Monochorionic twin pregnancies are at higher risk of adverse outcome than dichorionic twin pregnancies (Leduc et al., Reference Leduc, Takser and Rinfret2005; Morikawa et al., Reference Morikawa, Yamada, Yamada, Sato, Cho and Minakami2012; Sebire et al., Reference Sebire, Snijders, Hughes, Sepulveda and Nikolaides1997). Placental vascular anastomoses can lead to specific pregnancy complications, such as twin-to-twin transfusion syndrome (TTTS), selective intrauterine growth restriction, and death or neurologic damage of the surviving twin after sFD.
sFD is a serious complication and affects about 6–7% of all monochorionic twin pregnancies (Hillman et al., Reference Hillman, Morris and Kilby2011; McPherson et al., Reference McPherson, Odibo, Shanks, Roehl, Macones and Cahill2012). The risk for co-twin demise has been reported to be 12–17%, the risk for neurological abnormality in the survivor 18–26% (Hillman et al., Reference Hillman, Morris and Kilby2011; O’Donoghue et al., Reference O’Donoghue, Rutherford, Engineer, Wimalasundera, Cowan and Fisk2009; Ong et al., Reference Ong, Zamora, Khan and Kilby2006). Simonazzi et al. (Reference Simonazzi, Segata, Ghi, Sandri, Ancora and Pilu2006) reported that up to 67% of survivors develop neurological injury. Fetal cerebral lesions are thought to be caused by acute cerebral hypoperfusion because of exsanguination of the survivor into the dead fetus or thromboembolic events (Bajoria et al., Reference Bajoria, Wee, Anwar and Ward1999; Okamura et al., Reference Okamura, Murotsuki, Tanigawara, Uehara and Yajima1994; van Heteren et al., Reference Van Heteren, Nijhuis, Semmekrot and Merkus1998). Structural cerebral abnormalities observed in survivors include hypoxic-ischemic lesions of the white matter (multicystic encephalomalacia), microcephaly (cerebral atrophy), porencephaly, hemorrhagic lesions of white matter, and post-hemorrhagic hydrocephalus (Blickstein & Perlman, Reference Blickstein and Perlman2013; Hoffmann et al., Reference Hoffmann, Weisz, Yinon, Hogen, Gindes, Shrim and Lipitz2013).
Typical MRI findings after sFD are hypoxic-ischemic parenchymal lesions and malformations of cortical development, such as polymicrogyria and hemorrhage (de Laveaucoupet et al., Reference De Laveaucoupet, Audibert, Guis, Rambaud, Suarez, Boithias-Guérot and Musset2001; Elchalal et al., Reference Elchalal, Yagel, Gomori, Porat, Beni-Adani, Yanai and Nadjari2005; Glenn et al., Reference Glenn, Norton, Goldstein and Barkovich2005; Levine et al., Reference Levine, Barnes, Robertson, Wong and Mehta2003; Righini et al., Reference Righini, Salmona, Bianchini, Zirpoli, Moschetta, Kustermann and Triulzi2004; Sonigo et al., Reference Sonigo, Rypens, Carteret, Delezoide and Brunelle1998).
The aim of our study was to evaluate the rate of cerebral lesions detected at fetal MRI and to correlate those findings with neurological outcome of the survivors of monochorionic twin pregnancies after sFD.
Materials and Methods
This retrospective study was performed at a tertiary referral center, including all monochorionic twin pregnancies with sFD after the first trimester and subsequent fetal MRI between 2005 and 2012. Study approval was obtained from the institutional review board.
We used the obstetric database of our center to identify pregnancies fulfilling the inclusion criteria. Inclusion criteria were monochorionic twin pregnancies determined by ultrasound before 14 weeks of gestation, sFD after the first trimester, and subsequent fetal MRI examination of the survivor. Pregnancies complicated by fetal malformations or chromosomal abnormalities of the survivor were excluded. During the study period, 384 monochorionic twin pregnancies were identified. Eleven pregnancies fulfilled inclusion criteria and were included in the study (Figure 1).
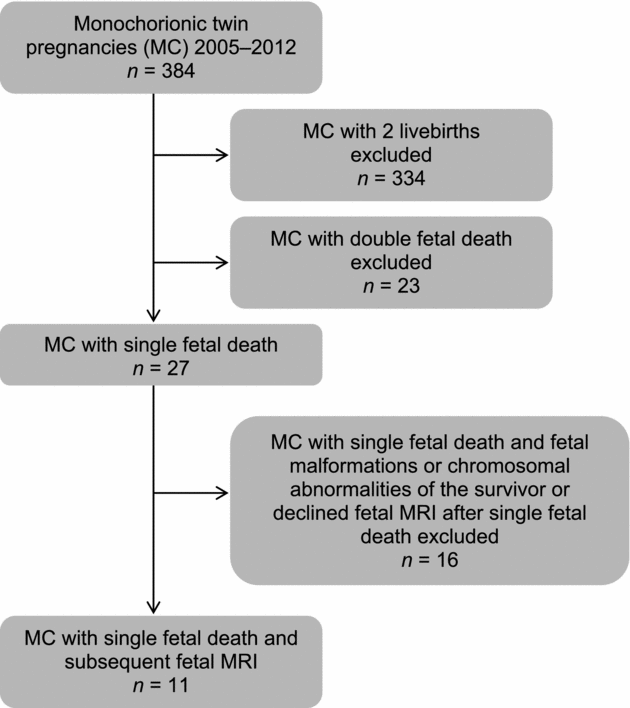
FIGURE 1 Process to identify monochorionic twin pregnancies with single fetal death after the first trimester and subsequent fetal MRI.
Demographic characteristics and pregnancy outcome were collected from the hospital maternity records. All women underwent first trimester ultrasound screening. Chorionicity was determined in accordance with the presence or absence of placental tissue extending into the base of the inter-twin membrane before 14 weeks of gestation (Sepulveda et al., Reference Sepulveda, Sebire, Hughes, Odibo and Nicolaides1996).
At 20–22 weeks, all women underwent anatomy scan. Fetal brain evaluation was done according to the ISUOG guidelines for the second trimester anatomy scan by transabdominal ultrasound. Women with monochorionic pregnancies were routinely scheduled every 2 weeks from 16 weeks of gestation onward at our institution's outpatient clinic. At each appointment, ultrasound was routinely performed for assessment of fetal growth and the amount of amniotic fluid. Fetal Doppler ultrasound examinations of the umbilical artery, the middle cerebral artery, and the ductus venosus were performed, if appropriate. Women with fetal or maternal complications were scheduled more frequently. TTTS was diagnosed ultrasonographically by demonstrating a polyhydramnios/oligohydramnios sequence and treated by laser photocoagulation of placental anastomoses (Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004).
After diagnosis of sFD, subsequent fetal MRI was performed for evaluation of the survivor in all patients of the study population. MRI was performed on a 1.5 Tesla superconductive unit, using a five-element cardiac surface coil. Women were positioned in the supine or lateral decubitus position and no sedation of either the mother or the fetus was given. Standard examination protocols included the following sequences: axial, coronal, and sagittal T2-weighted single shot turbo spin echo sequences, T1 weighted sequences, echoplanar sequences, and diffusion-weighted sequences.
After birth, all newborns underwent comprehensive neurologic examination performed by a consultant pediatrician. Neurologic examination included general assessment with evaluation of vital signs, determination of whether an infant's birth weight was appropriate for gestational age, and thorough examination of the head and spine. Motor function was assessed upon evaluation of passive tone and posture, and active motor activity. Reflex examination to evaluate integrity of the central and peripheral nervous system was performed, including for the developmental reflexes. Neonatal behavior was evaluated, including consolability and habituation, to assess higher cortical functions. All twins born preterm underwent a cranial ultrasound examination after birth. All infants of the study population were invited for long-term neurologic assessments using the Denver Developmental Screening Test II (Frankenburg et al., Reference Frankenburg, Dodds, Archer, Shapiro and Bresnick1992). It is a screening test including multiple items to examine four major categories (gross motor, fine motor-adaptive, language, and personal–social). The test is easily administered and predicts adverse outcomes accurately.
The main outcome parameter of the study was to evaluate the rate of cerebral lesions at fetal MRI in the survivor of monochorionic twin pregnancies after sFD and to correlate with neurologic assessments. Furthermore, we evaluated if the rate of cerebral lesions differed between survivors of pregnancies with spontaneous fetal demise and those with fetal demise after intervention.
Statistical analyses were performed with SPSS software (version 18.0; SPSS, Chicago, IL). Parametric continuous variables are summarized as means (±SD), non-parametric continuous variables as medians (minimum and maximum), and categorical data as percentages. Categorical variables were analyzed using Fisher's exact test.
Results
Between 2005 and 2012, 11 women with sFD and subsequent fetal MRI examination were included in our study. Mean gestational age at sFD was 20.9 (± 2.9) weeks of gestation. In 45% (5/11) of the pregnancies sFD occurred spontaneously and in 55% (6/11) they occurred after intervention (lasercoagulation of placental anastomoses or umbilical cord occlusion) because of TTTS.
Fetal MRI to evaluate the surviving twin was performed 14 (1–42) days after diagnosis of sFD at 23.5 (±2.3) weeks of gestation; 55% (6/11) of women underwent follow-up MRI examinations at 27.3 (±3.3) weeks of gestation. Follow-up MRI examinations showed cerebral lesions similar or worse than those described at the first MRI examination (Table 1).
TABLE 1 Findings on Fetal Magnetic Resonance Imaging and Neurologic Outcome of the Survivors of Monochorionic Twin Pregnancies After Single Fetal Death
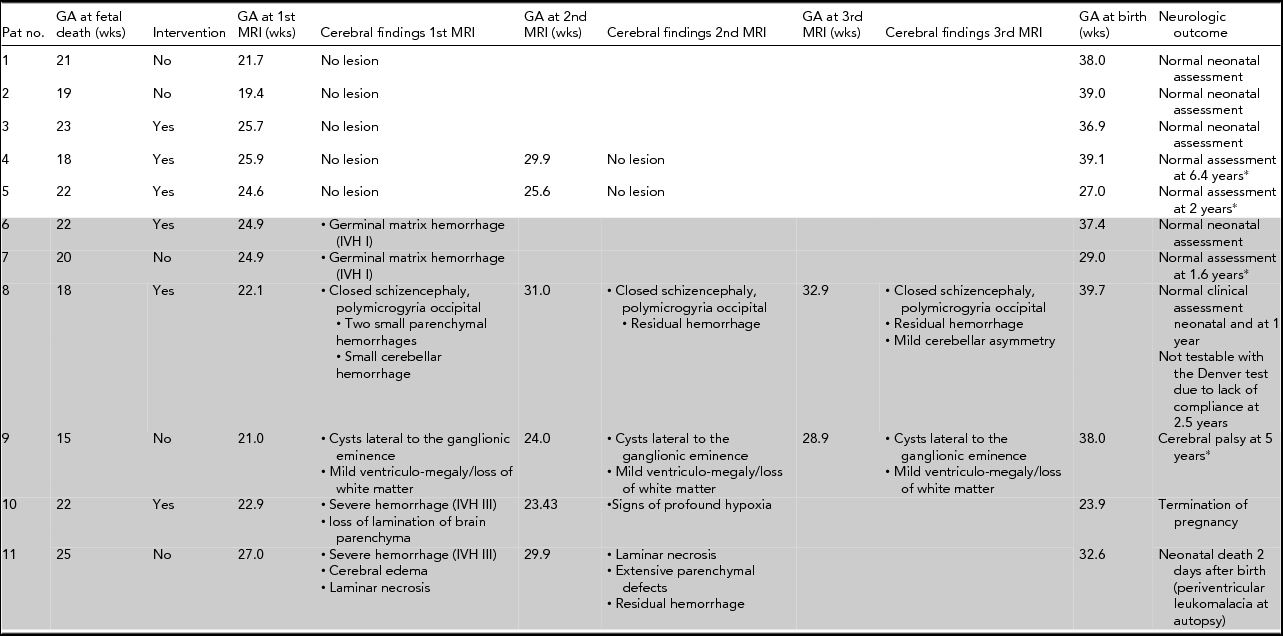
*Denver test, Pat No. = Patient number, GA = gestational age, wks = weeks, Intervention = laser coagulation of placental anastomoses or cord clamping, MRI = magnetic resonance imaging.
Overall, 55% (6/11) of the survivors showed cerebral lesions at MRI (Table 1), which are described in detail: Two survivors showed small intraventricular hemorrhage (IVH I) at fetal MRI and had normal neurologic outcomes (Table 1, Patient Nos. 6 and 7). Another survivor with closed schizencephaly and several small parenchymal hemorrhages at fetal MRI had a normal clinical assessment at one year but was not testable with the Denver test due to lack of compliance at the age of 2.5 years (Table 1, Patient No. 8). One survivor with cysts lateral to the ganglionic eminence and mild ventriculomegaly with loss of white matter at fetal MRI showed cerebral palsy at the age of five years (Table 1, Patient No. 9, Figure 2). In one pregnancy, fetal MRI of the survivor showed severe intracerebral hemorrhage (IVH III); a follow-up MRI showed signs of profound hypoxia and the pregnancy was terminated (Table 1, Patient No. 10). Another survivor showed severe IVH III at fetal MRI and extensive parenchymal defects at follow-up MRI. This twin died two days after birth (cesarean section was performed at 32 weeks of gestation due to pre-eclampsia) and autopsy revealed periventricular leukomalacia (Table 1, Patient No. 11, Figure 3). However, 72% (8/11) of all survivors had normal neonatal neurologic outcome: All survivors with normal fetal MRI (5/5) and 50% (3/6) of survivors with cerebral lesions detected at fetal MRI had normal neonatal neurologic assessments.

FIGURE 2 Axial (upper row) and coronal (lower row) T2-weighted MR images of case 9, imaged at 21 weeks of gestation (left row), 28 weeks of gestation (middle row), and postnatal at the age of 2 years. A classic lesion of the left ganglionic eminence (encircled), detected at the first prenatal MRI (left row) resulted in a significantly reduced volume of the thalamus and basal ganglia. Note the imaging appearance of periventricular leukomalacia postnatal (right row), which was less conscious at the prenatal examinations.

FIGURE 3 Fetal MR imaging appearance of case 11 at 27 weeks of gestation (upper row): an axial T2-weighted sequence (left upper corner) indicates a large hemorrhage of the right-sided ganglionic eminence. The diffusion weighted image (middle, upper row) shows diffusion restriction/infarction of the middle cerebral artery territory (arrow). The T1 weighted image depicts hyperintensities in the fetal brain parenchyma consistent with necrotic changes. T2-weighted sequences (lower row, left, and middle) at the follow-up at 29GW show large destructive lesions of both hemispheres and laminar necrotic changes at T1 weighted imaging (arrowhead).
The rate of cerebral lesions at fetal MRI did not significantly differ between those with spontaneous sFD and those with sFD after intervention (60% [3/5] vs. 50% [3/6]; p = .99 by Fisher's exact test). Sixty-four percent (7/11) of the pregnancies included in the study were complicated by TTTS. Mean gestational age at diagnosis of TTTS was 20.6 (± 2.3) weeks. Four patients had unfavorable Quintero stages: one fetus was already dead at diagnosis and three patients underwent cord occlusion (Table 1, Patient Nos. 1, 3, 7, and 9). The rate of cerebral lesions did not significantly differ between survivors of pregnancies with TTTS and those without (43% vs. 60%; p = .99 by Fisher's exact test).
Median gestational age at birth was 38 (27–39.7) weeks. Three newborns were admitted to the neonatal intensive care unit (NICU): Two were admitted due to prematurity (delivery at 27 and 29 weeks of gestation respectively) and had normal neurologic assessments. One newborn was admitted because of severe intracranial hemorrhage and died two days after delivery (Table 1, Patient No. 11). All other newborns (73% (8/11) were born after 37 weeks of gestation and were discharged from hospital with normal neonatal neurologic assessments.
Long-term neurologic assessments using Denver Developmental Screening Test II could be performed in 56% (5/9) of surviving infants at a median age of 2.5 (1.6–6.4) years (Table 1). Long-term neurologic assessment was normal in all (2/2) tested patients with normal fetal MRI and in one of three tested patients with cerebral lesions at fetal MRI.
Discussion
In our study, 55% of survivors of monochorionic twin pregnancies after sFD showed cerebral lesions at fetal MRI. However, 72% (8/11) of all survivors had normal neonatal neurologic outcome: All survivors with normal fetal MRI (5/5) and 50% (3/6) of survivors with cerebral lesions at fetal MRI. Long-term neurologic assessment was normal in all (2/2) tested patients with normal fetal MRI but only in one of three of tested patients with cerebral lesions at fetal MRI. Patient No. 6 had no long-term neurologic assessment but similar abnormalities at fetal MRI as patient No 7, who had normal neurologic assessment at 1.6 years. Although it is speculative, neurologic long-term outcome might be normal in patient No. 6 as well. However, long-term neurologic outcome of patient No. 8, who had severe cerebral lesions at fetal MRI and could not be tested with Denver test at age 2.5 years due to lack of compliance, remains uncertain.
The rate of cerebral lesions at fetal MRI did not significantly differ between those with spontaneous sFD and those with sFD after intervention (60% vs. 50%). Furthermore, the rate of cerebral lesions did not significantly differ between survivors with TTTS and those without (43% vs. 60%).
Prenatal follow-up MRI examinations showed cerebral lesions appropriate or worse than those described at the first MRI examination.
One major strength of our study is the combination of fetal MRI and neurologic outcome data, including long-term neurologic assessments. All patients were examined by radiologists with extensive expertise in fetal MRI and had neurologic assessments by consultant pediatricians. There were several limitations to the current study. First, this was a retrospective analysis. Furthermore, only 50% of the study population had follow-up MRI examinations during pregnancy. Long-term neurologic assessments were performed at a median age of 2.5 (1.6–6.4) years, though neuropsychologic consequences of periventricular leukomalacia could remain undetected before school age (Fazzi et al., Reference Fazzi, Bova, Giovenzana, Signorini, Uggetti and Bianchi2009). One potential bias of the study is that the radiologists were not blinded to the diagnosis of TTTS at the time MRI was assessed.
It has been demonstrated that acquired pathology of the fetal brain can be well recognized with fetal MRI (Elchalal et al., Reference Elchalal, Yagel, Gomori, Porat, Beni-Adani, Yanai and Nadjari2005; Levine et al., Reference Levine, Barnes, Robertson, Wong and Mehta2003). Acute changes such as hemorrhage or ischemic lesions have an appearance similar to that postnatal (Prayer et al., Reference Prayer, Brugger, Kasprian, Witzani, Helmer, Dietrich and Langer2006). Previous studies analyzing fetal MRI of the survivor in monochorionic twin pregnancies after sFD reported lower rates of cerebral lesions at fetal MRI compared to our results. Beside a few case reports (Glenn et al., Reference Glenn, Norton, Goldstein and Barkovich2005; Righini et al., Reference Righini, Salmona, Bianchini, Zirpoli, Moschetta, Kustermann and Triulzi2004; Reference Righini, Kustermann, Parazzini, Fogliani, Ceriani and Triulzi2007), Jelin et al. (Reference Jelin, Norton, Bartha, Fick and Glenn2008) reported a rate of 33% of intracranial abnormalities in 21 monochorionic twin pregnancies with spontaneous sFD; they reported no neurologic outcome. Hoffmann et al. (Reference Hoffmann, Weisz, Yinon, Hogen, Gindes, Shrim and Lipitz2013) reported a rate of 24% of abnormal prenatal cerebral findings at fetal MRI in 34 monochorionic twin pregnancies with sFD, including spontaneous sFD as well as sFD after intervention. In their study, most pregnant women with abnormal cerebral findings elected to terminate the pregnancy; therefore, they could not evaluate the true association between abnormal findings and neurodevelopment abnormalities. In contrast to the studies above, O’Donoghue et al. (Reference O’Donoghue, Rutherford, Engineer, Wimalasundera, Cowan and Fisk2009) reported a rate of only 6.6% of cerebral abnormalities at fetal MRI and 60% (3/5) of those patients had normal neurologic outcomes. However, in their study, fetal MRI examinations were performed in different centers and reviewed by one radiologist.
In our study, all fetal MRI examinations were performed at one expert center. All fetuses with normal MRI after co-twin demise showed normal neonatal neurologic outcome and all infants with normal prenatal MRI who were available for testing showed normal long-term neurologic outcome. Pregnancies with severe cerebral lesions at fetal MRI had an unfavorable outcome, resulting in termination of pregnancy, neonatal death, or severe handicap. Our data suggest that fetal MRI is a very sensitive tool to detect cerebral lesions. To evaluate the significance of the MRI findings, long-term neurologic examinations are required.
It has been reported that sFD after intervention could be protective for the survivor against cerebral injury (Lewi et al., Reference Lewi, Gratacos, Ortibus, Van Schoubroeck, Carreras, Higueras and Deprest2006; O’Donoghue et al., Reference O’Donoghue, Rutherford, Engineer, Wimalasundera, Cowan and Fisk2009; Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004). Our study showed a similar trend: the rate of cerebral lesions at fetal MRI was 60% after spontaneous sFD compared to 43% after intervention, but the difference was not statistically significant. Some of the interventions were done at an unfavorable stage of TTTS, which might have an impact on the neurologic outcome.
In conclusion, cerebral lesions of the survivor of monochorionic twin pregnancies after sFD detected at fetal MRI are frequent. The importance of cerebral lesions at fetal MRI in survivors after sFD in monochorionic twin pregnancies is uncertain. All tested survivors with normal fetal MRI showed normal neurologic outcome, but only one of three survivors with cerebral lesions at fetal MRI showed normal long-term neurologic outcome. This may be useful information for patient counseling and planning of care. Further studies including long-term neurologic outcome are necessary.






