Selective intra-uterine growth restriction (sIUGR) and twin-to-twin transfusion syndrome (TTTS) complicate monochorionic (MC) twin pregnancies, and are associated with a high risk of intra-uterine demise and neurological adverse outcome (Adegbite et al., Reference Adegbite, Castille, Ward and Bajoria2004, Reference Adegbite, Castille, Ward and Bajoria2005; Gratacos et al., Reference Gratacos, Carreras, Becker, Lewi, Enriquez, Perapoch and Deprest2004; Sebire et al., Reference Sebire, Snijders, Hughes, Sepulveda and Nicolaides1997; Victoria et al., Reference Victoria, Mora and Arias2001). Natural history of sIUGR in MC twin pregnancies is different to that in dichorionic (DC) twins and singletons because of the presence of placental anastomoses (Denbow et al., Reference Denbow, Cox, Taylor, Hammal and Fisk2000).
In 2007, Gratacos et al. (Reference Gratacos, Lewi, Munoz, Acosta-Rojas, Hernandez-Andrade, Martinez and Deprest2007) proposed a classification of sIUGR into three types according to the umbilical artery (UA) Doppler of the smaller twin.
Monochorionic twin management has always been challenging, and stratification into three different groups supported the decision-making process in the management of these high-risk patients. Although this classification is widely accepted in scientific community, proper studies testing its reliability are missing.
We report our experience on the application of this classification in a cohort of complicated MC twins with an abdominal circumference (AC) at or below the 10th percentile for gestational age (GA), followed from the first trimester onwards in a single fetal medicine center.
Materials and Methods
A retrospective observational study was conducted on 52 cases of MC twin pregnancies, in which one twin presented an abdominal circumference at or below the 10th percentile for GA. All cases were followed at the Department of Maternal and Child Health of Careggi University Hospital in Florence, a tertiary referral Center for Fetal Medicine, between June 2006 and June 2013. Ultrasound scans were performed from 14 weeks onwards, for every 2 weeks. In each examination, the following parameters were evaluated: fetal biometry and growth (with growth discrepancy), amniotic fluid volume in both sacs, expressed as the deepest vertical pocket (DVP), and Doppler in the UA, middle cerebral artery (MCA), and ductus venosus (DV). Discrepancy in weight was determined as (A - B) × 100/A, where A is the estimated fetal weight (EFW) of the larger twin and B is the EFW of the smaller twin. The UA Doppler was evaluated for several times in both twins in the free loop of umbilical cord with an angle of insonation <30° (Figure 1). The MCA Doppler was measured using the standard technique described previously (Mari et al., Reference Mari, Deter, Carpenter, Rahman, Zimmerman, Moise and Blackwell2000). Chorionicity was determined at the first trimester scan and confirmed by postpartum placental anatomo-pathological examination. Cases were classified into three types according to UA Doppler in the smaller twin: Type I with positive end diastolic flow in the UA; Type II with stable absent/reverse end diastolic flow (AREDF) in the UA, and Type III with intermittent AREDF (iAREDF) in the UA. Subsequently, patients were divided into two groups: the sIUGR group, and the TTTS group.

FIGURE 1 Umbilical artery Doppler measurement in a free loop of umbilical cord with an angle of insonation <30°.
Twin-to-twin transfusion syndrome was diagnosed in cases of twin oligo-polyhydramnios sequence (TOPS); sIUGR was diagnosed in the absence of oligo-polyhydramnios sequence. There were no cases of twin anemia-polycytemia sequence (TAPS), according to Lopriore's classification (Slaghekke et al., Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010). Patients were admitted for intensive fetal monitoring or delivery in cases of severe Doppler deterioration following the protocol used at our institution. If intra-uterine death (IUD) of one twin occurred, the survivor twin was followed by expectant management with MCA Doppler surveillance to detect fetal anemia, and magnetic resonance imaging (MRI) to detect brain damage. In accordance with our standard protocol, TTTS cases at stage III or higher had anastomosis laser ablations, while stages I and II were followed with expectant management, including a strict ultrasound surveillance. All neonates had an ultrasound brain scan after birth, and abnormal findings suggesting parenchymal brain damage were recorded. Postnatal angiography was not done for any case, not being a part of standard protocol at our institution.
We performed two different statistical analyses: one on the total population, including TTTS cases (total group), and the other after excluding TTTS cases (sIUGR group). The Fisher exact test and t-Student test were used to compare categorical and continuous variables, respectively. The ANOVA test (or the Welch Anova test, where indicated) was used for comparisons among study groups. For the post-hoc analysis, the Tukey test, for equal variances, or the Games–Howell test, in case of unequal variances, were used. SPSS version 20 (SPSS Inc, Chicago, IL) was used for statistical analysis. A p-value < .05 was considered significant.
Results
Fifty-two cases of MC twin pregnancies with AC ≤ 10th percentile in one twin were diagnosed during the study period. Of these, 37 were classified as Type I, 12 as Type II, and 3 as Type III cases. Pregnancy course and perinatal outcome in different groups are reported in Table 1.
TABLE 1 Pregnancy Course and Perinatal Outcome According to Umbilical Artery Doppler Classification in Different Groups of the Total Group

Categorical variables are presented as % (n/total), continuous variables are presented as mean values (interquartile range).
Statistics: Chi-square test, Kruskall Wallis test, One-way Anova or Welch Anova test. Post-hoc analysis performed using the Games–Howell test or the Tukey post-hoc test.
*p < .01 versus Type I; † p < .05 versus Type I; § p < .001 versus type I.
IUFD = Intra-uterine fetal demise.
Types II and III cases were diagnosed at a lower GA compared with Type I cases (p < .01). Types II and III cases were delivered significantly earlier than Type I cases (p < .05), with a mean gestational age of 34.1 weeks for Type I, 31.8 weeks for Type II, and 30.6 weeks for Type III cases. The birth weight of the larger twin was significantly lower in Type II and III cases compared with Type I cases (p < .01). The birth weight of the smaller twin was significantly lower in Type II and III cases compared with Type I cases (p < .001). The fetal weight discordance was significantly higher in Type II than in Type I cases (p < .001). Progressive fetal deterioration of the smaller fetus requiring active management was observed in 66.7% of Type II and in 11.1% of Type I cases (p < .001), and in no case of Type III. Unexpected fetal death of the smaller twin was observed more frequently in Type III (two cases, 66.7%) than in Types I and II cases (5% and 33%, respectively). In those two cases, the previous ultrasound scan showed normal MCA and DV Doppler. In one case, a cesarean section was performed at 29 weeks for maternal indication (maternal hypertension with nephropathy). In the other case, the survivor twin had a multi-cystic encephalopathy confirmed by a prenatal MRI, and the couple opted for termination of pregnancy (TOP). Unexpected death of the larger twin occurred only in three Type II cases.
Among the 52 cases, TTTS with oligo-polyhydramnios sequence was diagnosed in 10 cases. The remaining 42 cases were therefore defined as sIUGR. Thirty-one were classified as Type I, eight as Type II, and three as Type III cases. Pregnancy course and perinatal outcome in different groups are reported in Table 2.
TABLE 2 Pregnancy Course and Perinatal Outcome According to Umbilical Artery Doppler Classification in Different Groups of Fetuses With sIUGR
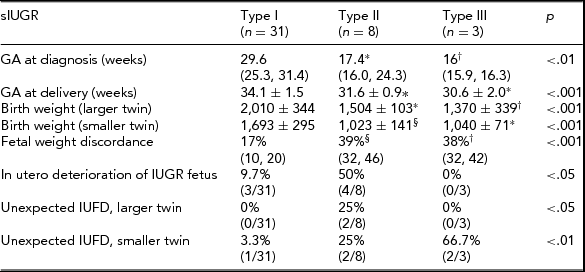
Categorical variables are presented as % (n/total), continuous variables are presented as mean values (interquartile range).
Statistics: Chi-square test, Kruskall Wallis test, One-way ANOVA or Welch ANOVA test. Post-hoc analysis performed using the Games–Howell test or the Tukey post-hoc test.
*p < .01 versus Type I; † p < .05 versus Type I; § p < .001 versus Type I.
IUFD = Intra-uterine fetal demise.
In the sIUGR group, results were similar to those obtained for the whole population (all cases of sIUGR and TTTS). Type II and Type III sIUGR cases were delivered significantly earlier than Type I cases (p < .001). The birth weight of the larger twin was significantly lower in Types III and II cases compared with Type I cases (p = .001). In Types II and III cases, the birth weight was significantly lower than in Type I cases (p < .001) for both twins. Fetal weight discordance was significantly higher in Types II and III than in Type I cases (p < .001). Progressive fetal deterioration of the IUGR fetus requiring active management was observed in 50% of Type II, 9.7% of Type I cases, and in no case of Type III. Unexpected fetal death was observed in 66.7% (2/3) cases of Type III compared with 3.3% and 25% cases of Types I and II respectively. In both cases, the smaller twin died, and at the previous ultrasound scan, the MCA and DV Doppler were normal.
Considering the neonatal outcome for babies who survived, the only structural anomaly identified was a double aortic arch, which was detected antenatally.
An ultrasound brain scan performed after birth revealed normal findings in all neonates except one, who presented a choroid plexus hemorrhage at 10 months of life, which was resolved by 14 months of age. Another baby from the sIUGR group presented motor impairment at 18 months of age, with no evidence of brain lesion in brain ultrasound.
Discussion
Our study confirms that the UA Doppler pattern in the smaller twin is a reliable way to classify complicated MC twins and to predict their clinical course and perinatal outcome. The use of UA Doppler in singletons and dichorionic twins complicated by IUGR is well established for their surveillance and timing of delivery. In fact, the finding of absent end diastolic flow (AEDF) in the UA artery in singletons has been associated with poor perinatal outcome (Vanderheyden et al., Reference Vanderheyden, Fichera, Pasquini, Tan, Wee, Frusca and Fisk2005), and it has been shown (Arduini et al., Reference Arduini, Rizzo and Romanini1993) that the mean latency between diagnosis of AEDF and delivery in singletons is 7–10 days, and this has also been confirmed in dichorionic twins (Vanderheyden et al., Reference Vanderheyden, Fichera, Pasquini, Tan, Wee, Frusca and Fisk2005). Further, it has been demonstrated that the UA Doppler waveforms, which reflect the adequacy of trophoblastic invasion, have similar resistance indices in MC and dichorionic twin pregnancies (Yu et al., Reference Yu, Papageorghiou, Boli, Cacho and Nicolaides2002). However, the interpretation of UA waveforms in MC twin pregnancies is different from that in dichorionic because UA Doppler does not simply reflect trophoblastic invasion but also the presence and the kind of cotiledonary or AA anastomoses, and the balance between both twins’ circulation. For this reason, the evaluation of a UA Doppler pattern in this type of twin pregnancy is difficult, and Gratacos's classification, based on UA patterns, allows for risk stratification and proper counseling.
In our experience, and in agreement with previous reports (Gratacos et al., Reference Gratacos, Lewi, Munoz, Acosta-Rojas, Hernandez-Andrade, Martinez and Deprest2007), Type III group is the most difficult one to evaluate and counsel in both cases: whether we consider the whole group or only the sIUGR group. In fact, Type III had the lowest GA at presentation (total group p < .05; sIUGR p < .05) and at delivery (total group p < .05; p < .01), and this group was characterized by the absence of in utero deterioration until birth or IUD. This specific UA Doppler pattern has been attributed to retrograde transmission of a large arterio-arterial anastomoses (AAA; Wee et al., Reference Wee, Taylor, Vanderheyden, Talbert and Fisk2003). In our experience, we had only three cases of Type III UA Doppler: in two cases, the smaller twin died unexpectedly, with normal UA, MCA, and DV Doppler at the previous scan. Our study confirms that Type III is characterized by being rare (3/42); it does not show deterioration during pregnancy and has a high rate of unexpected IUD determining an unpredictable outcome. For this reason, it cannot be easily monitored based on signs of fetal deterioration in the smaller twin.
Type II group had a much worse prognosis than Type I, with earlier GA at diagnosis (total group p < .05; sIUGR group p < .01) and at delivery (total group p < .01; sIUGR group p < .01), the lowest mean birth weight of smaller (total group p < .01; sIUGR group p < .001) and larger twins (total group p < .01; sIUGR group p < .01), and a high rate of IUD (total group: smaller twin 33% and larger twin 25%; sIUGR group: smaller twin 25% and larger twin 25%). These results are similar to those of Ishii et al. (Reference Ishii, Murakoshi, Takahashi, Shinno, Matsushita, Naruse and Nakata2009), who reported 29.6% IUD of smaller twin and 22.2% IUD of larger twin. This group had a high rate of deterioration (total group 67%; sIUGR group 50%), but differently from group III, this could be predicted by Doppler, allowing prenatal treatment or delivery. As described by other authors, fetal deterioration could be predicted in the majority of cases (Gratacos et al., Reference Gratacos, Lewi, Munoz, Acosta-Rojas, Hernandez-Andrade, Martinez and Deprest2007). There was also the highest weight discordance (39%) present in this group.
Type I was the group with the best outcome, with a mean GA at delivery of 34.1 ± 1.5 weeks for the total group and 34.1 ± 1.6 weeks for the sIUGR group, the highest fetal weight and the lowest rate of unexpected IUD. The strong point of our work is to give more evidence to the use of UA Doppler in complicated MC pregnancies. The strengths of our study are the relatively large number of cases included and the validation of Gratacos’ classification in the Italian population.
We acknowledge the retrospective nature of the study as a limitation. However, our study has similar results as that of the previous ones (Denbow et al., Reference Denbow, Cox, Taylor, Hammal and Fisk2000; Ishii et al., Reference Ishii, Murakoshi, Takahashi, Shinno, Matsushita, Naruse and Nakata2009), and highlight that the results are similar when we consider the whole group (total group) and the sIUGR group.
In conclusion, classification of complicated MC twins based on UA Doppler is particularly important for counseling, even when we include TTTS cases, and permits the prediction of clinical evolution and perinatal outcome. If Type I pattern is observed and confirmed at subsequent scans, intensive monitoring is necessary but parents can be reassured about the probable good prognosis of pregnancy. In Type II cases, parents should be aware of possible progression and possible poor prognosis because of the risk of in utero deterioration and IUD. In Type III cases, parents should be aware that to predict and avoid IUD is not always possible.





