Introduction
Rice (Oryza sativa L.) is one of the main staple foods worldwide, grown on all continents except Antarctica (Prasad et al. Reference Prasad, Shivay, Kumar, Chauhan, Jabran and Mahajan2017). It is the staple food for nearly half of the world’s population, most of whom live in developing countries. The crop occupies one-third of the world’s total area planted for cereal and provides 35% to 67% of the calories consumed by 3.5 billion people (GRiSP 2013). As well as being agriculturally important, rice fields provide many ecosystem services, including wildlife habitat for more than 230 species (Sterling and Buttner Reference Sterling2011). In the United States, rice is grown on 1.5 million ha, predominantly in the south-central region (Arkansas, Mississippi, Missouri, Louisiana, and Texas) and northern California (USDA-NASS 2019). In California, more than 200,000 ha are grown in Sacramento−San Joaquin Valley using a water-seeded, continuously flooded system (USDA-NASS 2019). In 2010, rice production contributed approximately $1.7 billion to California’s gross domestic product (GDP) (Richardson and Outlaw Reference Richardson and Outlaw2010). Rice growers flood their fields at the beginning of the growing season, and then pre-germinated rice seed is broadcast by airplane. A flood depth of 10 to 15 cm is maintained throughout the growing season.
One of the rising concerns in the California rice system is the prevalence of early-season blooms of nuisance algae (Lundy et al. Reference Lundy, Spencer, Van Kessel, Hill and Linquist2012; Spencer et al. Reference Spencer, Lembi and Blank2006). Algal communities in California rice are composed of diverse green algae and cyanobacteria species that take advantage of high levels of nitrogen and phosphorous within rice fields (Ohadi et al. Reference Ohadi, Madsen and Al-Khatib2019; Spencer and Linquist Reference Spencer and Linquist2014). Algal blooms in rice fields will become problematic if they interferes with rice seedling growth during the first month of the season (Spencer et al. Reference Spencer, Lembi and Blank2006). During this early stage, algal mat–forming species change their composition rapidly from green and golden algae (Carteria, Euglena, Scenedesmus, Kirchneriella, Actinastrum, Chlorogonium, Dysmorphococcus, Gonium, Tetraspora, Ankistrodesmus, Oedogonium, Rhizoclonium, Sphaeroplea, Tribonema, Hydrodictyon) to diatoms (Nauicula) and then cyanobacteria (Anabaena, Nostoc, Phormidium) (Spencer et al. Reference Spencer, Lembi and Blank2006). Cyanobacteria (Nostoc spongiforme Agardh ex Bornet) is the main concern among algal species identified in California rice; it has innate tolerance to conventional methods of algal control such as application of copper sulfate (Spencer et al. Reference Spencer, Lembi and Blank2006).
Successful and uniform rice stand establishment relies on physiological rice characteristics (e.g., seed vigor), field management decisions (e.g., seeding rate and seeding date), and biological (e.g., weed competition) and environmental factors (e.g., rainfall). In California, rice seeds are primed for 24 to 48 h (i.e., wet seeding) and then broadcast into the flooded fields. This method was adapted to suppress weeds (e.g., Echinochloa spp.) in the standing water (Gibson et al. Reference Gibson, Fischer, Foin and Hill2002). In addition, the pre-germinated rice seeds seem to be more competitive than their weedy counterparts and will emerge rapidly to develop a uniform ground cover (Gibson et al. Reference Gibson, Fischer, Foin and Hill2002). However, rice planted with this method is more sensitive to the occurrence of algal bloom. Accordingly, rice seedlings can be uprooted by the algal mat, and seedlings entangled in algae cannot develop roots deeper into the soil (Spencer et al. Reference Spencer, Lembi and Blank2006). In the case of large, thick algal mats, the emergence of rice seedlings through the mat is delayed. However, once the rice has emerged above water, the presence of algae could be beneficial due to their nitrogen-fixing capacity (Roger et al. Reference Roger, Santiago-Ardales, Reddy and Watanabe1987).
Although there are anecdotal observations that the early bloom of algae could interfere with rice, no quantitative research has investigated the extent of algal damage to rice. Here we evaluated the impact of algae on rice seedling emergence and establishment by manipulating the algal density. Further, a modeling approach was used to describe the relationship between the intensity of algal infestation and rice seedling emergence patterns. Results from this study can be used as a baseline for understanding the extent of early algal bloom impact on California rice.
Materials and Methods
Because of the difficulties of obtaining a uniform algae-infested field, the experiment was conducted under semi-controlled outdoor conditions at the USDA-ARS Invasive Species and Pollinator Health Research Unit, Davis, from June to August 2019. Treatments were conducted in 57-L tubs (Tuff Stuff Products, Terra Bella, CA 93270, USA). Tubs were filled with equal amounts of soil collected from a rice field at the Rice Experimental Station, Biggs, CA, allowing an algal profile similar to natural field conditions. The soil type was Esquon clay (fine, smectitic, thermic Xeric Epiaquerts and Duraquerts) consisting of 50% clay, 30% silt, 18% sand, and 2% organic matter with pH 5.85. The soil fertility content was 3.15 μg N L−1, 13.85 μg P L−1, and 250 μg K L−1.
To stimulate algal growth, fertilizers (N in the form of urea and P in the form of triple superphosphate) were added to the soil surface before adding water. The algal treatments included: (1) low algae, no fertilizers were added, and any growth of algae was controlled by copper sulfate (1 µg Cu L−1); (2) medium algal infestation, with nitrogen and phosphorous added at 75 and 37.5 kg ha−1 respectively; and (3) high algal infestation, with fertilizers added at a full rate similar to field conditions (150 kg N ha−1 and 75 kg P ha−1).
Next, tubs were flooded with water, and the water level in tubs was kept at 10 cm during the experiment, similar to the flood level in California rice fields. The water in the experiment was sourced from the groundwater well and contained 0.23 µg N L−1 and 0.059 µg P L−1. Sixty rice (‘M-206’) seeds were soaked for 24 h at 37 C and spread on the soil surface on the day tubs were flooded.
Each treatment had 10 replicates, and the experiment was performed as a completely randomized design. The experiment was repeated three times (hereafter “runs”), and each run took about a month.
Measurements
Chlorophyll a is one of the main characteristics that has been used widely to determine algal density in the water bodies. To be able to quantify the differences in the intensity of algae between treatments, the extracted chlorophyll a from the filtered water was used to quantify the intensity of the algae for each treatment throughout the experiment. Chlorophyll a was measured by the method described by Arar and Collins (Reference Arar and Collins1997). Accordingly, 15 ml of water sample containing algae was collected from each tub once a week. Water samples were vacuum filtered through glass-fiber filter papers (47 mm, pore size 0.7 µm; Whatman®, Maidstone, UK). Filters containing algae were homogenized in 90% acetone, stored for 2 h at 4 C in the dark, and then centrifuged for 5 min at 5,000 × g to remove filter paper debris. The chlorophyll a concentration was measured using a handheld fluorometer at 436 nm of wavelength (AquaFluor®, Turner Designs, San Jose, CA 95112-4220, USA). Although we measured chlorophyll a concentration weekly, the mean chlorophyll a concentration over time was calculated and used for further data analysis (Figure 1).
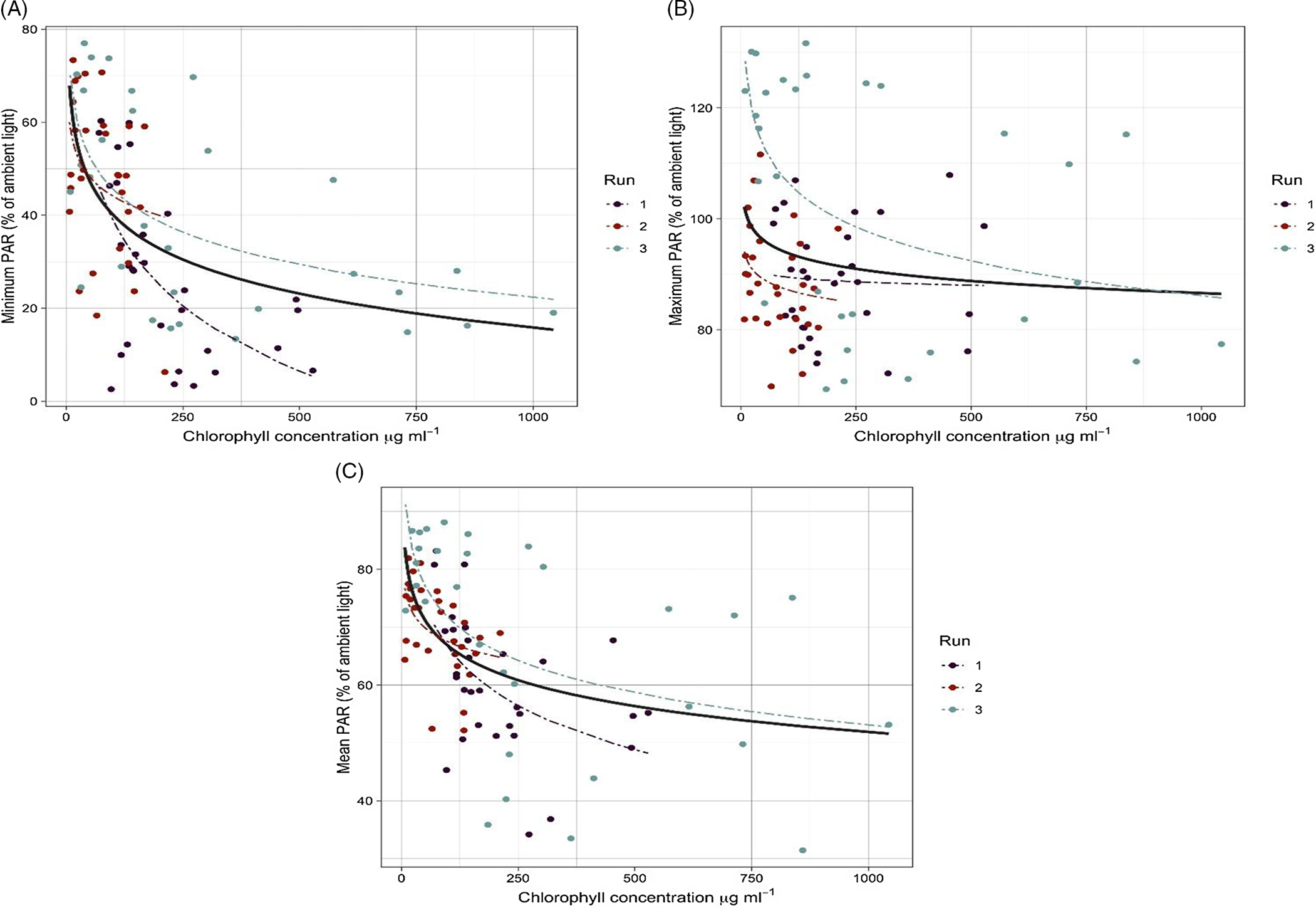
Figure 1. The relationship between chlorophyll a concentration (µg ml−1) and (A) minimum, (B) maximum, and (C) mean under the water PAR (relative to ambient PAR). The solid line shows data obtained from three experimental runs fit to the model, and dashed lines show data for each run fit to the model.
To estimate light intensity changes inside the water caused by algal infestation, photosynthetic active radiation (PAR) inside each tub was measured every other day and corrected relative to the outside PAR using a handheld PAR meter (underwater quantum light PAR meter, model MQ-210, Apogee Instruments, Logan, UT 84321, USA). Water temperature was recorded hourly using the HOBO® ONSET (Bourne, MA 02532, USA) temperature data logger to see if algal infestation would change the water temperature.
The number of rice seedlings that emerged from the water was counted every second day during the experiment till no new emergence was observed. Given that rice seedlings can entangle in algae and float on the surface of the water, only the seedlings that were upright and attached to the soil were considered as true emerging seedlings. The number of floating seedlings was also recorded during the experiment and used for adjusting the true number of seedlings that emerged and were established. All established rice seedlings were harvested in each tub separately at the end of each run, oven-dried at 70 C for 1 wk, and weighed to determine dry biomass.
Data Analysis
Data were tested for assumptions of normality and random distribution of the residuals. The data for mean chlorophyll a concentration and rice seedling emergence biomass were subjected to a log(x) + 1 transformation. ANOVA using PROC GLM in SAS (v. 9.4; SAS Institute, Cary, NC, USA) was initially performed to test the significance of experimental run and algal intensity level on mean chlorophyll a concentration; minimum, maximum, and mean PAR; percent of seedling emergence; and rice seedling biomass data. Comparison of means was performed using the LSMEANS statement of PROC GLM. When the interaction of run by algal intensity levels was significant, we used the SLICE feature of PROC GLM to study the interaction.
Further, a modeling approach was used to describe the relationship between chlorophyll a concentration (as a measure of algal infestation) and PAR (minimum, maximum, and mean), temperature, rice seedling emergence, and rice seedling biomass (g plant−1). All nonlinear regression analysis was performed using the drc package (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015) and its drcSeedGerm extension (Onofri et al. Reference Onofri, Benincasa, Mesgaran and Ritz2018) in RStudio (v. 1.2.1335; R Studio Team 2018).
The relationship between chlorophyll a and PAR (minimum, maximum, and mean) was described using a logarithmic function:
where y is the PAR inside the water relative to the ambient PAR, x is the mean chlorophyll a concentration, and a and b are the intercept and slope of the model, respectively.
To determine whether there were any changes in the water temperature under algal infestation, hourly temperature data were measured and cumulative growing degree days (GDD) were calculated from the day seeds were broadcast.
The relationship between rice seedling emergence and algal infestation levels (no, medium, and high algal infestation) over time was described using a nonlinear exponential function:
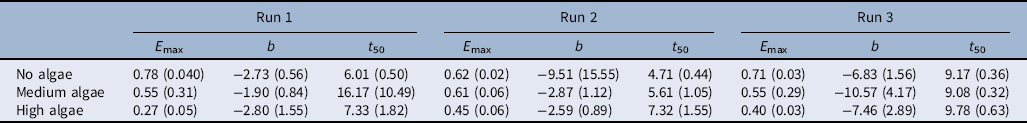
where E max is the maximum emergence, t 50 is time to median emergence (50%), and b is the slope about the inflection point. To obtain the relationship between the rice seedling emergence rate and chlorophyll a concentration, Equation 2 was fit and t 50 was calculated for individual replicates. Further, a linear method was fit to the relationship between t 50 and mean chlorophyll a concentration.
The relationship between chlorophyll a concentration and rice seedling biomass was described by an exponential function (Equation 3):
where q and c are the model parameters and meanchla is the mean chlorophyll a concentration.
Results and Discussion
The addition of different amounts of fertilizers promotes algal growth to different levels (i.e., low, medium, and high). There were significant interactions between run and algal intensity levels for chlorophyll a concentration; minimum, maximum, and mean PAR; rice seedling emergence percentage; and rice seedling biomass (Table 1). Therefore, comparisons of means were done separately for each run (Table 2).
Table 1. ANOVA for the effect of experimental run and algal intensity levels on mean chlorophyll a concentration; minimum, maximum, and mean photosynthetic active radiation (PAR); rice seedling emergence percentage; and rice seedling biomass.
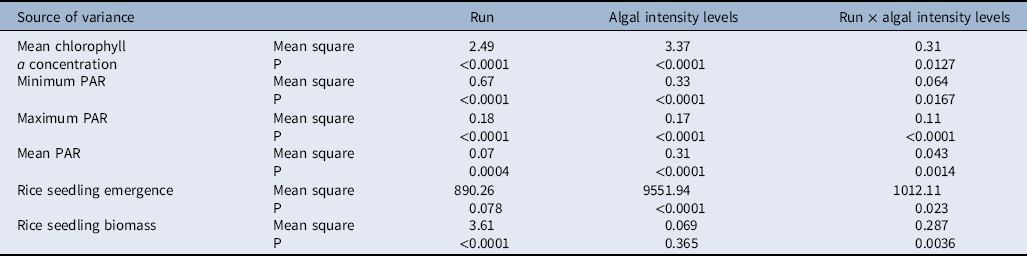
Table 2. Means differences between the algal intensity levels (no, medium, and high) and chlorophyll a concentration; minimum, maximum, and mean photosynthetic active radiation (PAR; relative to ambient PAR); rice seedling emergence percentage; and single rice seedling biomass.a
a Values in parentheses are the 95% confidence limits. Values for chlorophyll a concentration and rice seedling biomass were back-transformed from (log x) + 1. Letters indicate the comparison of means between levels of algal intensity and each trait within an experimental run.
The presence and amount of nutrients (nitrogen and phosphorous) in the rice field were shown to change the intensity of algal infestation (Ohadi et al. Reference Ohadi, Madsen and Al-Khatib2019). In this experiment, we used this knowledge to produce different levels of algal infestation. When fertilizers were added at full rice field rate (75 kg P ha−1 and 150 kg N ha−1), algae grew more rapidly and densely than when fertilizers were added at half the field rate (35.5 kg P ha−1 and 75 kg N ha−1).
Chlorophyll a Concentration Response
Chlorophyll a concentration, an indicator for algal intensity level, was at its lowest in the beginning of the experiment for all the treatments and increased rapidly within 2 to 3 d in medium and high algal intensity treatments. The lowest chlorophyll a concentration was 7 μgml−1 for the low algal treatment and the highest was 1,043 μgml−1 for the high algal treatment (Figure 1).
Low, medium, and high algal infestation levels were successfully produced by addition of nitrogen, phosphorous, or copper sulfate into the experimental mesocosms (Table 2). At Run 1, the mean chlorophyll a concentration for low, medium, and high algal intensities was 109.11, 161.12, and 317.88 µg ml−1, respectively (Table 2). The chlorophyll a concentration for Run 2 was 24.49 µg ml−1 at low, 44.42 µg ml−1 at medium, and 139.84 µg ml−1 at high algal intensities. The same patterns as in Runs 1 and 2 were also observed for Run 3, as chlorophyll a concentration was 47.33, 218.64, and 319.29 µg ml−1 at low, medium, and high algal intensities.
Relationship between Algal Infestation and PAR
Comparison of means showed a significant difference between the level of PAR penetrating under the water and algal intensity levels (Table 2). Plotting the minimum, maximum, and mean PAR against the chlorophyll a concentration also showed a similar pattern (Figure 1). Overall, the amount of PAR measured in the water dramatically decreased with the increase of chlorophyll a concentration. Minimum PAR under the water reached between 0% and 20% of ambient PAR when chlorophyll a concentration was more than 500 µg ml−1 (Figure 1A). In addition, the mean PAR penetrating under the water showed a decline (50% of the ambient PAR) over time during the experiment (Figure 1C).
Changes of maximum PAR relative to chlorophyll a concentration (i.e., algal infestation level) was smaller than minimum PAR (Figure 1; Table 3), suggesting algae can significantly reduce light penetration into the water when the outside light intensity is low (e.g., cloudy days). The PAR requirement for algae and cyanobacteria species is much lower than that of terrestrial plants, and high-intensity light could destroy their photosynthetic pigments (Singh and Singh Reference Singh and Singh2015). This is more common among motile green algae species such as Chlamydomonas spp., which change their position in the water column relative to light intensity (Muller Reference Muller1995). Once the outside light intensity dropped, the algal mat became more visible on the water surface, resulting in a significant reduction of minimum PAR.
Table 3. Parameter estimates of model (Equation 1) fit to photosynthetic active radiation (PAR; minimum, maximum, and mean) and chlorophyll a concentration.
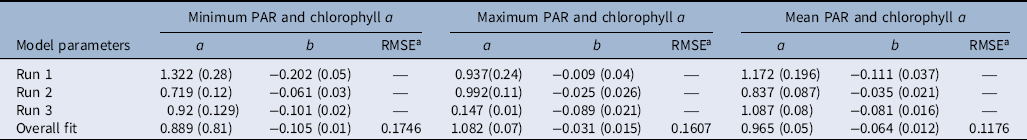
a RMSE, root mean-square error.
Algal bloom’s effect on rice is similar to its interaction with aquatic plants in a lake ecosystem. Accordingly, algal species forming the mat could be found at a different position (i.e., top vs. middle vs. bottom). Depending on their position in the mat structure, they could filter light received into the water (Kirk Reference Kirk1975). Morphological characteristics of algae such as size, shape, and pigment concentration can change light irradiance and attenuation (Kirk Reference Kirk1975). Light attenuation can influence PAR and photosynthesis, growth, and biomass production in rooted vascular plants (Scheffer et al. Reference Scheffer, Hosper, Meijer, Moss and Jeppesen1993). Therefore, a prolonged and uncontrolled algal bloom can rapidly remove plants from the system while phytoplankton species become dominant (Scheffer et al. Reference Scheffer, Hosper, Meijer, Moss and Jeppesen1993).
Previous studies showed that light intensity (i.e., quantity) can change the pattern of germination and emergence of plants (Ohadi et al. Reference Ohadi, Rahimian Mashhadi, Tavakkol-Afshari and Mesgaran2010). When the light intensity is low, the duration of receiving low light intensity becomes critical for seed germination and emergence (Ohadi et al. Reference Ohadi, Rahimian Mashhadi, Tavakkol-Afshari and Mesgaran2010). This phenomenon is more common among weed seeds, while crop seeds have no or less sensitivity to light for their emergence. Light quality, red–far-red (R:FR) ratio is another factor that can act on the seed germination and seedling emergence. When the R:FR is high, phytochromes tend to be active and promote emergence (Batlla and Benech-Arnold Reference Batlla and Benech-Arnold2014). Reduced R:FR under low-growing weed canopy altered corn (Zea mays L.) seedling growth even before interspecific competition began (Rajcan et al. Reference Rajcan, Chandler and Swanton2004). Reduction of PAR under the algal mat and low rice emergence imply a similar pattern.
Rice Seedling Emergence
Algal intensity had a significant effect on rice seedling emergence in all three runs (Tables 1 and 2). The maximum seedling emergence occurred at low algal treatment, and the minimum emergence was observed at high algal intensity treatments (Table 2); however, differences in rice seedling emergence between medium and high algal levels were small in all three runs (Table 2).
The nonlinear exponential model (Equation 2) was a suitable fit for the percent cumulative seedling emergence over time (Table 4). The germination profile was followed for at least 25 d for each run until no new emergence was observed (Figure 2). Rice started to emerge out of the water from the second (in Run 1 and Run 2) to fifth day (Run 3) after seeds were broadcast. No differences in seedling emergence were observed between the algal intensity levels at this stage (i.e., lag phase). However, the rate of seedling emergence changed dramatically between the algal infestation levels at the exponential stage. This was reflected in the slope (b) and time to 50% emergence (t 50) (Table 4). When there were no algae, rice seedling emergence reached maximum faster than in medium or high algal infestation levels (Figure 2; Table 4).
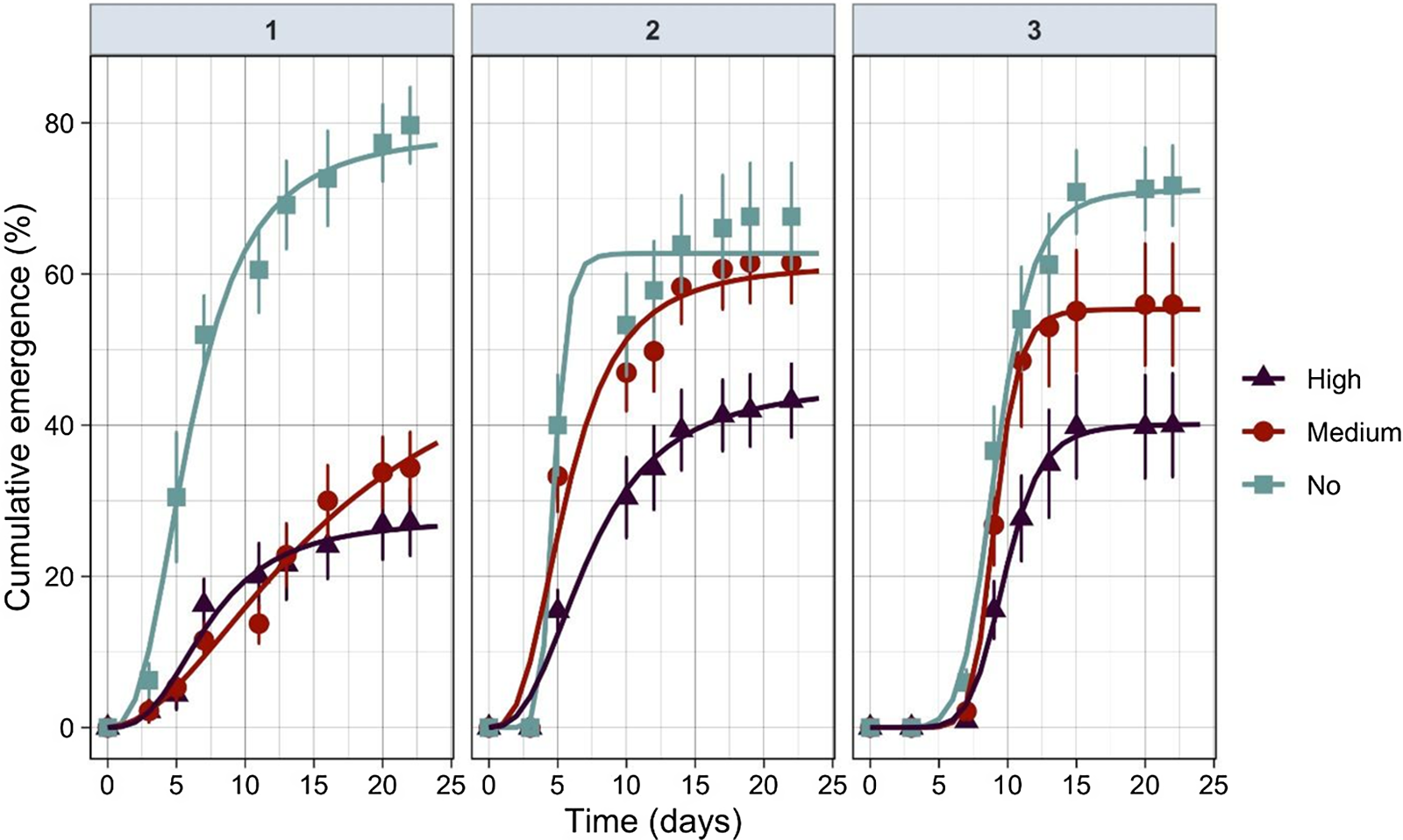
Figure 2 Relationship between cumulative emergence percentage and algal infestation levels (no, medium, and high) for each experimental run over time.
Table 4. Parameter estimates of Equation 2 fit to percent seedling emergence over time.
When plotting time to 25% and 50% seedling emergence against the chlorophyll a concentration, seedling emergence rate declined as chlorophyll a concentration increased (Figure 3). One unit increase in chlorophyll a concentration delayed rice seedling emergence by 0.005 and 0.0037 of a day. Once the chlorophyll a reached 250 µg ml−1, rice seedling emergence was delayed by 1 d. Primed rice seeds emerge within 3 to 5 d after dispersal into the rice field (Moldenhauer and Gibbons Reference Moldenhauer, Gibbons and Dilday2003). Therefore, any delay in rice seedling emergence and establishment could give an advantage to weeds in occupying the space before rice seedling emergence and decrease rice stand per area (Gibson et al. Reference Gibson, Fischer, Foin and Hill2002). Although rice may be able to compensate low density with tillering, interspecific competition with weeds may lead to yield reduction (Gibson et al. Reference Gibson, Fischer, Foin and Hill2002; Moldenhauer and Gibbons Reference Moldenhauer, Gibbons and Dilday2003).
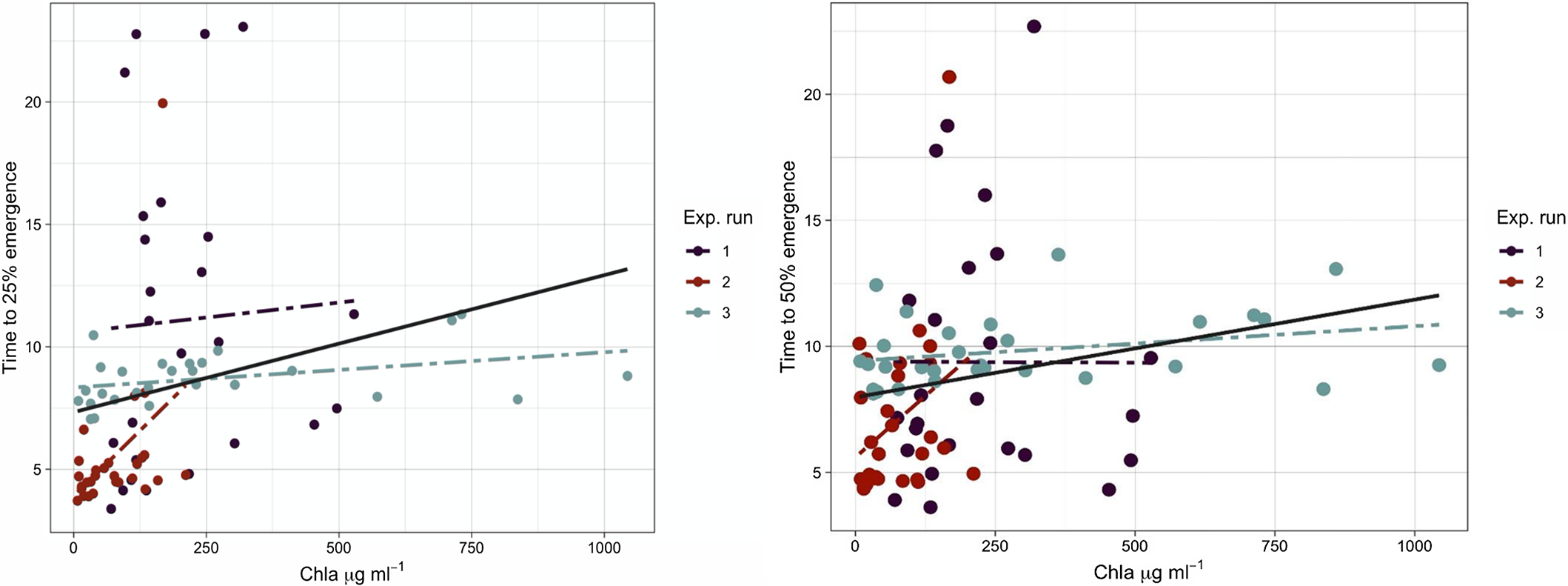
Figure 3 The relationship between rice seedling emergence rate (t 25 and t 50) and the mean chlorophyll a concentration. The solid line shows data obtained from three experimental runs fit to the model, and dashed lines show data for each run fit to the model. The regression model fit for t 25 is y = b + 0.0037x (R2 = 0.046) and for t 50 is y = b + 0054x (R2 = 0.054).
The occurrence of early algal bloom in rice fields seems to strongly effect light availability in the water, but has little effect on water temperature. We did not find any differences in water temperature between the treatments. The base temperature for rice seed germination is 10 C, and the emergence was above the optimum temperature ranges between 20 and 37 C (Moldenhauer and Gibbons Reference Moldenhauer, Gibbons and Dilday2003).
Some cyanobacteria species are known for producing toxins called cytotoxins. Microcystins, the most prominent cytotoxin group, can affect plant cell growth by DNA fragmentation and inhibiting activity of protein phosphatases 1 and 2A (MacKintosh et al. Reference MacKintosh, Beattie, Klumpp, Cohen and Codd1990; Lakshmana Rao et al. Reference Lakshmana Rao, Bhattacharya, Parida, Jana and Bhaskar1998). In a controlled experiment, microcystin concentrations above 0.12 ppm showed an inhibitory effect on rice seed germination and root elongation (Chen et al. Reference Chen, Song, Dai, Gan and Liu2004). To determine whether the inhibitory effect of algae was caused by cytotoxins, water from the experiment was sampled and tested for the amount of toxin using the Microcystins Strip Test (Eurofins Abraxis, Warminster, PA, USA). The amount of microsystin detected was less than 2.5 ppb, which did not affect rice seedling emergence in our experiment.
Rice Seedling Biomass
The relationship between mean chlorophyll a concentration and individual seedling biomass was explained by an exponential equation (Equation 3). Accordingly, individual rice seedling biomass was lower at high algal level (reflected in mean chlorophyll a concentration) than at the medium and no algae treatment (Figure 4). Given that fertilizers were added to high and medium algal level treatments, it was expected that rice seedlings would perform better in treatments with fertilizers (i.e., high and medium algae) than the control treatment (i.e., no fertilizers added). This may be because of nutrient consumption by algae rather than rice seedlings. While rice seedlings are consuming seed storage nutrients, algae are actively thriving on the nutrient resources in the environment. Further, the growth of rice seedlings out of the algal mat would require energy (i.e., competition), which would lead to rice seedling biomass reduction. Whether early rice seedling biomass reduction would be reflected in the final rice yield still needs to be explored. Our experimental conditions could not provide enough support for rice to reach maturity. Although it is tempting to conduct field experiments and test this hypothesis, uniform growth of the algae under field conditions is challenging.

Figure 4 The relationship between the mean chlorophyll a (Chla) concentration and individual seedling biomass. The parameter estimates for the fitted model are q = 0.387 (SE = 0.04), a = 646.78 (SE = 295.62), and root mean-square error (RMSE) = 0.236.
Another plausible explanation is that the excessive growth of algae can increase the water pH while reducing CO2 concentration in the water (Simpson and Eaton Reference Simpson and Eaton1986). Rice seedlings and algae, therefore, may compete for limited CO2 in the water. Reduced PAR and CO2 concentration together could impact rice seedling photosynthesis and, consequently, biomass reduction. Unfortunately, we did not measure the CO2 concentration, but pH was recorded occasionally where it varied between 8 and 10.
California rice-growing region has characteristics similar to seasonal shallow-water wetlands where algal communities occur during the wet phase of the season from late spring to early fall (Spencer et al. Reference Spencer, Lembi and Blank2006). Algal communities in rice are very dynamic and undergo temporal changes in generic composition and abundance (Spencer et al. Reference Spencer, Lembi and Blank2006). As a consequence, rice growth would be impacted either positively or negatively by this temporal shift. This research showed and quantified how algae can negatively interfere with rice in the beginning of the growing season.
Algal infestation can change the environmental properties of the water such as temperature, pH and irradiance, and PAR. In this study, we measured water temperature and light intensity (PAR) under the water, the two main factors required for seed germination and emergence. We did not find any differences in water temperature between the treatments. However, light intensity (PAR) was reduced dramatically under algal infestation. Our results showed that excessive growth of algae could impact rice by reducing the number of emerged seedlings, delaying their emergence, and resulting in biomass loss. Under natural conditions, the impact of algae on rice seedling emergence and establishment could vary greatly depending on environmental conditions and agronomic practices. This is the first study to try to quantify the negative impact of algal bloom on rice seedling emergence and establishment. In the early season, green algae and cyanobacteria blooms act similar to weeds and directly reduce the rice stand. In addition, the loss of rice stands could provide empty niches for other weeds to grow.
Acknowledgments
The authors would like to thank K.A-K.’s Weed Science Lab members for their support and assistance. Further acknowledgment goes to Mohsen Mesgaran for his statistical assistance. The funding for this project was provided by a grant from USDA–ARS entitled “Aquatic Weeds Associated with Agricultural Water Supply,” NACA agreement 58-2030-7-039. No conflicts of interest have been declared.










