 26°23′N; longitude 104°47′W). Photoperiod in this region varies from 13 h 41 min of light at summer solstice to 11 h 19 min of light at winter solstice. Three groups of local female and male goats born in different months of the year were used. In the first groups, mean (±s.e.) birth dates of females (n = 9) and males (n = 12) were January 3 ± 0.5 days and 5 ± 0.4 days, respectively. In the second groups, females (n = 18) were born in May 25 ± 1.9 days and males (n = 20) in May 29 ± 1.7 days. In the third groups, females (n = 10) and males (n = 10) were born in October 30 ± 0.8 days and 28 ± 1.3 days, respectively. In each season, animals were weaned at the age of 30 days and fed ad libitum with alfalfa hay and 100 g of a commercial concentrate containing 14% of crude protein, 10% of fibre, 2.5 Mkcal/kg and 62% of total digestible nutrients. Animals had free access to mineral blocks and water. Female and males were separated and allocated in shaded open pens from weaning to puberty.
Measurements of reproductive activity and body and testicular weights
Does
In all the three experimental groups, live weight was determined every 2 weeks from birth until the onset of puberty. The determination of the onset of ovarian activity commenced from 3 months of age. This determination was carried out once in a week by measurement of the progesterone plasma concentration (Terqui and Thimonier, Reference Terqui and Thimonier1974). Progesterone levels greater than 1 ng/ml in two consecutive samples were considered as indicative of luteal activity, which in turn indicates the onset of puberty.
Bucks
In the three experimental groups, live weight was determined every 2 weeks from birth until the onset of puberty. The testicular weight, an index of spermatogenetic activity (Delgadillo et al., Reference Delgadillo, Hochereau-de Reviers, Daveau and Chemineau1995), was determined every 2 weeks from birth to the end of the study by comparative palpation with an orchidometer (Oldham et al., Reference Oldham, Adams, Gherardi, Lindsay and McKintosh1978). Puberty can be defined as the ability of males to mount and intromit an oestrus doe and the presence of spermatozoa in the ejaculate. From 3 months of age, individual sexual behaviour was assessed once in a week. Males had 5 min following presentation to mount an intact doe (same body size as that of males) artificially induced into oestrus by weekly intramuscular injections of 100 μg oestradiol benzoate. One week after the occurrence of mounting, males were allowed to ejaculate into an artificial vagina to determine the presence of spermatozoa.
Statistical analyses
Changes in body weight of females and males and testicular weight in males were analysed by a two-way ANOVA with repeated measurements (month of birth and time of experiment). Body weight at birth, average daily gain, body and testicular weights, and age at puberty were analysed by a one-way ANOVA (month of birth). When differences between groups were found, these variables were compared by a Fisher’s protected LSD test.
Results
Females
Body weight
The evolution of body weight of the three groups from birth to the onset of puberty is shown in Figure 1. The data for body weight and age at the time of puberty are shown in Table 1. Season of birth had a strong effect on live weight at birth (P < 0.05). Females born in October weighed less than those born in January and May (P < 0.05). No difference was found between the females born in January and May (P > 0.05). Effect of time of experiment (P < 0.001) and an interaction between time and group (P < 0.001) on live weight were observed, which indicated that groups showed varying responses with time. Pair-wise comparisons showed that the evolution of body weight of females born in May was different from those born in January and October (P < 0.001, both comparisons). In contrast, no difference was found between the January and October groups. Differences in body weight recorded on a monthly basis are indicated in Figure 1. Live weight at puberty varied according to season (P < 0.01). The weights of females of the October group were higher at puberty than those of January and May groups (P < 0.01, both comparisons). The weight of females of the January group did not differ (P > 0.05) from that of the May group. The average daily gain from birth to puberty was also different between groups (P < 0.001). This average daily gain was greater in animals born in May than in those born in October and January (P < 0.001). The average daily gain in animals born in October did not differ from those born in January (P > 0.05).

Figure 1 Increase (mean ± s.e.) of body weight of female goats from subtropical Mexico, which were born in January (●), May (▪) and October (○) (*P < 0.05).
Table 1 Body weights and age at puberty (mean ± s.e.) of female goats from subtropical Mexico, which were born in January, May and October
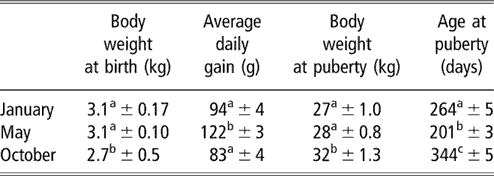
a,b,c Means with different superscripts in the same column are significantly different (P < 0.05).
Onset of ovarian activity
The percentages of females attaining puberty are shown in Figure 2. The data concerning the age at puberty are shown in Table 1. There was a highly significant effect of the season on age at first ovulation (P < 0.001). In females of the May group, ovulatory activity commenced earlier compared with females of the January and October groups (P < 0.001). In females born in January, ovulatory activity commenced earlier compared with females of the October group (P < 0.001). The onset of puberty was observed in September, December and October for January, May and October groups, respectively.
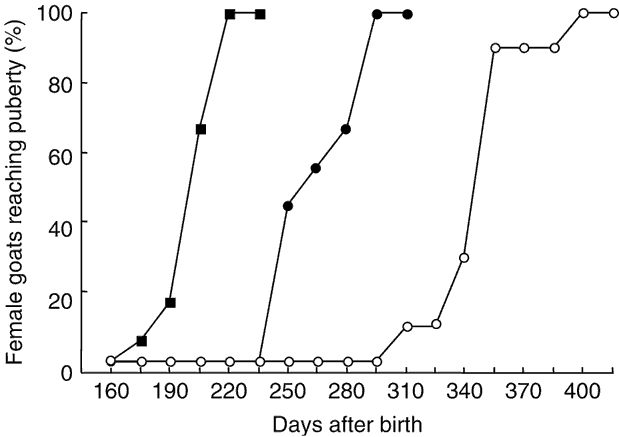
Figure 2 Cumulative percentage of female goats from subtropical Mexico attaining puberty, which were born in January (●), May (▪) and October (○).
Bucks
Body weight
The evolution of body weight of males of the three groups from birth to puberty is shown in Figure 3. The data for body weight are shown in Table 2. Season had a strong effect on live weight at birth (P < 0.001). The body weights of males born in October were less than those born in January (P < 0.01) and in May (P < 0.001). No difference was found between males of these two groups (P > 0.05). An effect of time of experiment (P < 0.001) and an interaction between time and group (P < 0.05) on live weight were observed, which indicated that groups showed varying responses with time. The evolution of body weight of the males when compared two by two groups was different throughout the experiment (P < 0.05). Pair-wise comparisons showed that body weight of males of the May group differed from those of the January and October groups (P < 0.05). There were no differences between the January and October groups (P > 0.05). The differences that were observed on a monthly basis are indicated in Figure 3. Live weight at puberty varied according to season (P < 0.001). The live weights of males of the October group at puberty were less than those of the January (P < 0.01) and May groups (P < 0.001). The live weights of males of January did not differ (P > 0.05) from those of May group. The average daily gain from birth to puberty was also different between groups (P < 0.05). This average was greater in animals born in May than in animals born in October. The average daily gain did not differ (P > 0.05) between males born in January and May.

Figure 3 Increase (mean ± s.e.) of body weight of male goats from subtropical Mexico, which were born in January (●), May (▪) and October (○) (P < 0.05).
Table 2 Body and testicular weights, and age at puberty (mean ± s.e.) of the local male goats from subtropical Mexico, which were born in January, May and October
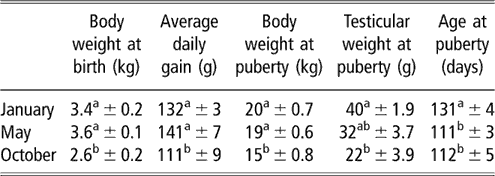
a,b Means with different superscripts in the same column are significantly different (P < 0.05).
Testicular weight
The evolution of testicular weight of males of the three groups from birth to puberty is shown in Figure 4. The data for testicular weight are shown in Table 2. An effect of time of experiment (P < 0.001) and an interaction between time and group (P < 0.01) on testicular weight were observed, indicating that groups showed varying responses with time. Pair-wise comparisons showed that the evolution of testicular weight differed only between the January and May groups (P < 0.01). The differences recorded on a monthly basis are indicated in Figure 4. Testicular weight at puberty varied according to season (P < 0.05). Males of the October group that attained puberty had smaller testicular weights compared with those born in January (P < 0.01). No difference was registered between males born in October v. May, or between males born in May v. January.

Figure 4 Increase (mean ± s.e.) of unpaired testicular weight of male goats from subtropical Mexico, which were born in January (●), May (▪) and October (○) (**P < 0.01).
Attainment of puberty
Age at first mounting with intromission varied significantly with the season of birth (P < 0.001; Table 2). The onset of puberty occurred earlier in May- and October-born males than in January-born males (P < 0.001). Puberty did not differ (P > 0.05) between May- and October-born males. All males showed presence of spermatozoa in the first ejaculate obtained 1 week after the first mount. The spermatozoa did not show mobility in any of the ejaculate.
Discussion
The results of this study show that in male and female goats from the local areas of subtropical Mexico, the season of birth considerably modified the onset of puberty. The effect of season of birth on puberty was more pronounced in females than in males. Regardless of the season of birth, male goats attained puberty at a younger age than did females.
Females in the January and May groups attained puberty in September and December (the same year of birth), respectively. The body weight at puberty was similar in both groups (about 27 kg) but females in the January group were about 9 weeks older than those in the May group (approx. 38 v. 29 weeks old). Onset of puberty in both groups occurred probably because they had perceived enough increasing days, allowing the onset of puberty during the decreasing days of the autumn, after having reached a sufficient body weight. The difference of age in attaining puberty in the January and May groups was probably because of the difference in body weight at the beginning of the natural breeding season, which lasts from September to March (Delgadillo et al., Reference Delgadillo, Fitz-Rodriguez, Duarte, Véliz, Carrillo, Flores, Vielma, Hernández and Malpaux2004a). Females in the January group with higher mature body weight attained puberty in September, whereas those in the May group should have attained a body size so that puberty commenced in December, similar to those reported in Suffolk ewes and Alpine goats (Ricordeau et al., Reference Ricordeau, Bouillon, Gaillard, Lajous and Lajous1984; Foster, Reference Foster1994). October-born females also reached puberty in the autumn (October) of the subsequent year. In fact, they were approximately 20 and 10 weeks older than females in the January and May groups, respectively, with highest (32 kg) body weight. Delayed puberty in this group was probably attributed to the fact that they required to perceive the increasing days from spring and summer to start puberty during the short days of the autumn (Foster, Reference Foster1994). In females, the effect of season on puberty observed in our study agrees with data reported in reproductive seasonal breeds of goats and sheep, in which puberty starts only during the breeding season of the adult females (Ricordeau et al., Reference Ricordeau, Bouillon, Gaillard, Lajous and Lajous1984; Forcada et al., Reference Forcada, Abecia and Zarazaga1991; Greyling, Reference Greyling2000). In these seasonal breeds, the long days of spring and summer followed by short days of autumn provide the stimulatory signal to initiate puberty, when the animals have achieved the appropriate body growth to initiate ovulation (Foster, Reference Foster1994; Adam et al., Reference Adam, Findlay, Kyle and Young1998).
In our study, despite the fact that all animals were fed ad libitum, there was an effect of season on average body daily gain. This was already reported in ad libitum-fed young or adult animals when subjected to increasing or long days, compared with those raised in decreasing or short days (Greyling and Van Niekerk, Reference Greyling and Van Niekerk1990; Wood et al., Reference Wood, Ebling, I’Anson and Foster1991; Delgadillo et al., Reference Delgadillo, Cortez, Duarte, Chemineau and Malpaux2004b). The differences in growth rates cannot explain the difference in puberty between the January and October groups because they had a similar growth rate, but puberty appeared later in the October group. In spring-born Soay ewe lambs receiving a diet ad libitum or at maintenance, puberty was not delayed and both groups started ovulatory cycles in autumn inspite of a different body weights (Adam et al., Reference Adam, Findlay, Kyle and Young1998). In our study, the difference in age and body weight at puberty were probably because of the season of birth, then the different photoperiodic conditions in which they were raised: January- and May-born groups received enough long days during winter and spring and attained puberty during the short days of autumn in the same year. In contrast, the October group must first perceive increasing days to reach puberty during the decreasing days of autumn of the following year, as reported in other seasonal breeds (Foster, Reference Foster1994). In Alpine does, for example, the onset of puberty occurs at about 45 and 36 weeks for females born in October and January, respectively (Ricordeau et al., Reference Ricordeau, Bouillon, Gaillard, Lajous and Lajous1984). The age at the onset of puberty of female goats from subtropical Mexico born in winter, spring or autumn is similar to that reported in different breeds of small ruminants from temperate and subtropical latitudes (Foster, Reference Foster1994; Lassoued and Rekik, Reference Lassoued and Rekik2001; Freitas et al., Reference Freitas, Lopes-Junior, Rondina, Salmito-Vanderley, Salles, Simplício, Baril and Saumande2004).
In males, puberty was slightly modified by the season of birth. May- and October-born males attained puberty at the same age with a different body weight and similar testicular weight despite being born during increasing or decreasing day length, respectively. These two groups attained puberty earlier than January-born males, which were born during increasing days. This weight of January group at puberty was higher than that of May and October groups. The lower body weight at birth and the lower average body daily gain of October-born males does not appear to have influenced the onset of puberty because the males of this group attained puberty at the same age as the May group, which showed a higher growth rate. This conclusion is reinforced by their similar average daily gain: the January group was older at puberty than the May group having similar body and testicular weights. These data suggest that in subtropical Mexican male goats, puberty was not strongly dependent on photoperiodic changes, although adult males display a reproductive seasonality controlled mainly by photoperiodic changes (Delgadillo et al., Reference Delgadillo, Canedo, Chemineau, Guillaume and Malpaux1999 and Reference Delgadillo, Cortez, Duarte, Chemineau and Malpaux2004b). In contrast to females, puberty of Mexican male goats does not appear to be controlled by photoperiodic changes, as reported previously in other breeds. In gonad-intact Ile-de-France and Suffolk rams, changes in testicular weight, an index of spermatogenesis, or the rise in LH secretion are not modified when subjected after birth to increasing or decreasing photoperiod, or to constant long days (Colas et al., Reference Colas, Guerin, Brios and Ortavant1987; Herbosa and Foster, Reference Herbosa and Foster1996). In spring-born gonadectomised oestradiol-treated lambs raised in natural or artificially reversed photoperiod, rise of pubertal LH is observed to be similar or slightly delayed for approximately 3 weeks in reverse natural simulated-photoperiod (Wood et al., Reference Wood, Ebling, I’Anson and Foster1991; Herbosa et al., Reference Herbosa, Wood and Foster1995). Considered together, these results suggest that at least in the above-mentioned breeds and in young males from subtropical Mexico, puberty started indifferently during increasing or decreasing days, which indicates that the influence of photoperiod on the onset of puberty was less. More generally, the age at puberty in local male goats from subtropical Mexico born in winter, spring or autumn is lower than that reported in various breeds of small ruminants from temperate and subtropical latitudes (Elwishy and Elsawaf, Reference Elwishy and Elsawaf1971; Chakraborty et al., Reference Chakraborty, Stuart and Brown1989; Özsar et al., Reference Özsar, Güeven, Celebi, Kalkandelen and Van de Wiel1990; Ahmad and Noakes, Reference Ahmad and Noakes1996).
The difference in puberty between males and females may be because of a different sensibility of the hypothalamo-pituitary axis to steroid negative feedback. In both the sexes, the onset of puberty originates in a decrease in the sensitivity to the negative feedback of oestradiol in females and testosterone in males (Claypool and Foster, Reference Claypool and Foster1990; Foster, Reference Foster1994). It was shown that this decreased sensitivity to steroids occurred later in females than in males, provoking an earlier onset of puberty in males (Claypool and Foster, Reference Claypool and Foster1990; Foster, Reference Foster1994; Herbosa et al., Reference Herbosa, Wood and Foster1995). This difference is probably because of a different response of males and females to photoperiodic information. In females, decreasing day length is necessary to attain puberty, whereas in males, it appears that decreasing day length is not necessary to attain the puberty (Wood et al., Reference Wood, Ebling, I’Anson and Foster1991).
Results of this study show that in these subtropical conditions, the season of birth affects the onset of puberty. This fact must be taken into account because it is usually hypothesised that in subtropical conditions, level of nutrition is the main environmental factor controlling the time of the breeding season rather than that of puberty (Walkden-Brown and Bocquier, 2000). Our data challenged this conclusion by showing that the onset of puberty is dependent on season of birth and that this effect of season is not obtained through seasonal changes in availability of food (birth is reached at different weights depending on the season). Our data, therefore, clearly indicate that, in addition to the availability of food, other environmental factors such as photoperiod must be considered.
In conclusion, these data show that in local goats from subtropical Mexico, season of birth influences the onset of puberty. In does, puberty depends on photoperiodic changes, and the onset of puberty occurs only during the short days of autumn during the natural breeding season. In contrast, puberty in male goats does not appear to depend on photoperiodic changes. Bucks attain puberty either during the long or during the short days.
Acknowledgments
This work was supported by the International Foundation of Science (Grant B/2071-3F). We express our thanks to the Assay Laboratory of the Station de Physiologie de la Reproduction et des Comportements of Nouzilly, France, for carrying out the radio-immunoassays, to B. Malpaux and P. Chemineau for their comments to improve the manuscript, and to D. López for her secretarial help. M.A. De Santiago was supported by a CONACyT scholarship for her Postgraduate studies.








