Heat stress is of great concern in all types of poultry operations, as the poultry are very susceptible to high environmental temperature owing to the lack of sweat glands and fast metabolic rates. High environmental temperature negatively influenced the performance of commercial laying hens( Reference Mashaly, Hendricks and Kalama 1 ) and broiler breeders( Reference McDaniel, Bramwell and Wilson 2 , Reference Zhu, Xie and Li 3 ) by reducing feed intake, egg production performance and eggshell quality. In addition to altered productive performance, heat stress could also disturb the redox balance and induce oxidative stress, with the production of reactive oxygen species (ROS), in broiler breeders( Reference Xie, Tang and Lu 4 ) and commercial hens( Reference Lin, De Vos and Decuypere 5 ). In addition, heat stress increases the synthesis of heat-shock proteins (HSP), which are thought to play an important role in cellular protection under high ambient temperature, with a proposed relationship between the development of thermotolerance and HSP synthesis( Reference Burdon 6 ). Several methods are available in alleviating the negative effect of high environmental temperature on the performance of broiler breeders. Because it is expensive to cool buildings, such methods have focused mostly on dietary manipulations. The Zn has an important role in numerous biological processes, and it has both structural and catalytic functions in more than 300 metalloenzymes( Reference Vallee and Auld 7 ). One of the most important functions of Zn is related to its antioxidant role and its participation in the antioxidant defence system. Oxidative damage of the cell membrane by free radicals occurs during Zn deficiency( Reference Oteiza, Olin and Fraga 8 ), thus altering the status of antioxidant enzymes and substances( Reference Zago and Oteiza 9 ). Two of the most common inorganic Zn supplements in broiler diets are ZnSO4 and ZnO. However, recent studies in our laboratory have demonstrated that the organic Zn with a moderate chelation strength (Q f value=30·7) had higher absorption and bioavailability than the inorganic ZnSO4, and both liver and pancreas are sensitive tissues to reflect the Zn status of broilers( Reference Huang, Lu and Li 10 – Reference Liu 15 ). Nevertheless, it is not clear whether dietary supplementation with Zn, especially the organic Zn with moderate chelation strength, can reduce oxidative damage and increase heat stress resistance with the enhancement of antioxidant ability in broiler breeders. As mentioned above, the broiler breeders are very susceptible to heat stress, and a continuous environmental temperature of 32°C was used for successfully setting up the heat stress model of Arbor Acres broiler breeders in our previous study( Reference Xie, Tang and Lu 4 ). Moreover, the dietary Zn requirement (110 mg/kg) of Arbor Acres broiler breeders is the highest among the different types of breeders( Reference Leeson and Summer 16 ). Therefore, the heat stress model of Arbor Acres broiler breeders may be a good heat stress model, which could be used to evaluate the effect of dietary Zn source. It is hypothesised that dietary Zn supplementation might alleviate the adverse effect of heat stress on laying broiler breeders by enhancing the antioxidant response. Therefore, the objective of this study was to investigate the effects of environmental temperature and dietary Zn on egg production performance, egg quality, tissue Zn content, antioxidant status and mRNA levels of HSP in tissues of laying broiler breeders so as to determine whether dietary supplementation with Zn, especially the organic Zn with a moderate chelation strength, can reduce the detrimental effect of high temperature on laying broiler breeders.
Methods
Experimental design and treatments
A completely randomised design involving a 2 (environmental temperatures)×3 (dietary Zn sources) factorial arrangement of treatments was used in this experiment. The two environmental temperatures were a normal temperature of 21±1°C (NT) and a high temperature of 32±1°C (HT). The three dietary Zn sources were a semi-purified basal diet without Zn supplementation (CON) and the basal diet supplemented with 110 mg of Zn/kg of diet on an as-fed basis as either the inorganic Zn sulphate (ZnSO4.7H2O, iZn) or the organic Zn proteinate with a moderate chelation strength (oZn). Thus, there were a total of six different treatments (NT–CON, NT–iZn, NT–oZn, HT–CON, HT–iZn and HT–oZn).
Birds and diets
All experimental procedures were approved by the Animal Management Committee (in charge of animal welfare issue) of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS-CAAS, Beijing, China), and performed in accordance with the guidelines. Ethical approval on animal survival was given by the animal ethics committee of IAS-CAAS. We have followed the ARRIVE guidelines for reporting animal research( Reference Kilkenny, Browne and Cuthill 17 ). In all, 144 23-week-old female Arbor Acres broiler breeders with similar body weights (2720 (sem 41) g) were purchased from a commercial company (Huadu Broiler Company) and randomly allotted to one of six treatments with six replicates of four birds per replicate for each treatment. Four birds in each replicate were kept in two neighbouring cages, with two birds per cage. All broiler breeders were handled in accordance with the Arbor Acres breeder management guidelines for lighting and feeding and allowed ad libitum access to tap water containing 0·12 μg Zn/ml during the adaptation period from 23 to 29 weeks of age. The birds were housed in electrically heated, thermostatically controlled room with fibreglass feeders, waterers and stainless-steel cages coated with plastic. After the adaptation period (7 weeks), all broiler breeders were fed the maize starch–soya isolated protein meal purified diet (Table 1) without Zn addition to deplete the storage of Zn from 30 to 32 weeks. During the depletion, broiler breeders were adjusted appropriately to maintain similar laying rates in each treatment at the beginning of the formal experiment. After the depletion, the room temperature for the broiler breeders of NT–CON, NT–iZn and NT–oZn was maintained at 21±1°C, whereas the room temperature for broiler breeders of HT–CON, HT–iZn and HT–oZn was increased stepwise from 21 to 32°C over 3 d for these birds to acclimatise to the experimental chronic heat challenge and then maintained at 32±1°C for the rest time of the experiment. Relative humidity was kept at 40±5 % for the two rooms during the experimental period of 9 weeks (33–42 weeks of age).
Table 1 Composition and nutrient levels of the basal diets for laying broiler breeders (as-fed basis)
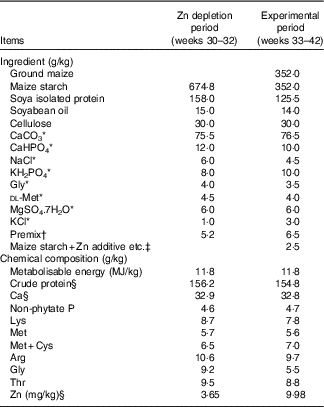
* Reagent grade.
† Provided per kg of diet during Zn depletion period: retinol, 1·5 mg; cholecalciferol, 0·01 mg; α-tocopheryl acetate, 50·0 mg; menadione, 1·5 mg; thiamin, 13·4 mg; riboflavin, 15·0 mg; pyridoxine, 4·5 mg; cyanocobalamin, 0·02 mg; pantothenate, 18·0 mg; niacin, 50·0 mg; folic acid, 6·0 mg; biotin, 0·60 mg; choline, 1500 mg; Cu (CuSO4.5H2O), 10 mg; Fe (FeSO4.7H2O), 50 mg; Mn (MnSO4.H2O), 120 mg; iodine (KI), 1·2 mg; Se (NaSeO3), 0·30 mg; Mo (Na2MoO4.2H2O), 8·3 mg. Provided per kg of diet during experimental period: retinol, 3·8 mg; cholecalciferol, 0·09 mg; α-tocopheryl acetate, 50·0 mg; menadione, 4·4 mg; thiamin, 6·6 mg; riboflavin, 12·0 mg; pyridoxine, 4·5 mg; cyanocobalamin, 0·02 mg; pantothenate, 15·5 mg; niacin, 50·0 mg; folic acid, 2·0 mg; biotin, 0·22 mg; choline, 2000 mg; Cu (CuSO4.5H2O), 10 mg; Fe (FeSO4.7H2O), 50 mg; Mn (MnSO4.H2O), 120 mg; iodine (KI), 1·2 mg; Se (NaSeO3), 0·30 mg; Mo (Na2MoO4.2H2O), 8·3 mg.
‡ Zn additive, Lys-HCl or dl-Met were added to diets by replacing an equal weight of maize starch.
§ These values were determined by analysis based on triplicate determinations; other values in the table are as formulated.
Both body weight and rectal temperature of broiler breeders were measured at 08.30 hours on the start and the last day of different periods to check whether these broiler breeders were maintained in the standard body weight range and heat exposure stage, respectively. The rectal temperature was monitored using a thermo-code electric gauge (JM222; JinMing) with an accuracy of 0·1°C. Eggs were collected daily at 14.30 hours and the number of eggs and egg weight in each replicate were recorded. The feed intake of broiler breeders in each replicate was recorded each day. When broiler breeders in HT had lower feed intake than those in NT, to eliminate the potential effect of reduced feed intake under HT, broiler breeders in NT were pair-fed the same amount of feed consumed by broiler breeders with feed restriction in HT on the previous day.
The basal diets for the depletion period (maize starch-isolated soyabean protein purified diet) and experimental period (maize–maize starch-isolated soyabean protein semi-purified diet) were formulated to meet or exceed the nutrient requirements for laying broiler breeders (National Research Council, 1994)( 18 ), except for Zn (the Zn requirement is 110 mg/kg( Reference Leeson and Summer 16 ), Table 1). The Zn sulphate (ZnSO4.7H2O) was reagent grade (Beijing Chemical Company) and contained 22·5 % Zn on a basis of analysis (purity>99 %). The Zn proteinate was provided by a special commercial company and contained 10·9 % Zn on a basis of analysis (purity>99 %). The chelation strength (Q f value) of the Zn proteinate was analysed to be 30·7, which is categorised as a moderate chelation strength based on the classification of Holwerda et al.( Reference Holwerda, Albin and Madsen 19 ). A single batch of basal diet was mixed and then divided into three aliquots according to the experimental treatments. Lysine and methionine levels in the control diet or the diet supplemented with the Zn sulphate were balanced by adding synthetic lysine-HCl and dl-methionine based on supplemental amounts of lysine and methionine from the Zn proteinate source. The analysed Zn concentrations in the diets were 9·98, 116 and 120 mg/kg for CON, iZn and oZn, respectively.
Sample collections and preparations
Samples of the Zn sources, diets and tap water were collected for analyses of Zn, Ca or dietary crude protein (CP) contents. In each replicate, two eggs based on the average egg weight were collected on the last 3 consecutive days of weeks 40 and 42 for the measurements of egg quality. At the end of the experiment, two birds from each replicate were selected based on body weight and slaughtered humanely by carbon dioxide asphyxiation. Liver and pancreas samples were collected immediately. A set of tissue sub-samples were snap-frozen in liquid N2 and then stored at −80°C for the mRNA level analysis, whereas another set of sub-samples were kept on ice and stored at −20°C for subsequent measurements of Zn content, malondialdehyde (MDA) and metallothionein (MT) levels, as well as Cu Zn superoxide dismutase (CuZnSOD) activity. All samples from two birds in each replicate were pooled into one sample in equal ratios before analysis.
Measurements of zinc, calcium, crude protein and Q f value of zinc proteinate
The Zn concentrations in Zn sources, diets, water and tissues were determined by inductively coupled plasma emission spectroscope (model IRIS Intrepid II; Thermal Jarrell Ash) after wet digestions with HNO3 and HCIO4, as described by Huang et al. ( Reference Huang, Lu and Li 10 ). The lowest limit of Zn detection is 0·05 mg/kg. Validation of the Zn analysis was conducted concurrently using bovine liver powder (GBW (E) 080193; National Institute of Standards and Technology) as a standard reference material (SRM). The actual Zn recovery rates for the bovine liver SRM were determined to be about 99 % in the present study. Contents of CP and Ca in feed ingredients and diets were determined using Association of Official Analytical Chemists methods( 20 ). The quotient of formation (Q f) value of Zn proteinate was determined using polarography( Reference Holwerda, Albin and Madsen 19 , Reference Li, Luo and Liu 21 ).
Determinations of malondialdehyde and metallothionein levels and copper zinc superoxide dismutase activity in tissues
The liver and pancreas were homogenised in 10 % (w/v) ice-cold physiological saline, and then sonicated with an ultrasonic wave cell grinder (JY92-11; Ningbo Xinzhi Bio-technology Co., Ltd) for 1 min (1 s with 2-s interval). The homogenates were centrifuged at 1500 g for 15 min at 4°C and supernatants were collected to determine total protein contents, and MDA and MT levels and CuZnSOD activity. Total protein contents were determined using a BCA protein assay kit (Cat no. 23225; Pierce). The MDA levels in the supernatants were determined using a commercial assay kit (Cat no. A003-1; Nanjing Jiancheng Bioengineering Institute). The total superoxide dismutase (TSOD) and Mn superoxide dismutase (MnSOD) activities were measured according to the nitrite method described by Li et al.( Reference Li, Luo and Liu 21 ), and CuZnSOD activity was calculated by subtracting MnSOD activity from TSOD activity. The MT concentration was measured by Cd2+–Hb affinity assay( Reference Eaton and Toal 22 ) with inductively coupled plasma emission spectroscope (Model IRIS Intrepid II). The concentration of MT was calculated using a Cd-MT binding stoichiometry of 7:1.
RNA extraction, reverse transcription and real-time quantitative PCR
Total RNA was isolated from liver and pancreas using Trizol reagent (Invitrogen) according to the manufacturer’s instruction. The concentration of each isolated RNA sample was determined using a NanoDrop Spectrophotometer (ND-2000; Gene Company Ltd), and the integrity of the RNA was checked using denatured RNA electrophoresis. A total of 1 μg of RNA was used to obtain complementary DNA by reverse transcription using the Super Script First-Strand Synthesis System (Invitrogen). Real-time quantitative PCR reactions were performed on an ABI 7500 real-time quantitative PCR system using SYBR-Green PCR Master Mix (Applied Biosystems). The primer sequences for CuZnSOD, MT, HSP70, HSP90, β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are given in Table 2. The protocol of PCR was as follows: denaturation at 95°C for 10 min, followed by forty cycles of 94°C for 15 s and 60°C for 1 min. The
![]() $$2^{{{\minus}\Delta \Delta C_{T} }} $$
was used to calculate the mRNA level of each target gene(
Reference Livak and Schmittgen
23
). The geometric mean of internal reference genes, β-actin and GAPDH, was used to normalise the expression level of the targeted gene. The run was performed in triplicate.
$$2^{{{\minus}\Delta \Delta C_{T} }} $$
was used to calculate the mRNA level of each target gene(
Reference Livak and Schmittgen
23
). The geometric mean of internal reference genes, β-actin and GAPDH, was used to normalise the expression level of the targeted gene. The run was performed in triplicate.
Table 2 Primers used for the target and reference genes

CuZnSOD, copper zinc superoxide dismutase; MT, metallothionein; HSP70 and HSP90, heat-shock protein 70 and heat-shock protein 90;GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analyses
The sample size calculation was performed using software PASS 13 (NCSS, LLC). In our previous study( Reference Suo, Lu and Zhang 24 ), the means of MT mRNA expression levels in pancreas of broilers at 21 d of age for CON and supplemental oZn (90 mg/kg) treatments were 0·88 and 1·81 with a pooled sd of 0·51. On the basis of the same effect size, five replicates per treatment are needed to achieve 80 % power using a two-sided two-sample t test at a significance level of 0·05. Our proposed sample size of six replicates per treatment may provide more power when considering multiple comparisons( Reference Chow, Wang and Shao 25 ). Data from the present study were subjected to two-way ANOVA using the general linear model procedure of SAS 9.2 (SAS Institute Inc.), and the model included the main effects of environmental temperature, dietary Zn and their interaction. The replicate of four birds for egg production performance or two birds for other indices served as the experimental unit. Percentage data were transformed to arcsine for analysis. Differences among means were tested by the least significant difference method, and the statistical significance was set at P≤0·05.
Results
Rectal temperature
Before heat exposure, environmental temperature, dietary Zn and their interactions did not affect (P>0·14) the rectal temperature of broiler breeders at 224 d of age (Table 3). After heat exposure, dietary Zn and the interaction between environmental temperature and dietary Zn did not affect (P>0·89) the rectal temperature of broiler breeders at 291 d of age; however, environmental temperature affected (P<0·0001) it. Broiler breeders at 291 d of age had greater (P<0·0001) rectal temperature value in HT than in NT.
Table 3 Effects of environmental temperature (TEMP) and dietary zinc on rectal temperature (°C) of laying broiler breeders (Mean values with their standard errors)
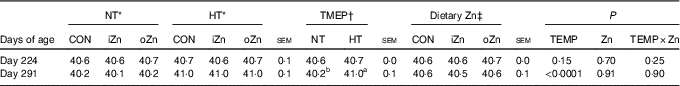
NT, normal temperature; HT, high temperature; CON, Zn-unsupplemented basal diet; iZn, basal diet supplemented with 110 mg Zn/kg as the inorganic Zn sulphate; oZn, basal diet supplemented with 110 mg Zn/kg as the organic Zn proteinate with a moderate chelation strength (Q f value=30·7).
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·0001).
* The values represented the means of six replicates (n 6).
† The values represented the means of eighteen replicates (n 18).
‡ The values represented the means of twelve replicates (n 12).
Egg production performance
Broiler breeders in NT and HT groups were pair-fed and had similar feed intakes (Table 4). Egg weight, laying rate, broken egg rate, misshapen egg rate and feed:egg ratio were affected (P≤0·01) by environmental temperature, but not (P>0·25) by dietary Zn and the interaction between environmental temperature and dietary Zn. Soft-shell egg rate and body weight were not affected (P>0·26) by environmental temperature, dietary Zn and their interaction. Compared with NT, HT decreased (P<0·0003) egg weight and laying rate, but increased (P<0·02) broken egg rate, misshapen egg rate and feed:egg ratio.
Table 4 Effects of environmental temperature (TEMP) and dietary zinc on egg production performance of laying broiler breeders (Mean values with their standard errors)
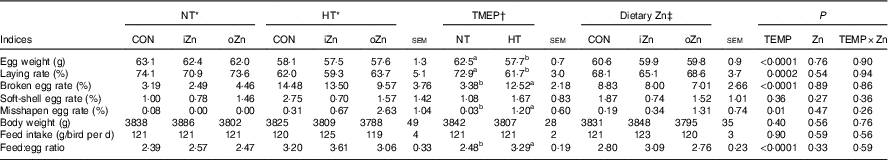
NT, normal temperature; HT, high temperature; CON, Zn-unsupplemented basal diet; iZn, basal diet supplemented with 110 mg Zn/kg as the inorganic Zn sulphate; oZn, basal diet supplemented with 110 mg Zn/kg as the organic Zn proteinate with a moderate chelation strength (Q f value=30·7).
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·02).
* The values represented the means of six replicates (n 6).
† The values represented the means of eighteen replicates (n 18).
‡ The values represented the means of twelve replicates (n 12).
Egg quality
Dietary Zn and the interaction between environmental temperature and dietary Zn did not affect (P>0·11) eggshell strength, thickness and weight of broiler breeders at 40 and 42 weeks of age; however, environmental temperature affected (P≤0·001) them (Table 5). Environmental temperature, dietary Zn and their interaction had no effect (P>0·11) on the haugh unit and yolk colour of broiler breeders at 40 and 42 weeks of age. Compared with NT, HT decreased (P<0·002) eggshell strength, thickness and weight.
Table 5 Effects of environmental temperature (TEMP) and dietary zinc on egg quality of laying broiler breeders (Mean values with their standard errors)

NT, normal temperature; HT, high temperature; CON, Zn-unsupplemented basal diet; iZn, basal diet supplemented with 110 mg Zn/kg as the inorganic Zn sulphate; oZn, basal diet supplemented with 110 mg Zn/kg as the organic Zn proteinate with a moderate chelation strength (Q f value=30·7).
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·002).
* The values represented the means of six replicates (n 6).
† The values represented the means of eighteen replicates (n 18).
‡ The values represented the means of twelve replicates (n 12).
Zinc contents
Environmental temperature and the interaction between environmental temperature and dietary Zn did not affect (P>0·17) Zn content in the liver; however, dietary Zn affected (P=0·006) it (Table 6). Environmental temperature, dietary Zn and their interaction affected (P<0·04) Zn content in the pancreas. Broiler breeders fed the oZn diet had higher (P<0·003) Zn content in the liver compared with those fed the CON diet, with no differences (P>0·63) between the two Zn sources and between the CON and the iZn. No difference (P>0·13) in pancreas Zn content was observed among all treatment groups under NT; however, under HT, broiler breeders fed either the iZn or the oZn diet had higher (P<0·03) Zn content in the pancreas compared with those fed the CON diet, and broiler breeders fed the oZn diet had higher (P<0·05) Zn content in the pancreas compared with those fed the iZn diet.
Table 6 Effects of environmental temperature (TEMP) and dietary zinc on tissue zinc contents of laying broiler breedersFootnote * (Mean values with their standard errors)

NT, normal temperature; HT, high temperature; CON, the Zn-unsupplemented basal diet; iZn, the basal diet supplemented with 110 mg Zn/kg as the inorganic Zn sulphate; oZn, the basal diet supplemented with 110 mg Zn/kg as the organic Zn proteinate with a moderate chelation strength (Q f value=30·7).
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* The Zn contents in liver and pancreas were on a fresh-weight basis.
† The values represented the means of six replicates (n 6).
‡ The values represented the means of eighteen replicates (n 18).
§ The values represented the means of twelve replicates (n 12).
Tissue copper zinc superoxide dismutase activity and malondialdehyde and metallothionein levels
CuZnSOD activities and MDA and MT levels in liver and pancreas of broiler breeders are listed in Table 7. No interactions (P>0·14) between environmental temperature and dietary Zn were observed in all of the above-mentioned indices. Environmental temperature affected (P≤0·05) CuZnSOD activities in liver and pancreas, as well as MT level in the pancreas, but did not affect (P>0·05) MDA and MT levels in the liver and MDA level in the pancreas of broiler breeders. Dietary Zn affects (P≤0·04) MT levels, but had no effect (P>0·24) on CuZnSOD activities and MDA levels in the liver and pancreas of broiler breeders. Compared with NT, HT increased (P≤0·05) CuZnSOD activities in liver and pancreas, as well as MT level in the pancreas. Broiler breeders fed the diet supplemented with either iZn or oZn had higher (P<0·04) MT level in the liver compared with those fed the CON diet, with no difference (P>0·84) between the two Zn sources. Broiler breeders fed the oZn diet had higher (P<0·01) MT level in the pancreas compared with those fed the CON diet, with no differences (P>0·08) between the iZn group and CON and between the two Zn sources.
Table 7 Effects of environmental temperature (TEMP) and dietary zinc on tissue copper zinc superoxide dismutase (CuZnSOD) activity and malondialdehyde (MDA) and metallothionein (MT) levels of laying broiler breeders (Mean values with their standard errors)

NT, normal temperature; HT, high temperature; CON, Zn-unsupplemented basal diet; iZn, basal diet supplemented with 110 mg Zn/kg as the inorganic Zn sulphate; oZn, basal diet supplemented with 110 mg Zn/kg as the organic Zn proteinate with a moderate chelation strength (Q f value=30·7); NU, nitrite units.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* The values represented the means of six replicates (n 6).
† The values represented the means of eighteen replicates (n 18).
‡ The values represented the means of twelve replicates (n 12).
§ One NU was defined as the amount of enzyme needed to obtain 50 % inhibition of nitrite formation.
mRNA levels of copper zinc superoxide dismutase, metallothionein and heat-shock proteins in tissues
Dietary Zn and the interaction between environmental temperature and dietary Zn did not affect (P>0·16) the mRNA levels of CuZnSOD, MT, HSP70 and HSP90 in liver and pancreas (Table 8). Environmental temperature affected (P≤0·05) HSP70 mRNA levels in liver and pancreas, but not (P>0·06) mRNA levels of other indices. Compared with NT, HT up-regulated (P≤0·05) HSP70 mRNA levels in liver and pancreas.
Table 8 Effects of environmental temperature (TEMP) and dietary zinc on mRNA levels (relative quantities (RQ)) of copper zinc superoxide dismutase (CuZnSOD), metallothionein (MT) and heat-shock proteins (HSP) in the tissues of laying broiler breedersFootnote * (Mean values with their standard errors)

NT, normal temperature; HT, high temperature; CON, Zn-unsupplemented basal diet; iZn, basal diet supplemented with 110 mg Zn/kg as the inorganic Zn sulphate; oZn, basal diet supplemented with 110 mg Zn/kg as the organic Zn proteinate with a moderate chelation strength (Q f value=30·7).
a,b Mean values within a row with unlike superscript letters were significantly different (P≤0·05).
* The mRNA expression were calculated as the RQ of the target gene mRNA to the geometric mean of β-actin mRNA and glyceraldehyde-3-phosphate dehydrogenase, RQ=
![]() $$2^{{{\minus}\Delta \Delta C_{T} }} $$
(C
T
=threshold cycle).
$$2^{{{\minus}\Delta \Delta C_{T} }} $$
(C
T
=threshold cycle).
† The values represented the means of six replicates (n 6).
‡ The values represented the means of eighteen replicates (n 18).
§ The values represented the means of twelve replicates (n 12).
Discussion
Heat stress has a highly detrimental effect on growth performance of broilers and egg production of laying hens( Reference Smith 26 , Reference Yalcin, Ozkan and Turkmut 27 ). Moreover, researches on heat stress in laying hens have indicated a consistent decrease in egg weight and eggshell thickness( Reference Wolfenson, Frei and Snapir 28 , Reference Emery, Vohra and Ernst 29 ). The results from the present study also showed that HT negatively influence the egg production performance and egg quality of laying broiler breeders. The reduced egg production, quality and feed efficiency in heat-exposed broiler breeders might be owing to the reduction in utilisation of nutrients. Moreover, it was found that the declined reproductive performance in the acutely heat-stressed hens was mediated by reduced luteinising hormone-releasing ability of the hypothalamus( Reference Donoghue, Krueger and Hargis 30 ). The Zn is an important component of many enzymes, and it is required for optimum performance. It is also a component of the carbonic anhydrase enzyme, which is crucial for supplying the carbonate ions during eggshell formation. Inhibition of this enzyme resulted in lowered bicarbonate ion secretion and, consequently, greatly reduced eggshell weight( Reference Nys, Gautron and Mckee 31 ). However, there are conflicting reports on the influence of Zn on performances in stressed birds. Sahin & Kucuk( Reference Sahin and Kucuk 32 ) reported linear increases in feed intake and egg production and improved feed efficiency and egg quality upon ZnSO4 supplementation to quail reared under heat stress condition. Moreng et al.( Reference Moreng, Balnave and Zhang 33 ) found that dietary supplementation of Zn as Zn-methionine improved eggshell weight and reduced eggshell defects in hens exposed to high temperatures. However, in the present study, dietary Zn addition did not influence egg production performance and egg quality of broiler breeders. Tabatabaie et al.( Reference Tabatabaie, Aliarabi and Saki 34 ) also reported that Zn source or Zn level had no effect on egg production performance, egg weight or feed:egg ratio of laying hens. These above inconsistencies may have been owing to the differences in diet type, dietary Zn treatment, feed intake, experimental duration time and other factors.
The negative effect of heat stress on mineral balance in broilers has been previously reported( Reference Belay, Wiernusz and Teeter 35 ). Heat stress may exacerbate a marginal mineral deficiency or lead to increased mineral requirements. Dietary supplementation of Zn could increase Zn content in the liver and pancreas of laying hens( Reference Klenholz, Sunde and Hoekstra 36 ) and heat-stressed broilers( Reference Bartlett and Smith 37 ). In the present study, it was also found that dietary supplementation of oZn increased the Zn content in liver or either Zn source increased Zn content in the pancreas of broiler breeders under heat stress. Specifically, the organic Zn proteinate with the moderate chelation strength demonstrated greater bioavailability than the inorganic Zn sulphate in the pancreas of broiler breeders under heat stress. A series of studies from our laboratory have also shown that the organic Zn had higher absorption and bioavailability for broilers than the inorganic Zn sulphate, and the organic Zn with a moderate chelation strength exhibited the greatest bioavailabilities( Reference Huang, Lu and Li 10 – Reference Yu, Lu and Li 13 ).
Many studies have demonstrated that heat stress could produce ROS and induce oxidative damage in broilers( Reference Lin, Decuypere and Buyse 38 ) and laying hens( Reference Lin, De Vos and Decuypere 5 ). Synthesised antioxidant enzymes, such as SOD and glutathione peroxidase, play important roles in anti-heat stress of animals. However, this anti-heat stress response will be effective only if co-factors such as Se for glutathione peroxidase and Zn, Cu and Mn for SOD are available( Reference Sahin, Sahin and Kucuk 39 ). In the current study, HT elevated CuZnSOD activities in liver and pancreas, as well as MT level in the pancreas, of laying broiler breeders, which may allow superoxide and hydroxyl radicals to be scavenged and protect cells against toxic free radicals. The damage to the liver and pancreas membrane resulting from free radicals may have induced corresponding increases in CuZnSOD activity and MT level owing to the self-protection function during heat stress. In addition, the present study showed that dietary supplementation of either Zn source increased MT level in liver and the oZn increased MT level in pancreas of broiler breeders. Our previous results also indicated that the MT level in the pancreas of broilers increased linearly as the dietary Zn level increased( Reference Huang, Lu and Li 10 , Reference Huang, Lu and Xie 11 , Reference Liao, Li and Lu 40 ). Therefore, the above results suggest that HT may disturb the redox balance, whereas dietary Zn, especially the oZn, may promote the antioxidative ability of laying broiler breeders, but the interaction between environmental temperature and dietary Zn treatment had no effect on these antioxidative indices.
At the cellular level, elevated ambient temperature and other stress factors increase the synthesis of HSP, also known as stress proteins. Increased HSP protect cells against the additional stress, by protecting the cells against harmful insults and making the cells resistant to apoptosis( Reference Coronato, Di Girolamo and Salas 41 ). Evidence suggests that HSP might be involved in the development of thermotolerance in broiler chickens( Reference Wang and Edens 42 , Reference Soleimani, Zulkifli and Hair-Bejo 43 ). The up-regulation of HSP70 mRNA in the liver and pancreas of broiler breeders exposed to HT might favour an anti-heat stress response. This up-regulation might be dependent upon the presence of ROS and/or oxidative stress induced by heat stress( Reference Wu 44 ). Furthermore, it was found that a lower HSP70 expression level was observed in quails fed the Zn picolinate diet under NT( Reference Sahin, Tuzcu and Ozercan 45 ). However, it was found in the present study that dietary Zn did not affect the mRNA expression levels of HSP70 and HSP90 in liver and pancreas. These inconsistent results might be due to different types of birds, Zn sources, basal diets or growth phases.
Heat shock, as a promoter of oxidative stress, creates a redox imbalance by increasing the generation of ROS( Reference Lin, Decuypere and Buyse 38 ). Subsequent cellular damage caused by accumulation of ROS has been suggested as a key factor for activation of HSP expression( Reference Sahin and Kucuk 32 , Reference Mahmoud and Edens 46 ). When cells are subjected to heat shock with an increase in oxidative damage, HSP70 accumulates and might serve as a tissue biomarker for potential stress damage( Reference Tedeschi, Kennington and Berry 47 ). Thus, constitutive and inducible HSP70 expression might be regarded as a response to damage resulting from a strong stress to the organism( Reference Burdon, Gill and Rice-Evans 48 ). In addition to HSP, antioxidant enzymes are induced by stressors and provide the organism with multiple protective options( Reference Wu 44 , Reference Stephanou and Latchman 49 ). The increased activities of SOD and catalase likely scavenge free radicals that inhibit expression of HSP and improve cell survival( Reference Omar and Pappolla 50 ). In the present study, HT induced the synchronised increases between CuZnSOD activities and MT levels, as well as HSP70 expressions, in liver and pancreas, suggesting that HT might disturb the redox balance and HSP homoeostasis, and thus impair egg production performance and eggshell quality of broiler breeders. These findings also provide scientific experimental bases for how to alleviate the negative impact of HT on other animals. However, dietary supplementation of Zn did not alleviate the negative effect of HT on egg production performance, eggshell quality, antioxidant status and HSP expressions, except for pancreas Zn content of laying broiler breeders, probably owing to the similar feed intake of laying broiler breeders among all treatment groups. However, the exact reasons and mechanisms need to be further studied in future experiments.
In conclusions, the results from the present study indicated that HT impaired egg production performance and eggshell quality, which might be associated with the disturbed redox balance and HSP homoeostasis; dietary supplementation of oZn increased Zn content in the liver and MT levels in liver and pancreas regardless of environmental temperatures, whereas oZn is more available than the iZn for pancreatic Zn retention of heat-stressed laying broiler breeders.
Acknowledgements
The authors would like to thank the personnel of these teams for their kind assistance. This study was supported by the Agricultural Science and Technology Innovation Program (project no. ASTIP-IAS08; Beijing, P. R. China), the Key International Cooperation Program of the National Natural Science Foundation of China (project no. 31110103916; Beijing, P. R. China) and the China Agriculture Research System (project no. CARS-41; Beijing, P. R. China).
The authors’ contributions are as follows: X. Luo and X. Liao designed the experiment; X. Liao drafted the manuscript; X. Luo and L. L. participated in writing and editing the manuscript; W. L. and Y. Z. conducted most of the experiments and analysed the data; L. Z. performed the Zn analysis; X. L. was involved in the experimental design and data interpretations; X. Luo had primary responsibility for the final content. All authors have read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.










