Introduction
Since the first description of the birds in the Junín area by Taczanowski (Reference Taczanowski1874), Lake Junín on the high plateau in the central Andes of Peru has been famous for its abundance of waterbirds. Surveys in the 1930s, 1960s (Morrison Reference Morrison1939, Dourojeanni et al. Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968) revealed a rich avifauna and gave Lake Junín a reputation as the principal site for waterbirds in the central Andes. Morrison (Reference Morrison1939) wrote in his account “probably because of the shallow depth, birdlife simply swarms. The numbers of Ibis, Coot, Ducks and other birds are simply amazing”. Dourojeanni et al. (Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968) estimated about a million waterbirds of 37 species. Surveys in the late 1970s confirmed the presence of large numbers of waterbirds (Harris Reference Harris1981, Fjeldså Reference Fjeldså1983b) although fewer than those claimed by Dourojeanni et al. (Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968). Fjeldså (Reference Fjeldså1983b) estimated about 100,000 waterbirds in 1977–1978.
Lake Junín is still today rich in its concentrations of waterbirds. The two endemic species: Junín Grebe Podiceps taczanowskii (Taczanowski Reference Taczanowski1874) and Junín Rail Laterallus tuerosi (Fjeldså Reference Fjeldså1983a) are both classified as globally threatened - The Junín Grebe as ‘Critically Endangered’ and Junin Rail as ‘Endangered’ (BirdLife International 2018). Moreover, the endemic subspecies of the White-tufted Grebe Rollandia rolland morrisoni (Simmons Reference Simmons1962) and the Northern Silvery Grebe Podiceps juninensis classified as ‘Near Threatened’ (BirdLife International 2018) are present. Grebes represent a group with high species extinction risk. Globally three species are recently considered extinct: The Colombian Grebe Podiceps andinus of the Eastern Andes of Colombia in the 1970s (Fjeldså Reference Fjeldså1993), the Atitlan Grebe Podilymbus gigas in Guatemala and Alaotra Grebe Tachybaptus rufolavatus in Madagascar in the 1980s; others are globally threatened (Reference Del Hoyo, Elliott and Sargataldel Hoyo et al. 1992, Roesler et al. Reference Roesler, Imberti, Casaňas, Mahler and Reboreda2012, BirdLife International 2018).
Generallly, aquatic ecosystems in Peru face degradation, with deterioration water quality due to contamination from mining industries and sewage, and manipulations of water levels (Ortega and Chang, Reference Ortega, Chang and Halfter1998, Ortega et al. Reference Ortega, Hidalgo, Trevejo, Correa, Corijo, Meza and Espino2012). Lake Junín is no exception and the lake is currently subject to a range of unsustainable land use pressures that seriously challenge its ability to sustain its previous biodiversity values and ecosystem functions (see later for an elaboration).
In order to preserve the rich wetland habitats including waterbirds the Junín National Reserve was designated in 1974 and covers an area of 530 km2 where hunting is restricted. This area was designated a wetland of international importance under the Ramsar Convention in 1996 (RIS 1996, Franke Reference Franke2006). Further, Lake Junín is identified as an Endemic Bird Area of the Junín puna (Stattersfield et al. Reference Stattersfield, Crosby, Long and Wege1998) and as an Important Bird Area (Devenish et al. Reference Devenish, Diaz Fernandez, Clay, Davidson and Yepez Zabala2009, BirdLife International 2017).
The history of increasing threats to this ornithologically important site raises the need for continued monitoring, with the aim of setting appropriate goals for environmental restoration of the lake. Birds are the most studied biological group and no quantitative data exist for other groups. Moreover, we consider waterbirds to be an indicator of the health of the wetland, and therefore aimed to assess long-term changes in waterbird numbers in the Junín basin and to propose appropriate management interventions.
Material and methods
Study area
Lake Junín (also known as “Chinchaycocha”) is situated in the high Andes of central Peru at 11°01’S, 76°07’W at about 4,080 m elevation (Figure 1). The main tributaries to the lake are the Colorado and San Juan rivers, supplemented by 10 smaller rivers and twenty streams (Valdivia and Alvarino Reference Valdivia and Alvarino1991). Mean average air temperatures range from 3° to 7°C with the coldest period being from May to September and annual rainfall is 940 mm, of which most falls between December and April (RIS 1996). The lake has a catchment basin of about 1,800 km2 in the treeless Puna zone (Harris Reference Harris1981).
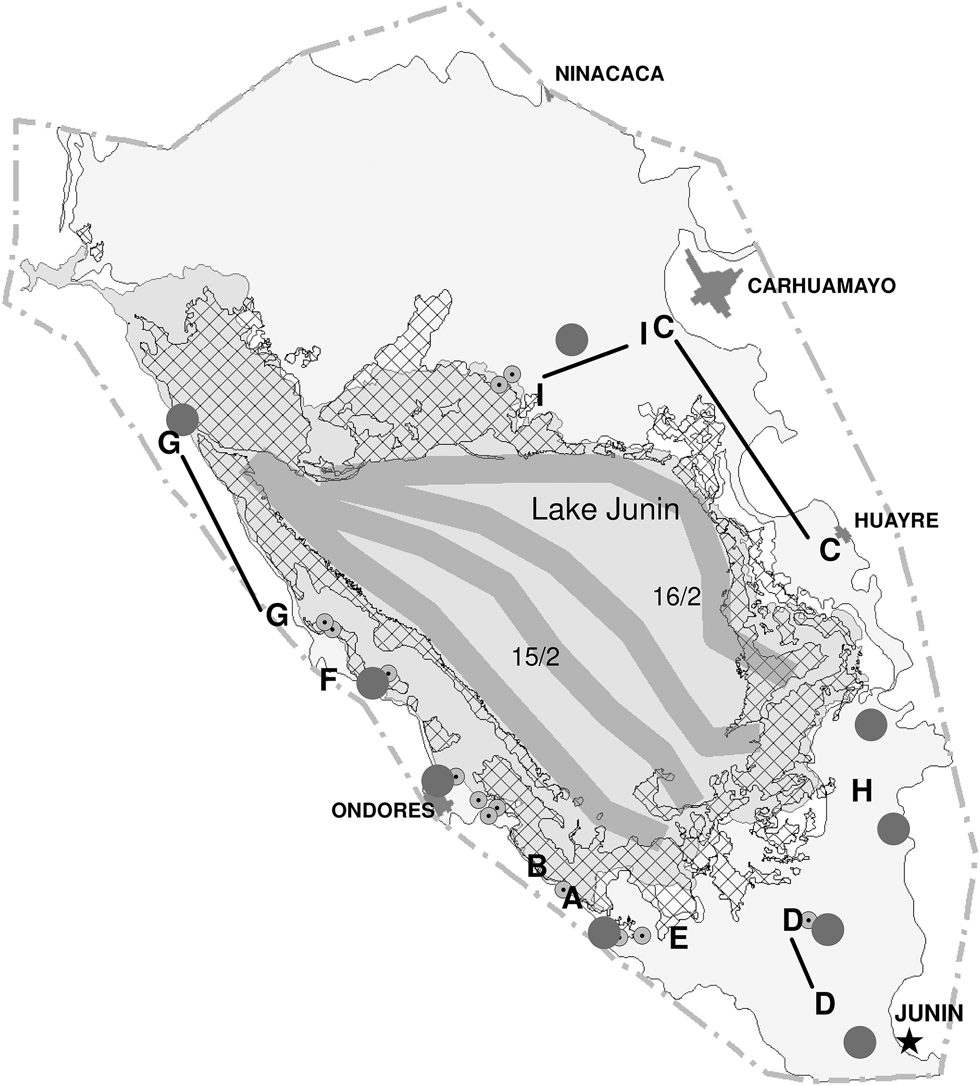
Figure 1. Map of Lake Junín, Peru showing extension of lake and marsh vegetation. The 2014 observations points and routes are indicated. Letters refer to Table 1 and black lines are either walking (I and D) or driving routes (G and C) and smaller circles with dots are areas visited on foot. Larger circles are general observation points and the boat routes used on 15 and 16 February are indicated by broad grey lines.
Lake Junín is the second largest lake in Peru after Lake Titicaca, extending to c.300 km2 (Fjeldså Reference Fjeldså1982) with a mean annual seasonal fluctuation in water level of 1–5 m (Harris Reference Harris1981 and later records). The abundant avifauna in Lake Junín is likely due to shallow water (3–4 m deep) over most of its area and 11–12 m at its deepest (Harris Reference Harris1981, ECOAN 2010). The lake is surrounded by a dense growth of Juncus balticus covering large areas of the shore to the point of making many areas almost impenetrable, with tall Schoenoplectus californicus in deeper waters in the lake. A description of the plant communities of the lake can be found in Dourojeanni et al. (Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968) and Fjeldså (Reference Fjeldså1981, Reference Fjeldså1983b). The peripheral marsh is up to 6 km wide covering an area of about 100 km2 (Dinesen et al. Reference Dinesen, Chamorro, Fjeldså and Aucca2017), providing food, shelter and breeding sites for waterbirds, although part of the marsh dries up between June and October (Fjeldså Reference Fjeldså1983b). Grass-covered hills reach down to the shorelines along the northern and western sections.
Literature review
The authors have good knowledge of the past publications presenting results from earlier surveys undertaken at Lake Junín due to a collective research and management involvement in the area over almost 40 years. An internet search added some references relating to specific studies or reports with management recommendations. In several of the previous papers on waterbirds at Lake Junín e.g. Harris (Reference Harris1981) and Fjeldså (Reference Fjeldså1983b) counts are compared and these comparisons are updated in the present paper based on our findings. Below brief summaries are given of the earlier key studies.
Morrison (Reference Morrison1939) lived at the eastern end at the lakeshore from 11 January to 11 May 1938 and he collected 122 specimens of 46 bird species and provided notes on the abundance of waterbirds without undertaking systematic counts. Most of his observations must have been from the eastern shore and around his hut 6 km from Carhuamayo and 20 km north of Junín town. Presumably, Morrison had access to a boat as many of his notes refer to bird abundance on the lake. Between July 1966 and March 1968, Dourojeanni et al. (Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968) surveyed the lake and undertook detailed habitat surveys and censused waterbirds in July 1967, which produced very high figures of certain species by extrapolating counts from small areas near the shoreline.
Fjeldså (Reference Fjeldså1983b) stayed in the village of Ondores on the western side and counted waterbirds over a larger area (1,800 ha in January) and extrapolated totals for the lake between 30 September and 28 October 1977 and 31 December and 17 January 1978. Much time was spent in the outer marsh and he carried out his waterbird census as part of a comparative study of grebes (Fjeldså Reference Fjeldså1981) and Andean Coot Fulica ardesiaca (Fjeldså Reference Fjeldså1982). Harris (Reference Harris1981) counted from the lake margin on 18–23 May and 5–10 October 1979 and used an inflatable boat to cover parts of the lake. His population estimates were based on extrapolations from counts of about 5–10% of the areas of different habitats using the total habitat area estimated from maps available at that time.
Asociación Ecosistemas Andinos (ECOAN) – the Peruvian NGO working in the Andes – has worked intensively in the Lake Junín area since 2007 including two of the co-authors, with a special focus on monitoring of Junín Grebe, Chilean Flamingo Phoenicopterus chilensis and Junín Rail as well as running conservation awareness campaigns and supporting management activities (ECOAN 2010, Chamorro and Aucca Reference Chamorro and Aucca2017). ECOAN continues a program at Lake Junín in support of community-based conservation awareness as well as supporting certain management interventions.
In summary, the estimated total numbers of different waterbird species at Lake Junín are largely based on intermittent counts in selected areas, which have been extrapolated (using different methods) based on best estimates of habitat extent at the time when censuses were performed. Probably the most comprehensive waterbird counts were carried out in 1977–1978 as reported by Fjeldså (Reference Fjeldså1983b), but the quality of historical records of avian abundance is highly heterogeneous, and we urge extreme caution in interpreting the reliability of past counts and in particular using them as a basis for comparisons with contemporary counts and estimates. Nevertheless, some of the changes in relative species abundance or changes in estimated numbers appear unambiguous and we feel confident in undertaking some interpretations.
Records of threats
The literature has been surveyed to generate information on threats to the ecological integrity of the lake and compared with observations by the authors and ECOAN in the 1970s and 2000s. Morrison (Reference Morrison1939) provided some notes on hunting and the effect of the dam constructed at the north-western end of the lake. Dourojeanni et al. (Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968) also remarked that pollution from the mines and manipulation of water levels posed threats, in addition to the effects of agriculture and hunting. Harris (Reference Harris1981) discussed threats to the lake and waterbirds in his 1979 surveys including pollution from mining. Surveys of Andean Goose Chloephaga melanoptera (Summers and Castro Reference Summers and Castro1988) and Junín Rail (Dinesen et al. Reference Dinesen, Chamorro, Fjeldså and Aucca2017) outline specific threats to these two species expected from current land use practices and fluctuating water level. In the Ramsar Information Sheet (RIS 1996) and reports by ParkWatch Peru and ECOAN (Shoobridge Reference Shoobridge2006, ECOAN 2010, Chamorro and Aucca Reference Chamorro and Aucca2017) mining activities upstream and eutrophication from towns and settlements are identified as key threats along with grazing and fluctuating water levels. Recent studies on eutrophication and contamination by heavy metals are available from European cases, which have been scrutinized with the aim of contributing to management options, include Ibelings et al. (2007), Klosowski et al. (Reference Klosowski, Tomaszewicz and Tomaszewicz2006), Lambert and Davy (Reference Lambert and Davy2010), Meijer et al. (Reference Meijer, De Boois, Scheffer, Portielje and Hosper1999), Van den Berg et al. (Reference Van den Berg, Scheffer, Coops and Simons1998b), Sooksawat et al. (Reference Sooksawat, Meetam, Kruatrachue, Pokethitiyook and Nathalang2013) and Solinska-Gornicka and Symonides (Reference Solinska-Gornicka and Symonides2001).
Waterbird counts
Waterbirds were counted between 6 and 20 February 2014 by LD and AC. We spent a total of about 155 hours in the field during two weeks (Table 1) observing waterbirds at different points around the lake, visited other areas on foot and spent two days on the lake surveying by motorboat (Figure 1). Bird counts were divided into four geographically defined sections and totals summed for each. From selected count vantage points at the western, southern and eastern sections the lake water and shores were scanned using a 20-40x Kowa telescope and 7x42 Swarovski binoculars. Several parts of the marsh in the southern, western and northern section were slowly walked for marsh birds (see Figure 1). The boat counts were undertaken on 15 and 16 February (eastern section and central-western sections respectively) and on 19 February the northern part was surveyed on foot (see Figure 1 and Table 1). Surveys were also undertaken during a parallel survey of the Junín Rail, implementing 46 point-counts while walking through sections of the marsh (Dinesen et al. Reference Dinesen, Chamorro, Fjeldså and Aucca2017).
Table 1. Timing of 2014 waterbird counts at Lake Junín
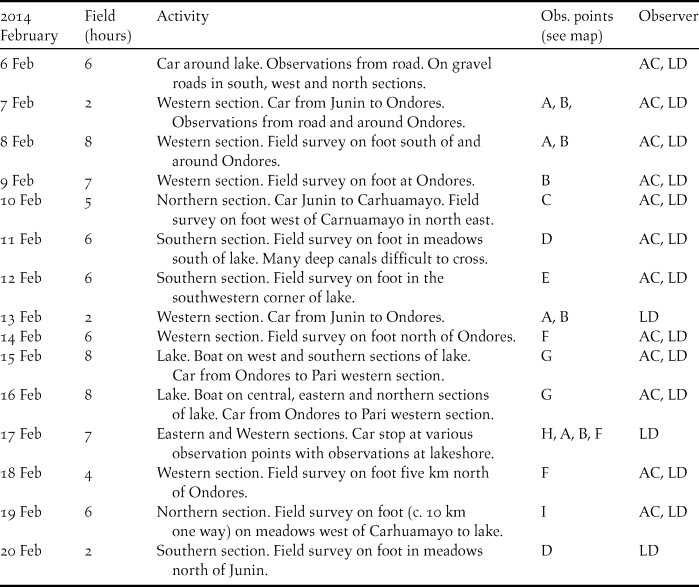
Surveys performed on different days in different portions of the wetland were generally assumed not to involve the same individuals. Generally, the waterbirds observed were feeding or resting and we did not observe large-scale movements or had other indications that could lead to considerations of serious double counting. While some species are resident year-round there may be significant turnover in the numbers of others, e.g. migratory shorebirds during a season, and other waterfowl disperse during the rainy season to small wetlands in the surrounding mountains (Harris Reference Harris1981, Fjeldså Reference Fjeldså1983b) not covered by this study. The sections in the south and west were visited more often because our activities were based in Junín and Ondores while the northern and the eastern sections were visited on fewer occasions (Table 1).
Results
The results of the 2014 counts are presented in Table 2 along with published studies in the 1930s, 1960s and 1970s (see above). The results from species-specific surveys of Junín Grebe and Chilean Flamingo in the 1990s and 2000s are summarized in ECOAN (2010) and Chamorro and Aucca (Reference Chamorro and Aucca2017) and below.
Table 2. Assessments of changes in abundance of waterbirds in Lake Junín based on information from six studies undertaken since the 1930s and compared with our 2014 survey. Language by previous authors to evaluate abundance e.g. “not common” or “extremely abundant” etc. is used in the table where no other estimations have been made. The top figure in each cell represents a count and the lower value an estimate/extrapolation or an abundance category. Based on these studies, the indications of long-term change are assessed where possible as either major decline (> 75% population reduction), decline (> 50% population reduction), stable/fluctuating or increase (> 50% population increase since the 1930s). Figures from 1966-68 are marked with an asterisk due to previous authors reservation about the accuracy of these figures.
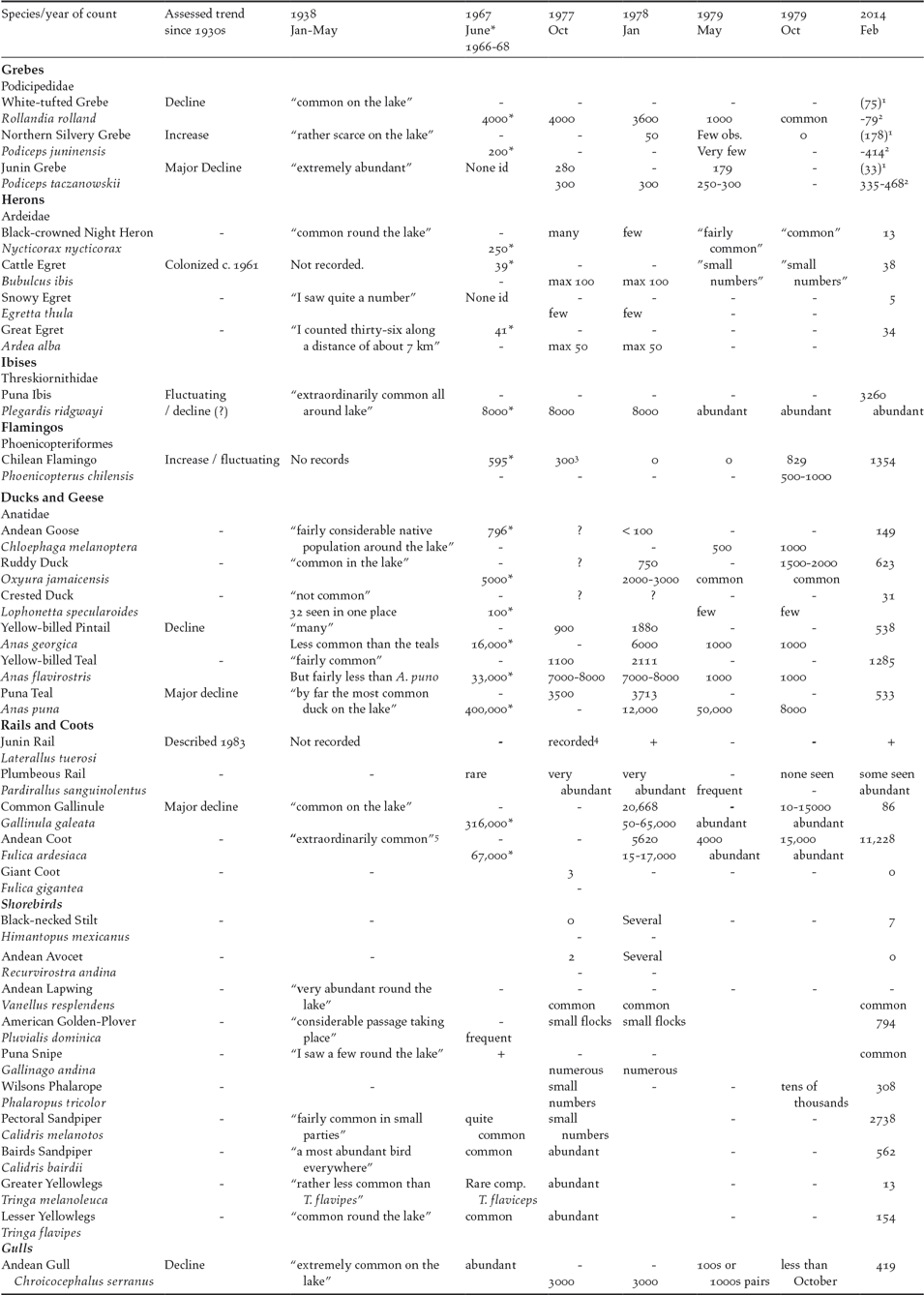
1 Targeted counts of grebes have been undertaken on a yearly basis by ECOAN and SERNANP since 2007. This figure does not represent a total survey of the lake.
2 Based on counts by ECOAN 2012 and 2016 (Chamorro and Aucca 2015, 2017).
3 Petterson (1977) mentions up to 5000.
4 By Fjeldså (1983b).
5 Morrison identified both F. ardesiaca and F. americana while later authors lumped them in one species (see Fjeldså 1982 for a review of its taxonomic status).
Literature review
Morrison (Reference Morrison1939) stated that the Junín Grebe was extremely abundant and that several other waterbirds were considered extraordinarily common (e.g. Andean Coot Fulica ardesiaca, Andean Gull Chroicocephalus serranus, Puna Ibis Puna Ibis Plegadis ridgwayi); common (e.g. White-tufted Grebe, Common Gallinule, Ruddy Duck Oxyura ferruginea, Black-crowned Night Heron Nycticorax nycticorax, Lesser Yellowlegs Tringa flavipes); or fairly common to common (e.g. Puna Teal labelled the most common duck and Yellow-billed Teal Anas flavirostris).
Dourojeanni et al. Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968 reported 37 species of waterbirds during their 1967 census and estimated altogether one million waterbirds including 400,000 Puna Teal, 316,000 Common Gallinule and 67,000 Andean Coot. The figures for these species have been considered an overestimate (Fjeldså Reference Fjeldså1983b). The extrapolations by Dourojeanni et al. (Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968) were derived from May counts when ducks are known to concentrate in certain zones close to the road near Ondores.
In 1977 and 1978 Fjeldså (Reference Fjeldså1983b) extrapolated from counts in an area of c.1,800 ha in the southern part of the lake in January 1978. This led to estimates of 50,000–65,000 Common Gallinule, 15,000–17,000 Andean Coot, 12,000 Puna Teal, 7,000–8,000 Yellow-billed Teal, 6,000 Yellow-billed Pintail Anas georgica, 8,000 Puna Ibis, 4,000 White-tufted Grebe and 3,000 Andean Gull (Table 2).
An estimated total of 75,000 waterbirds by Harris (Reference Harris1981) included 10,000–15,000 Common Gallinule, 15,000 Andean Coot and 8,000 and 50,000 Puna Teal in the two count periods in May and October respectively (Table 2). For Yellow-billed Teal and Yellow-billed Pintail the figures were 1,000 birds in both count periods. Harris (Reference Harris1981) considered that there had been a decline in the number of waterbirds between the mid-1960s and the late 1970s. Harris (Reference Harris1981) also reported tens of thousands Wilson’s Phalarope Phalaropus tricolor in small flocks.
Summers and Castro (Reference Summers and Castro1988) counted 1,887 Andean Goose in a species-specific study between 1 and 4 September 1984 and stated that large numbers gathered in winter at Lake Junín. Various counts of Junín Grebe have been undertaken since 1985 and the estimates have usually been 200-300 birds (ECOAN 2010) ranging from 50 to 304. Similar counts of Chilean Flamingo between 2003 and 2008 have documented breeding success and counts of 800 and 1,000 in 2007 and 2008 (ECOAN 2010).
Waterbird counts 2014
In total about 24,500 waterbirds of 32 species were counted including the 29 species listed in Table 2. The total numbers of waterbirds will be higher because the numbers of elusive species such as Plumbeous Rail Pardirallus sanguinolentus and Junín Rail are not included in this total (see Dinesen et al. Reference Dinesen, Chamorro, Fjeldså and Aucca2017). Moreover, single observations of Cocoi Heron Ardea cocoi – normally a lowland species – on 18 February near Pari, Blue-winged Teal Anas discors (one pair) and one immature Laughing Gull Larus atricilla on 6 February were also recorded. The most abundant species were Andean Coot comprising 46% of the individuals counted followed by Puna Ibis (13 %) and Pectoral Sandpiper Calidris melanotos (11%).
The three species of grebes were recorded. Northern Silvery Grebe was the most abundant (178 individuals observed was less than the numbers present) and the most widespread grebe in this survey, present also in the northern and most contaminated part by waste material from the mines, and along lake margins. Junín Grebe was the least common grebe in the 2014 survey with 33 individuals observed on open water mainly in the south and west. Counts revealed 75 individuals of White-tufted Grebe at the lake margins. None of these figures represent true total numbers of the species.
None of the heron species were particularly abundant. A total of 34 Great Egrets Ardea alba were counted and five Snowy Egrets Egretta thula as well as Black-crowned Night Heron. It is not possible to generate trends since the 1930s from the present material. The Puna Ibis was one of the most abundant waterbirds. Indication of breeding was recorded in the marshes, but this species foraged all over the adjacent Puna grassland and more than 3,200 were counted (Table 2), mostly in the grassland where birds gathered and were easy to count in the wet areas and sometimes close to settlements. The count of 1,354 Chilean Flamingo in 2014 included birds that were scattered along the lake margin in small and larger flocks of up to 130 birds.
In total 149 Andean Goose were counted; this species breeds in the surrounding highland and numbers at the lake are expected to be higher outside the breeding season. Although the 2014 count of about 533 Puna Teal does not represent a total lake estimate it certainly seems to be far less than earlier estimates. All ducks were distributed along the margins but were outnumbered by 1,285 Yellow-billed Teal in the same areas. A total of about 500 Yellow-billed Pintail were observed including flocks of birds moulting their flight feathers in the southern part of the lake.
A large number of Andean Coot were present with 11,228 counted both on the central lake and around the margins and about 25 dead birds were found in the lake or on pastures adjacent to the lake. Previous large counts of Common Gallinule were not be found during the 2014 survey, when less than 100 birds were recorded (mainly heard) in the Juncus vegetation. The Junín Rail was found in previously unsurveyed areas and the population estimated to be 6,200 individuals (Dinesen et al. Reference Dinesen, Chamorro, Fjeldså and Aucca2017) in Juncus and Festuca vegetation and the Plumbeous Rail was also abundant in the Juncus but numbers were not estimated.
Two resident shorebirds were common breeders: the Andean Lapwing, Vanellus resplendens and Puna Snipe Gallinago andina, both of which were found with nests in February 2014. The numbers of three migratory shorebirds in 2014 indicate that the Junín area is an important staging area for the American Golden Plover Pluvialis dominica, Pectoral Sandpiper and Baird’s Sandpiper Calidris bairdii. Numbers did meet the 1% of population criterion using minimum population estimates in Reference Del Hoyo, Elliott and Sargataldel Hoyo et al. (1996), but much below when using recent estimates by Wetlands International (2017). A minimum of 300 Wilson’s Phalaropes was counted on the lake during the 2014 study. Taking into consideration potential turnover rates and that meadow areas were not counted, it cannot be excluded that the 1% level will be approached for one or more of the shorebird species under targeted surveys. Shorebirds apart from the phalaropes were feeding on the extensive areas of surrounding pastures. Andean Gull was the only regularly occurring gull in Junín, and 419 individuals were counted including a few juveniles and colonial defence in the northern part of the lake.
Review of threats
Until 1933 the only water entering the lake came unregulated from the catchment. Since then the Mantaro river has been dammed for hydropower at its northern outflow to Lake Junín. Moreover, water from the Rio San Juan and Rio Colorado rivers has been diverted into the Upamayo pond, and the character of Lake Junín has changed dramatically. Silt and dissolved metals brought down by waters from the many large mines upstream has resulted in polluted river water overflowing into Lake Junín at times of flooding, with high concentrations of copper, iron and zinc from mineral processing (Harris Reference Harris1981, RIS 1996) to the inlet at the north-western end of the lake. Moreover, sewage, especially from the towns of Junín (15,400 inhabitants) and Carhuamayo (9,200 inhabitants), has caused eutrophication and depletion of oxygen in parts of the lake (RIS 1996, Shoobridge Reference Shoobridge2006, ECOAN 2010) and may have harmful effects on native fish populations such as Orestias sp. which is consumed by fish-eating grebes (O’Donnell and Fjeldså 1997). Additionally, enhanced fluctuations in lake water levels due to flow regulation that takes water from the Upamayo Dam for the Malpaso hydroelectric station constructed in 1933 (Harris Reference Harris1981, Shoobridge Reference Shoobridge2006) have caused rapid changes in flooding regimes in the marsh.
In the past the lake bottom was covered by extensive submergent and floating carpets of aquatic plants, mainly charophytes (Fjeldså Reference Fjeldså1981); however, the visibility in the water column was extremely poor throughout large parts of the lake in 2014, in particular in the north-western section, which is very likely associated with pollution from mining activities and sewage inflow. In these areas the extensive carpets of charophytes have disappeared (Fjeldså Reference Fjeldså1983b, RIS 1996). According to Harris (Reference Harris1981) upstream mines adopted a cementation process in 1958 by using iron to precipitate precious metals but this iron is later precipitated when the acid water from the mine washing meets the alkaline lake water. Moreover, Harris (Reference Harris1981) noted that fish were absent from the polluted shoreline in 1979 and most bottom-dwelling animals and plants were absent over perhaps a third of the lake area.
In addition, large areas of the wetlands were heavily grazed by herds of sheep and large numbers of cattle and to a lesser extent llamas Lama glama and alpacas Vicugna pacos (Dinesen et al. Reference Dinesen, Chamorro, Fjeldså and Aucca2017), while turf was dug up on rotational basis for use as fuel by people from the surrounding towns and villages (ECOAN 2010).
Former hunting pressure has declined considerably and perhaps ceased completely due to intensive campaigns conducted by ECOAN, SERNANP and Policía Nacional (ECOAN pers. comm.). Morrison (Reference Morrison1939) wrote: “During January and February the native fowlers were taking large quantities of eggs for eating purposes”. Dourojeanni et al. (Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968) estimated that more than 180 people made their livelihoods from hunting frogs and waterbirds in the area and Harris (Reference Harris1981) reported “there is much hunting both on waterfowl and for Giant Edible Frog” and reported that an estimated 700 people were involved in hunting and egg-collecting. Hunting and egg-collection were officially regulated in the 1970s when the area became a National Reserve (Harris Reference Harris1981) and especially since the first agreement made with the local communities in 2007 (ECOAN pers. comm.) the pressure from hunting has declined.
Discussion
The results strongly suggest that the community composition and abundance of individual waterbirds have changed dramatically since the first published survey in the 1930s by Morrison (Reference Morrison1939).
Grebes including fish-eating species
Junín Grebe and White-tufted Grebe subspecies morrisoni (Simmons Reference Simmons1962) both feed predominantly on fish (Reference Del Hoyo, Elliott and Sargataldel Hoyo et al. 1992, O’Donnell and Fjeldså Reference O’Donnell and Fjeldså1997). The Junín Grebe has declined considerably compared to the 1930s when the species was regarded as extremely abundant (Morrison Reference Morrison1939) and in 1961 when more than 1,000 were estimated (O’Donnel and Fjeldså 1997). A population of 50–304 birds was estimated between 1985 and 2007 (ECOAN 2010) and numbers are declining, with 304 in 2001, 249 in 2002 and 217 in 2007 (ECOAN 2010) and between 11 and 335 counted and 232 to 335 estimated between 2007 and 2013 (Chamorro and Aucca Reference Chamorro and Aucca2015). The species appears to be on the brink of extinction but survey results from 2016 provide some hope, with a total of 468 individuals in October 2016 (Chamorro and Aucca Reference Chamorro and Aucca2017) the highest number in more than 30 years. This apparent increase must most probably be seen as a result of targeted conservation work by ECOAN and SERNANP (Chamorro and Aucca Reference Chamorro and Aucca2017) including the reduction of illegal poaching, protection of nesting sites and not least cleaning up the effluents to ensure clean water for the spawning fish species forming a critical part of the grebe’s diet.
Indications of mass starvation of the White-tufted Grebe in Junín were reported by Scott and Carbonell (Reference Scott and Carbonell1986) and a count in October 2016 revealed low numbers (Chamorro and Aucca Reference Chamorro and Aucca2017). It was reported as common by Morrison (Reference Morrison1939) and estimates made of up to 4,000 in the 1960s and 1970s (Dourojeanni et al. Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968, Fjeldså Reference Fjeldså1983b). The original fish fauna includes Orestias sp. and the catfish Pygidium oroyae (Fjeldså Reference Fjeldså1983b, RIS 1996), but although some fish may still occur in the margins where clean water enters the lake, the native fish community is believed to have collapsed (ECOAN and SERNANP pers. comm.) and this grebe is most probably showing long-term decline as well, likely linked to reduced stocks of fish of suitable sizes. Moreover, there are reports of dead trout in the lake (ECOAN and SERNANP pers. comm.). This exotic fish is reported to be harmful in studies on other South American grebe species and their habitats (Fjeldså Reference Fjeldså1993, Roesler et al. Reference Roesler, Imberti, Casaňas, Mahler and Reboreda2012), which may be the case in Lake Junín as well.
In contrast the formerly scarce Northern Silvery Grebe is more dependent on small arthropods compared to fish, which may explain its more abundant status in 2014. This grebe has recently been split from Southern Silvery Grebe P. occipitalis categorised as ‘Near Threatened’ (BirdLife International 2018) due to an overall decreasing population (Guevara et al. Reference Guevara, Santander, Soria and Henrys2016) and Lake Junín is an important site for the species. Harris (Reference Harris1981) wrote in his account of Northern Silvery Grebe “this is the rarest of the resident grebes” seeing a few in May and none in October 1979 and Fjeldså (Reference Fjeldså1983b) stated that it was found only in the southern corner of the lake, and in small numbers. This is in contrast to the high relative numbers in 2014.
Ducks and herbivorous waterbirds
Our study indicates that several herbivore species are undergoing long-term decline. Puna Teal seems to have experienced a population crash which is most probably due to the disappearance of the submerged Chara communities, which in turn could be the consequence of eutrophication and siltation, possibly combined with effects of heavy metal (see also Scott and Carbonell Reference Scott and Carbonell1986). Morrison (Reference Morrison1939) reported it to be “by far the most common duck on the lake”. Fjeldså (Reference Fjeldså1983b) arrived at a total estimate of 12,000 and Harris (Reference Harris1981) at 50,000 and 8,000 in his two counts (Table 2). The 400,000 teals reported by Dourojeanni et al. (Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968) are questioned by e.g. Fjeldså (Reference Fjeldså1983b) but indicate large numbers.
In contrast, the Yellow-billed Teal was the most common duck found in the present study. The number of Yellow-billed Pintail was estimated at 7,000–8,000 in the 1970s (Fjeldså Reference Fjeldså1983b) and Morrison (Reference Morrison1939) reported “many” but fewer than the teal (see also Table 2) compared to 538 in our survey and it is difficult to draw conclusions based on this material. Moreover, it should be noted that ducks also breed in the surrounding highlands and concentrate in the lake outside their breeding season and undergo annual variations in their occurrence. A comprehensive total of 11,228 Andean Coot was counted in 2014, much less than the estimate of 67,000 by Dourojeanni et al. (Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968) and less than 15,000–17,000 by Fjeldså (Reference Fjeldså1983b) in January 1978. Morrison (Reference Morrison1939) reported Andean Coot as extraordinarily common in the 1930s and Harris (Reference Harris1981) 4,000 and 15,000 in his two counting periods respectively.
Primarily invertebrate feeders and other species
Lake Junín is still today one of the most important localities for Puna Ibis, which is endemic to the humid part of the Andean puna zone and the species appeared abundant in 2014. The Chilean Flamingo is at its northernmost breeding site in the Andes and there are indications of an increasing or fluctuating population compared with the earlier surveys. No flamingos were reported by Morrison (Reference Morrison1939) and 595 in June 1967 by Dourojeanni et al. (Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968). Fjeldså (Reference Fjeldså1983b) did not record any and Harris (Reference Harris1981) counted 829 in October 1979 and none in May. About 800 and 1,000 adults were counted in 2007 and 2008 respectively, increasing from 120 in 2003 (ECOAN 2010), and 1,354 in 2014. The species has been recorded breeding in several years since 2005 and individuals migrate seasonally to the Pacific coast (ECOAN 2010). The declining persecution may have benefitted both species in recent years.
There are no historical quantitative data for migratory shorebirds, but Baird’s and Pectoral Sandpipers and American Golden Plovers were also recorded in numbers in the 1930s (Morrison Reference Morrison1939) and they may be favoured in recent years by the intensive grazing. Moreover, as stated earlier the hunting pressure has decreased considerably in the last four decades and possibly ceased completely compared to earlier reports (Morrison Reference Morrison1939, Harris Reference Harris1981). The former pressure is illustrated e.g. by Morrison (Reference Morrison1939) on the American Golden Plover: “They were sufficiently common for the Indians to go out especially to snare them on the lake side flats. They catch them very brutally by putting out lines with a hook at the end baited with worms”.
The population of Common Gallinule seems to have crashed since accounts in the 1970s (see Fjeldså Reference Fjeldså1983b, Table 2) and few birds were recorded in 2014 compared to more than 20,000 in January 1978 in the south of the lake, which gave rise to an estimate of 50,000–65,000 for the entire lake (see also Scott and Carbonell Reference Scott and Carbonell1986). At that time, gallinules were recorded in large flocks out in open areas. The 316,000 birds reported by Dourojeanni et al. (Reference Dourojeanni, Hofman, Garcia, Malleaux and Tovar1968) may well have been an overestimate but demonstrate the implied abundance at that time. Harris (Reference Harris1981) noted that this moorhen is abundant all around the lake even in the contaminated water in the northern part. The 2014 count of c.400 Andean Gulls indicates a considerable decline compared to earlier reports e.g. an estimated 3,000 in the 1970s (Fjeldså Reference Fjeldså1983b) and the statement as “extremely common on the lake” in the 1930s (Morrison Reference Morrison1939).
Lake Junín still supported more than 25,000 waterbirds in 2014, meeting this particular Ramsar criterion for a wetland of international importance. The bird counts from 2014 are regarded as representing minimum estimates of the true numbers, although the magnitude depends on the species because of the sheer size of the lake and peripheral marsh, which makes many areas very difficult to access.
Despite our inability to make accurate assessments of trends in many cases, the overall pattern does nonetheless seem to be of falling or crashing numbers of several species compared to early to mid-last century especially those depending on native fish or directly or indirectly on the submerged Chara sp. community. Populations of e.g. primarily invertebrate feeders, for example species confined to the marsh and meadows surrounding the lake, such as rails and shorebirds, may be less affected existing in the marsh and meadow vegetation but are threatened by grazing and fluctuating water levels.
Recommended conservation actions
The overall decline in the waterbird populations strongly suggests there are serious challenges to the ecological character and integrity of the lake.
Eutrophication and a subsequent increase in water turbidity due to phytoplankton surface blooms have led to a pronounced decrease of charophytes in many shallow lakes in Europe (Van den Berg et al. Reference Van den Berg, Coops, Meijer, Scheffer, Simons, Jeppesen, Søndergaard, Søndergaard and Christoffersen1998a, Reference Van den Berg, Scheffer, Coops and Simons1998b, Klosowski et al. Reference Klosowski, Tomaszewicz and Tomaszewicz2006, Lambert and Davy Reference Lambert and Davy2010). Charophyte loss is both a symptom of degradation and an obstacle to recovery from eutrophication (Solonska-Gornicka and Symonides 2001, Lambert and Davy Reference Lambert and Davy2010). Experimental restoration of lakes where external phosphorus input and water turbidity were experimentally reduced, have led to the return of dense charophyte beds (Meijer et al. Reference Meijer, De Boois, Scheffer, Portielje and Hosper1999, Ibelings et al. Reference Ibelings, Portielje, Lammens, Noordhuis, Van den Berg, Joosse and Meijer2007), however, different charophyte species are involved and experience from the field are mixed (e.g. Solonska-Gornicka and Symonides 2001).
Moreover, we do not know in detail the current effects on lake function including the link to contamination from heavy metals such as zinc, copper and iron. Charophytes seem generally resistant to high zinc concentrations in the water (Sooksawat et al. Reference Sooksawat, Meetam, Kruatrachue, Pokethitiyook and Nathalang2013) and can be highly effective at removing heavy metals from the water column (Lambert and Davy Reference Lambert and Davy2010). However, charophytes cannot tolerate reduction in water transparency such as that associated with high levels of phosphate in human waste water, hence removal of sources of untreated sewage water being discharged into the lake is potentially a major contribution to the reestablishment of a clear water column, which is an essential precursor to restoring the charophyte community. Similarly, siltation from mines should be avoided too.
Specific conservation actions
It is recommended that clean inflowing watercourses are secured and protected from pollution, especially crucial and a matter of priority in the parts of the lake where the Junín Grebe persists. Such clean inflow areas should be classified as areas of strict protection, which would enhance the conservation status of these crucial inflows. In addition, legislation should be improved and include protection of water sources benefitting nature conservation.
Further, it is important for the ecological integrity of the lake that small and large sources of pollution are identified and attempts to reduce pollutants undertaken. This will be achieved in a dialogue with municipalities, private companies and landowners and include the establishment of various treatment plants at the relevant settlements. The major sources of pollution from towns around the lake have been identified and the municipalities are obliged to treat wastewater. The Agency for Environmental Assessment and Control (OEFA) ensures that this happens but enforcement needs to be strengthened.
The provision of scientific and technical expertise to mining companies will ensure the application of latest technologies to clean mine wastewater that currently flows unregulated into Lake Junín. In the vicinity of Junín Lake there are four mining companies, but these are not obliged to improve the water quality of the lake as long as the lake water is not used for humans. This task can be directed by the Chinchaycocha Environmental Management Committee, with the aim of restoring the lake and involving multiple government institutions and private companies including the mining companies. It is recommended that this important issue is given priority in the work of the Committee.
Artificial fluctuations in water level as a result of discharges from the hydropower dam should be reduced by establishing acceptable criteria for flooding regimes. Although the scale of this problem is not currently known, it is highly likely that nesting bird species including the endemic rail and grebe are negatively affected by such water fluctuations. The National Water Authority (ANA) is the entity in charge of setting flooding limits and failure to comply provides the possibility for sanctions to the hydropower companies. The Chinchaycocha Environmental Management Committee supervises compliance.
Intensive grazing occurs in many places around the lake and degrades the natural marsh vegetation. It is important that a balance is established between grazing areas and areas set aside for conservation of the natural vegetation and habitats. A land-use zonation system is currently being developed by the National Service of Natural Protected Areas (SERNANP), which is highly recommended. Research and monitoring of the biota including waterbirds and fish populations appear important. Moreover, chemical analysis of water and birds, and studies on the impacts of e.g. zinc on charophytes are recommended. Furthermore, it is recommended to establish research and monitoring in relation to human health, because it is possible that an accumulation of heavy metals happens, which eventually may be a risk to the health of humans and domestic animals.
In summary, the establishment of clear management objects should steer the process of implementing management actions for the catchment in order to balance competing interests. Such a plan will include the need to engage with relevant stakeholders and agree and adhere to priorities among competing interests. An existing plan is currently being implemented through the Chinchaycocha Environmental Management Committee, however actions are limited and implementation has not been very successful through the last 14 years (ECOAN pers. comm.).
Finally, it is recommended that Junín Lake is considered for listing as a Ramsar site on the Montreux Record until the ecological character of the lake has been restored and it is considered by the Peruvian Government to request a Ramsar Advisory Mission to provide detailed advice.
Acknowledgements
We are grateful to Dr Tony Fox, Aarhus University, who improved the manuscript and language. We thank Ronald Medrano, Servicio Nacional de Áreas Naturales Protegidas (SERNANP) for his support for the surveys. Fieldwork was carried out with invaluable assistance from Cesar Donato Zevallos Bashualdo. Moreover, Duanne Martinez and Rolando Tito Uribe, SERNANP, Shannon Behmke from the US Peace Corps joined us for shorter periods and the latter did invaluable translation. Thanks to the Mohamed bin Zayed Species Conservation Fund for sponsoring part of the project. ECOAN thanks the American Bird Conservancy and BirdLife International for support to their conservation activities. Many thanks to Signe Dinesen and Jens Dinesen for their great company in the field. A car and boat provided by National protected Areas Service of Peru (SERNANP) was used for part of the survey. Finally we thank two anonymous reviewers for constructive comments.





