Extensive research has shown that genetic factors have a role in the development of bipolar disorder. However, concordance rates of less than 100% in monozygotic twins (e.g. Reference Bertelsen, Harvald and HaugeBertelsen et al, 1977) imply that environmental factors may also be important in the aetiology of the disorder. Some researchers suggest that, rather like the neurodevelopmental hypothesis of schizophrenia (Reference Murray and LewisMurray & Lewis, 1986), exposure to obstetric complications increases vulnerability to bipolar disorder and that such exposure may account for some of the brain abnormalities reported in neuro-imaging studies of this disorder (Reference DalenDalen, 1965; Reference Pearlson, Garbacz and MobergPearlson et al, 1985; Reference NasrallahNasrallah, 1991). Although earlier review articles provide tentative support for such conclusions (e.g. Reference Buka and FanBuka & Fan, 1999), many of the purported studies of obstetric complications in bipolar disorder included samples of poorly defined affective disorders, selected populations with affective psychoses, or mixed samples of cases combined with varying proportions of cases of psychotic and/or non-psychotic unipolar disorders. Therefore, the empirical evidence for and against the hypothesis that exposure to obstetric complications increases the risk of bipolar disorder has not been fully elucidated.
We aimed, first, to systematically review studies comparing the obstetric histories of people with bipolar disorder and non-psychiatric control groups, and second, to systematically review studies comparing the obstetric histories of people with bipolar disorder and groups of people with other mental disorders. This quantitative review allowed the calculation of the pooled odds ratios for exposure to obstetric complications on the subsequent development of bipolar disorder.
METHOD
Search method
Definition of obstetric complications
Obstetric complications were defined according to McNeil (Reference McNeil, Helmchen and Henn1987) as:
‘the broad class of somatic deviations from an expected, normal course of events and offspring development during pregnancy, labour-delivery, and the early neonatal period.’
Inclusion criteria
All studies that stated the method used for measuring obstetric complications and for diagnosis of bipolar disorder (or other mental disorders) were eligible for inclusion in the review.
Exclusion criteria
The three exclusion criteria were:
-
(a) insufficient information to allow identification of a distinct subgroup of cases of bipolar disorder with obstetric complications that met the operational criteria defined;
-
(b) insufficient information to allow obstetric complications to be distinguished from other early developmental abnormalities or adverse events;
-
(c) review papers with no new empirical data.
Search strategy
The computerised databases searched were: Medline (1966 to January 2004), PreMedline (to January 2004), PsychINFO (1967 to January 2004), Cochrane Library (up to October 2003), Best Evidence (1991 to September 2003) and EMBASE (1980 to January 2004). The key words searched were [BIPOLAR AFFECTIVE DISORDER] or [MANIC-DEPRESSION] and [OBSTETRIC COMPLICATIONS]. The Thesaurus for Psychological Index Terms identified all terms mapping onto the key words, and an additional search was carried out using each of these terms (see Appendix 1 to the online version of this paper). All online abstracts were reviewed and relevant reports were obtained. Citations in relevant publications were also checked.
A total of 46 studies were identified, of which 45 were published papers or abstracts. Thirty-five were identified from electronic searches (Reference DalenDalen, 1965; Waters et al, Reference Waters, Marcenko-Bouer and Smiley1982, Reference Waters, Marchenko and Smiley1983; Reference Pearlson, Garbacz and MobergPearlson et al, 1985; Reference WilcoxWilcox, 1986; Reference Done, Johnstone and FirthDone et al, 1991; Kinney et al, Reference Kinney, Yurgelun-Todd and Levy1993, Reference Kinney, Yurgelun-Todd and Maureio1998; Reference Takei, O'Callaghan and ShamTakei et al, 1993; Verdoux & Bourgeois, Reference Verdoux and Bourgeois1993a ,Reference Verdoux and Bourgeois b ; Reference Cornelius, Fabrega and MezzichCornelius et al, 1994; Reference Gureje, Bamidele and RajiGureje et al, 1994; Reference Rifkin, Lewis and JonesRifkin et al, 1994; Brown et al, Reference Brown, Susser and Lin1995, Reference Brown, Van Os and Driessens2000; Reference Sacker, Done and CrowSacker et al, 1995; Cannon et al, Reference Cannon, Cotter and Coffey1996, Reference Cannon, Jones and Gilvarry1997, Reference Cannon, Casp and Moffitt2002a ; Reference Vocisano, Klein and KeefeVocisano et al, 1996; Machon et al, l997; Reference Morgan, Castle and PageMorgan et al, 1997; Reference Stober, Kocher and FranzekStober et al, 1997; Reference Marcelis, Van Os. and ShamMarcelis et al, 1998; Reference Buka and FanBuka & Fan, 1999; Reference Gunduz, Woerner and AlvirGunduz et al, 1999; Reference Hultman, Sparen and TakeinHultman et al, 1999; Reference TorreyTorrey, 1999; Reference Watson, Mednick and HuttunenWatson et al, 1999; Reference Bain, Juszczak and McInnenyBain et al, 2000; Reference Browne, Byrne and MorrisBrowne et al, 2000; Reference Eaton, Mortensen and FrydenbergEaton et al, 2000; Reference Torrey, Rawlings and YolkenTorrey et al, 2000; Reference Mortensen, Pedersen and MelbeyeMortensen et al, 2003).
A further 10 studies were identified following a manual search of reference lists and conference abstracts (Reference Taylor and AbramsTaylor & Abrams, 1981; Reference Lewis and MurrayLewis & Murray, 1987; Reference Schwarzkopf, Nasrallah and OlsonSchwarzkopf et al, 1989; Reference Buka, Tsuang and LipsittBuka et al, 1993; Reference Guth, Jones and MurrayGuth et al, 1993; Reference Byrne, Morris and BrowneByrne et al, 1996; Reference Sigurdsson, Fombonne and SayalSigurdsson et al, 1999; Reference Zornberg, Buka and TsuangZornberg et al, 2000; Reference Ogendahl, Agerbo and LichtOgendahl et al, 2002; Reference Wals, Reichart and ManonWals et al, 2003).
Five data-sets that met our inclusion criteria for ascertainment of obstetric complications and caseness were received from researchers in the field. Dr S. El-Badri of Hamilton, New Zealand, provided data and a draft of a paper ‘Family history, obstetric complications and age of onset in bipolar patients’ based on findings reported in his MD thesis ‘Neurobiological changes in bipolar affective disorder’ (University of Newcastle upon Tyne, UK) (El-Badri, personal communication, 1999). Dr H. Gunduz of Hillside Hospital, New York, USA, provided data that supplemented the research published as Gunduz et al (Reference Gunduz, Woerner and Alvir1999); this is referenced throughout the review as ‘Gunduz et al, updated’. Dr R. Murray and Dr M. Cannon of the Institute of Psychiatry, London, UK, provided an updated version of the database from the Camberwell Collaborative Psychosis Study (CCPS). Three publications report earlier findings from that project (Reference Cannon, Jones and GilvarryCannon et al, 1997; Reference Marcelis, Van Os. and ShamMarcelis et al, 1998; Reference Rifkin, Lewis and JonesRifkin et al, 1994), so the new data file is referred to as ‘CCPS, updated’ throughout this paper. Dr Cannon also provided additional data for the Dunedin cohort (Reference Cannon, Casp and MoffittCannon et al, 2002a ), and Dr M. Wals of the Department of Child/Adolescent Psychiatry, Erasmus MC-Sophia Children's Hospital, Rotterdam, The Netherlands, provided additional data to supplement that published from the Dutch study of 140 offspring of parents with bipolar disorder (Reference Wals, Reichart and ManonWals et al, 2003); these studies are referred to in this review as ‘Cannon et al, updated’ and ‘Wals et al, updated’ respectively.
Twenty-four publications were excluded from the systematic review. Seventeen papers were excluded because distinct groups of participants with bipolar disorder and obstetric complications could not be identified (in 11 papers bipolar disorder was included in a category termed ‘broad affective disorders’). Three papers (Reference Rifkin, Lewis and JonesRifkin et al, 1994; Reference Cannon, Jones and GilvarryCannon et al, 1997; Reference Marcelis, Van Os. and ShamMarcelis et al, 1998) from the CCPS were excluded because the findings were superseded by new data from the researchers, as noted above. Two papers (Reference Buka and FanBuka & Fan, 1999; Reference TorreyTorrey, 1999) were excluded because they were review articles. The paper of Mortensen et al (Reference Mortensen, Pedersen and Melbeye2003) was excluded, as information on obstetric complications in this sample was available from the published abstract and poster reported by Ogendahl et al (Reference Ogendahl, Agerbo and Licht2002). One paper (Reference Verdoux and BourgeoisVerdoux & Bourgeois, 1993b ) was excluded because it was written in French and the data about the sample were available in an English language publication (Reference Verdoux and BourgeoisVerdoux & Bourgeois, 1993a ).
A list of the included and excluded studies appears in Appendix 2 to the online version of this paper.
Data extraction
Reports were initially assessed independently by Y.M. and J.C. The findings were cross-checked and analysed by J.S., and further reviewed by M.C. and R.M. The following methodological factors were recorded in a structured pro forma: reported clinical diagnosis; method of assigning diagnosis (structured clinical questionnaire, interview with psychiatrist, information obtained from medical records); demography (age, gender and ethnicity); family history of bipolar disorder or other mental disorders (and method of ascertainment); source of data about obstetric history (maternal recall, birth records, birth registers); and the assessment scale or tool used to identify and quantify obstetric complications. The scales were categorised as ‘no scale used’; ‘Lewis scale’ (Reference Lewis, Owen, Murray, Schulz and TammingaLewis et al, 1989); ‘Parnas scale’ (Reference Parnas, Schulsinger and TeasdaleParnas et al, 1982); ‘McNeil-Sjostrom scale’ (Reference McNeil, Cantor-Graae and SjostromMcNeil et al, 1994); and Mirdal scale (Reference Mirdal, Mednick and SchulsingerMirdal et al, 1974). The reviewers also recorded whether healthy controls had been screened to exclude individuals with a possible or definite DSM-IV diagnosis and whether or not family history of mental disorders was assessed. Differences were noted between cases and controls with respect to gender distribution, age and socio-economic status. Study methodologies were classified as being of high quality (e.g. structured interview for assigning diagnosis and prospective measurement of obstetric complications), of medium quality or of lower quality (case-note information only, or no information on how diagnosis or complications were defined).
For the purposes of the review, studies were grouped into four categories in which the frequency and severity of exposure to obstetric complications were compared in:
-
(a) individuals with bipolar disorder and non-psychiatric healthy controls;
-
(b) individuals with bipolar disorder and individuals with another mental disorder;
-
(c) early-onset as compared with late-onset bipolar disorder; and among cases of bipolar disorder with and without a family history of the disorder;
-
(d) birth cohort or prospective longitudinal studies that examined the relative risk of developing bipolar disorder in individuals who did or did not experience prenatal exposure to a specified adverse event (the Dutch ‘hunger winter’, the Greater Helsinki influenza epidemic, and a prospective study of foetal and neonatal complications related to hypoxic ischaemia).
Statistical analysis
Chi-squared tests of exposure to obstetric complications, in the bipolar disorder group and the comparison group (or, in one study, differences between mean number of complications per individual) were recorded from the papers or calculated from data provided. Where possible, estimates of the odds ratio (OR) or relative risk (RR) and the 95% confidence interval were also recorded or calculated. As information on pairing in matched case-control studies was not available, these data were analysed as unmatched (a more conservative approach). Estimates of effect sizes in individual studies were calculated for exposure to obstetric complications and, when possible, for exposure to complications of different levels of severity.
Pooled data were analysed using the Comprehensive Meta-Analysis programme (Reference BiostatBiostat, 2000). For the purposes of the meta-analysis, exposure to obstetric complications was dichotomised into ‘exposed’ (definite complications) or ‘unexposed’ (equivocal or no complication reported). Pooled estimates for specific complications (e.g. low birth weight) were calculated if more than two studies reported on that individual complication in a comparable way. The analyses were then repeated using studies providing data that allowed adjustment for potential confounders (e.g. gender of baby, maternal age, socio-economic status) and again after investigating any interaction with study quality; a separate analysis of only those studies regarded as high-quality was also undertaken. Estimates of effect sizes and their variance did not change significantly in these repeated analyses so they are not reported here, although it should be noted that the analyses of confounders were hampered because relevant data were not reported in many studies.
Given the variability in sample sizes and in quality and quantity of the available data, we calculated random effects estimates for each association. A Mantel-Haenszel random effects model leads to wider 95% confidence intervals than a fixed model as it assumes a different underlying effect for each study. A homogeneity statistic (Q) was calculated for each pooled analysis. Funnel plots of study-specific estimates against sample size suggested some gaps in the region of large-scale negative studies (bipolar disorder v. healthy controls) or positive studies (bipolar disorder v. unipolar disorders).
RESULTS
Bipolar disorder cases v. healthy controls
Eight studies compared the obstetric complications experienced by individuals with bipolar disorder and by healthy controls: Kinney et al (Reference Kinney, Yurgelun-Todd and Levy1993, Reference Kinney, Yurgelun-Todd and Maureio1998); Verdoux & Bourgeois (Reference Verdoux and Bourgeois1993a ); Stober et al (Reference Stober, Kocher and Franzek1997); El Badri (1999, personal communication); Browne et al (Reference Browne, Byrne and Morris2000); CCPS (updated); Gunduz et al (updated).
The sample sizes were small and the majority of studies found no difference in rates of complications in cases or controls (Table 1). Only Kinney et al (Reference Kinney, Yurgelun-Todd and Levy1993, Reference Kinney, Yurgelun-Todd and Maureio1998) reported statistically significant differences between cases and healthy controls (who were the unaffected siblings of the cases), with odds ratios above 10 in each study. In their first study (Reference Kinney, Yurgelun-Todd and LevyKinney et al, 1993), patients with bipolar disorder were significantly (P=0.003) more likely than their healthy siblings to be exposed to a wide range of non-specific obstetric complications, such as maternal anaemia, rubella, prematurity, prolonged duration of labour and neonatal respiratory problems; the complications were also significantly more severe in the patient group (Wilcoxon test, P=0.034). In their later study (Reference Kinney, Yurgelun-Todd and MaureioKinney et al, 1998), patients with bipolar disorder had significantly greater exposure to prenatal (P=0.02) and perinatal obstetric complications (P=0.04) compared with their healthy siblings. However, the CCPS study (CCPS, updated) reported similar rates of definite obstetric complications in patients with bipolar disorder (n=49; 24) and controls (n=100; 28). Stober et al (Reference Stober, Kocher and Franzek1997) found an OR of 0.8 for exposure to obstetric complications in those developing bipolar disorder v. healthy controls. Likewise, Verdoux & Bourgeois (Reference Verdoux and Bourgeois1993a ), El-Badri (1999, personal communication), Gunduz et al (updated) and Brown et al (Reference Brown, Van Os and Driessens2000) did not report any significant between-group differences in number of individuals exposed to obstetric complications. As shown in Fig. 1, the pooled OR for the exposure to obstetric complications on subsequent development of bipolar disorder was 1.15 (95% CI 0.62-2.14). The Q statistic reflected the heterogeneity of the studies as it just failed to reach statistical significance (χ2=13.9, d.f.=7, P=0.053).
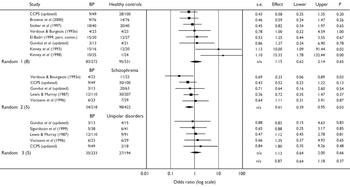
Fig. 1 Study-specific odds ratios (log scale) and pooled random effects models for obstetric complications and bipolar disorder (BP), healthy controls, schizophrenia and unipolar disorders.
Table 1 Studies comparing exposure to obstetric complications in individuals with bipolar disorder and healthy controls
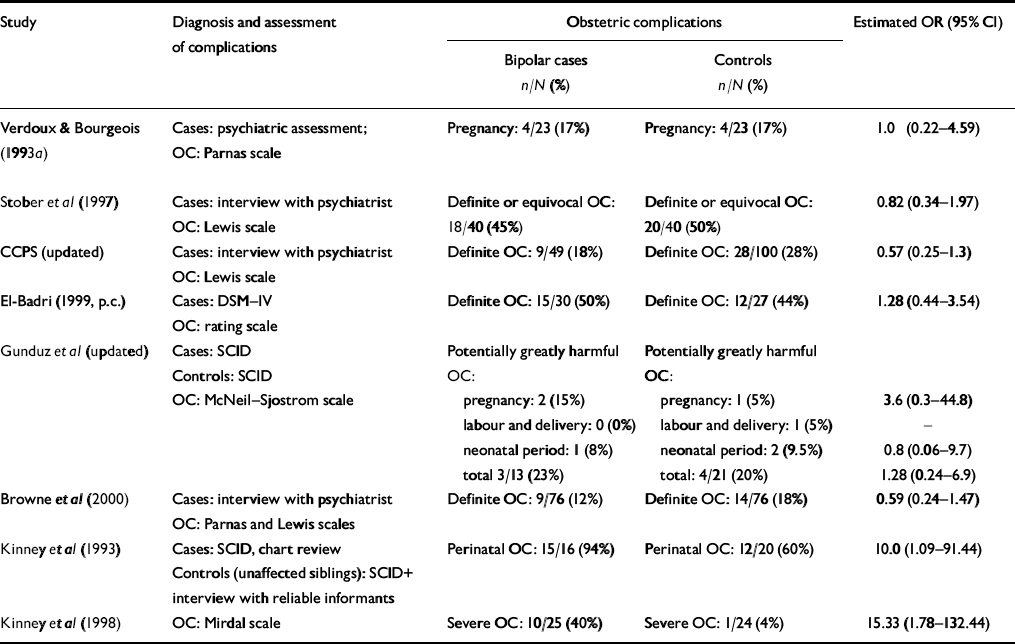
| Study | Diagnosis and assessment of complications | Obstetric complications | Estimated OR (95% CI) | ||
|---|---|---|---|---|---|
| Bipolar cases n/N (%) | Controls n/N (%) | ||||
| Verdoux & Bourgeois (Reference Verdoux and Bourgeois1993a ) | Cases: psychiatric assessment; | Pregnancy: 4/23 (17%) | Pregnancy: 4/23 (17%) | 1.0 (0.22–4.59) | |
| OC: Parnas scale | |||||
| Stober et al (Reference Stober, Kocher and Franzek1997) | Cases: interview with psychiatrist | Definite or equivocal OC: | Definite or equivocal OC: | 0.82 (0.34–1.97) | |
| OC: Lewis scale | 18/40 (45%) | 20/40 (50%) | |||
| CCPS (updated) | Cases: interview with psychiatrist | Definite OC: 9/49 (18%) | Definite OC: 28/100 (28%) | 0.57 (0.25–1.3) | |
| OC: Lewis scale | |||||
| El-Badri (1999, p.c.) | Cases: DSM–IV | Definite OC: 15/30 (50%) | Definite OC: 12/27 (44%) | 1.28 (0.44–3.54) | |
| OC: rating scale | |||||
| Gunduz et al (updated) | Cases: SCID | Potentially greatly harmful | Potentially greatly harmful | ||
| Controls: SCID | OC: | OC: | |||
| OC: McNeil–Sjostrom scale | pregnancy: 2 (15%) | pregnancy: 1 (5%) | 3.6 (0.3–44.8) | ||
| labour and delivery: 0 (0%) | labour and delivery: 1 (5%) | – | |||
| neonatal period: 1 (8%) | neonatal period: 2 (9.5%) | 0.8 (0.06–9.7) | |||
| total 3/13 (23%) | total: 4/21 (20%) | 1.28 (0.24–6.9) | |||
| Browne et al (Reference Browne, Byrne and Morris2000) | Cases: interview with psychiatrist | Definite OC: 9/76 (12%) | Definite OC: 14/76 (18%) | 0.59 (0.24–1.47) | |
| OC: Parnas and Lewis scales | |||||
| Kinney et al (Reference Kinney, Yurgelun-Todd and Levy1993) | Cases: SCID, chart review | Perinatal OC: 15/16 (94%) | Perinatal OC: 12/20 (60%) | 10.0 (1.09–91.44) | |
| Controls (unaffected siblings): SCID+interview with reliable informants | |||||
| Kinney et al (Reference Kinney, Yurgelun-Todd and Maureio1998) | OC: Mirdal scale | Severe OC: 10/25 (40%) | Severe OC: 1/24 (4%) | 15.33 (1.78–132.44) | |
CCPS, Camberwell Collaborative Psychosis Study; OC, obstetric complications; OR, odds ratio; p.c., personal communication; SCID, Structured Clinical Interview for DSM–IV
Data on exposure to obstetric complications of different levels of severity produced conflicting trends: Verdoux & Bourgeois (Reference Verdoux and Bourgeois1993a ) found that the mean number and severity of the complications were similar for both groups. Gunduz et al (updated) reported that exposure to potentially harmful complications during pregnancy was lower (OR=0.6, 95 CI 0.1-2.9), but exposure to potentially greatly harmful complications during pregnancy was higher in those who developed bipolar disorder (OR=3.6, 95 CI 0.3-44.8). Browne et al (Reference Browne, Byrne and Morris2000) found the opposite pattern.
It was possible to explore the odds ratios for only three specific complications: four studies reported on low birth weight (<2.5 kg) or being small for gestational age (Reference Ogendahl, Agerbo and LichtOgendahl et al, 2002; CCPS, updated; Cannon et al, updated; Wals et al, updated) and three studies on parity (Reference Ogendahl, Agerbo and LichtOgendahl et al, 2002; CCPS, updated; Cannon et al, updated). Wals et al (updated) demonstrated that low birth weight is independently associated with increased risk of developing a mood disorder (bipolar disorder, 16%) in the offspring of parents with bipolar disorder (hazard ratio 0.7, 95% CI 0.5-0.9). However, the pooled OR for low birth weight and later development of bipolar disorder was 0.91 (95% CI 0.51-1.6) and for babies who were small for gestational age it was 1.79 (95% CI 0.83-3.86). Only the pooled OR (1.39, 95% CI 1.08-1.95; P=0.04) for three or more previous pregnancies in the mothers of those in the bipolar disorder case groups was statistically significant and remained so when results were adjusted for baby gender and maternal age.
Bipolar disorder cases v. other mental disorders
Schizophrenia
Geddes et al (Reference Geddes, Verdoux and Takei1999) highlighted that complications of labour and pregnancy are among the most extensively studied putative risk factors for schizophrenia. However, only eight studies allow comparison of exposure to any obstetric complications (Reference Lewis and MurrayLewis & Murray, 1987; Reference Schwarzkopf, Nasrallah and OlsonSchwarzkopf et al, 1989; Reference Verdoux and BourgeoisVerdoux & Bourgeois, 1993a ; Reference Byrne, Morris and BrowneByrne et al, 1996; Reference Vocisano, Klein and KeefeVocisano et al, 1996; CCPS, updated; Gunduz et al, updated) or exposure to a specific complication (Reference Schwarzkopf, Nasrallah and OlsonSchwarzkopf et al, 1989; Cannon et al, updated; CCPS, updated) in individuals who develop schizophrenia or bipolar disorder. Table 2 presents data that can be summarised from six studies.
Table 2 Studies comparing exposure to obstetric complications in individuals with bipolar disorder and schizophrenia
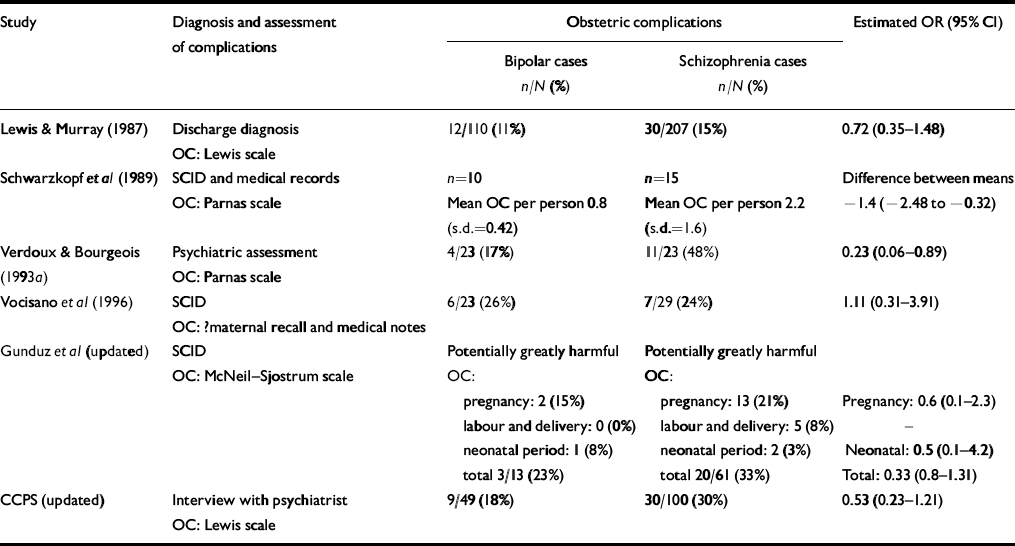
| Study | Diagnosis and assessment of complications | Obstetric complications | Estimated OR (95% CI) | ||
|---|---|---|---|---|---|
| Bipolar cases n/N (%) | Schizophrenia cases n/N (%) | ||||
| Lewis & Murray (Reference Lewis and Murray1987) | Discharge diagnosis | 12/110 (11%) | 30/207 (15%) | 0.72 (0.35–1.48) | |
| OC: Lewis scale | |||||
| Schwarzkopf et al (Reference Schwarzkopf, Nasrallah and Olson1989) | SCID and medical records | n=10 | n=15 | Difference between means | |
| OC: Parnas scale | Mean OC per person 0.8 | Mean OC per person 2.2 | –1.4 (–2.48 to –0.32) | ||
| (s.d.=0.42) | (s.d.=1.6) | ||||
| Verdoux & Bourgeois (Reference Verdoux and Bourgeois1993a ) | Psychiatric assessment | 4/23 (17%) | 11/23 (48%) | 0.23 (0.06–0.89) | |
| OC: Parnas scale | |||||
| Vocisano et al (Reference Vocisano, Klein and Keefe1996) | SCID | 6/23 (26%) | 7/29 (24%) | 1.11 (0.31–3.91) | |
| OC: ?maternal recall and medical notes | |||||
| Gunduz et al (updated) | SCID | Potentially greatly harmful | Potentially greatly harmful | ||
| OC: McNeil–Sjostrum scale | OC: | OC: | |||
| pregnancy: 2 (15%) | pregnancy: 13 (21%) | Pregnancy: 0.6 (0.1–2.3) | |||
| labour and delivery: 0 (0%) | labour and delivery: 5 (8%) | – | |||
| neonatal period: 1 (8%) | neonatal period: 2 (3%) | Neonatal: 0.5 (0.1–4.2) | |||
| total 3/13 (23%) | total 20/61 (33%) | Total: 0.33 (0.8–1.31) | |||
| CCPS (updated) | Interview with psychiatrist | 9/49 (18%) | 30/100 (30%) | 0.53 (0.23–1.21) | |
| OC: Lewis scale | |||||
CCPS, Camberwell Collaborative Psychosis Study; OC, obstetric complications; OR, odds ratio; SCID, Structured Clinical Interview for DSM–IV
Verdoux & Bourgeois (Reference Verdoux and Bourgeois1993a ) reported that 48% (n=11) of participants who developed schizophrenia were exposed to obstetric complications and/or more severe complications during pregnancy compared with 17% (n=4; Fisher's exact test, P=0.03) of those who developed bipolar disorder (OR=0.2, 95% CI 0.1-0.9); Byrne et al (Reference Byrne, Morris and Browne1996) reported similar statistically significant differences for men. Schwarzkopf et al (Reference Schwarzkopf, Nasrallah and Olson1989) reported a significantly higher mean rate of exposure to obstetric complications in individuals who developed schizophrenia compared with bipolar disorder (difference between means -1.4, 95% CI -2.48 to -0.32). The OR for two other studies demonstrated a non-significant trend for more frequent exposure to obstetric complications in individuals who developed schizophrenia rather than bipolar disorder (Reference Lewis and MurrayLewis & Murray, 1987; CCPS, updated), although Vocisano et al (Reference Vocisano, Klein and Keefe1996) found no difference and no clear pattern emerged in the study by Gunduz et al (updated). As shown in Fig. 1, the pooled OR for exposure to obstetric complications on subsequent development of bipolar disorder v. schizophrenia was 0.61 (95% CI 0.39-0.95; P=0.035).
Data on exposure to individual obstetric complications were extracted from three studies (Reference Schwarzkopf, Nasrallah and OlsonSchwarzkopf et al, 1989; Reference Cannon, Cotter and CoffeyCannon et al, 1996, updated; CCPS, updated). Although Schwarzkopf et al (Reference Schwarzkopf, Nasrallah and Olson1989) reported that individuals who developed bipolar disorder were significantly less likely to have experienced a prolonged labour compared with those who developed schizophrenia (bipolar disorder 1/10, schizophrenia 7/15; Fisher's exact test, P=0.02), the pooled OR for prolonged or difficult labour was not significant (OR=0.99, 95% CI 0.46-2.41). The CCPS (updated) study demonstrated that significantly more individuals who developed schizophrenia (n=9, 11%) had been exposed to maternal infections such as rubella during pregnancy compared with individuals who developed bipolar disorder (0%; Fisher's exact test, P=0.03). However, the pooled OR for exposure to infections during pregnancy was again not statistically significant (OR=0.69, 95% CI 0.19-2.37).
Major depressive disorder
Data on exposure to obstetric complications in individuals who developed bipolar or unipolar disorders was available from five studies (Reference Lewis and MurrayLewis & Murray, 1987; Reference Vocisano, Klein and KeefeVocisano et al, 1996; Reference Sigurdsson, Fombonne and SayalSigurdsson et al, 1999; CCPS, updated; Gunduz et al, updated). As shown in Table 3, the majority of studies reported trends for either more frequent exposure to obstetric complications (Reference Lewis and MurrayLewis & Murray, 1987; Reference Vocisano, Klein and KeefeVocisano et al, 1996; CCPS, updated) or exposure to more severe complications in those who developed bipolar compared with unipolar disorders (Gunduz et al, updated). However, there was a ten-fold variation in odds ratios (0.2 to 2.5) and no study found any statistically significant difference. Figure 1 shows that the pooled OR for exposure to obstetric complications on subsequent development of bipolar disorder v. unipolar disorder was 1.34 (95% CI 0.64-1.99).
Table 3 Studies comparing exposure to obstetric complications in individuals with bipolar disorder and major depressive disorder
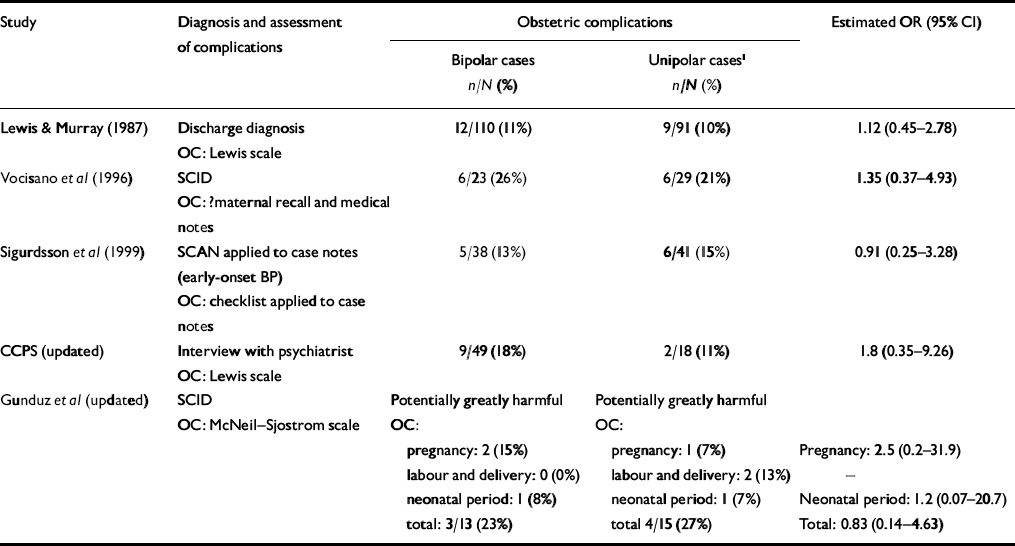
| Study | Diagnosis and assessment of complications | Obstetric complications | Estimated OR (95% CI) | ||
|---|---|---|---|---|---|
| Bipolar cases n/N (%) | Unipolar cases 1 n/N (%) | ||||
| Lewis & Murray (Reference Lewis and Murray1987) | Discharge diagnosis | 12/110 (11%) | 9/91 (10%) | 1.12 (0.45–2.78) | |
| OC: Lewis scale | |||||
| Vocisano et al (Reference Vocisano, Klein and Keefe1996) | SCID | 6/23 (26%) | 6/29 (21%) | 1.35 (0.37–4.93) | |
| OC: ?maternal recall and medical | |||||
| notes | |||||
| Sigurdsson et al (Reference Sigurdsson, Fombonne and Sayal1999) | SCAN applied to case notes | 5/38 (13%) | 6/41 (15%) | 0.91 (0.25–3.28) | |
| (early-onset BP) | |||||
| OC: checklist applied to case | |||||
| notes | |||||
| CCPS (updated) | Interview with psychiatrist | 9/49 (18%) | 2/18 (11%) | 1.8 (0.35–9.26) | |
| OC: Lewis scale | |||||
| Gunduz et al (updated) | SCID | Potentially greatly harmful | Potentially greatly harmful | ||
| OC: McNeil–Sjostrom scale | OC: | OC: | |||
| pregnancy: 2 (15%) | pregnancy: 1 (7%) | Pregnancy: 2.5 (0.2–31.9) | |||
| labour and delivery: 0 (0%) | labour and delivery: 2 (13%) | – | |||
| neonatal period: 1 (8%) | neonatal period: 1 (7%) | Neonatal period: 1.2 (0.07–20.7) | |||
| total: 3/13 (23%) | total 4/15 (27%) | Total: 0.83 (0.14–4.63) | |||
BP, bipolar disorder; CCPS, Camberwell Collaborative Psychosis Study; OC, obstetric complications; OR, odds ratio; SCAN, Structured Clinical Assessment of Neuropsychiatry; SCID, Structured Clinical Interview for DSM–IV
1. Major depressive disorder
Other psychoses or other disorders
Three studies reported non-significant trends for more individuals who developed schizoaffective disorder (Reference Schwarzkopf, Nasrallah and OlsonSchwarzkopf et al, 1989; Gunduz et al, updated) or cycloid psychosis (Reference Stober, Kocher and FranzekStober et al, 1997) to have been exposed to obstetric complications than those who developed bipolar disorder. Lewis & Murray (Reference Lewis and Murray1987) noted that exposure to definite obstetric complications was significantly more common in individuals with bipolar disorder (11%) compared with individuals who developed drug or alcohol dependence (3%; Fisher's exact test, P=0.04); marginally more individuals with anorexia nervosa (16%) had been exposed to such complications compared with individuals with bipolar disorder (11%). The data from these studies were not pooled, as few inferences can be drawn because of the mixture of diagnoses in the comparison groups, small sample sizes and inadequate statistical power.
Studies of subgroups of bipolar disorder cases
A small number of studies reported exposure to obstetric complications in individuals with early-onset (age <29 years) v. late-onset or familial v. non-familial bipolar disorder. Guth et al (Reference Guth, Jones and Murray1993) found that individuals with early-onset disorder were significantly (McNemar χ2=7.7; P=0.006) more likely to have been exposed to definite complications compared with individuals with late-onset disorder (OR=12.0, 95% CI 2.1-69.5). Taylor & Abrams (Reference Taylor and Abrams1981) reported a similar trend, with exposure to gestational or neonatal obstetric complications in 13% (10/78) of patients with early-onset disorder compared with only 4% (2/54) in patients with late-onset disorder. However, El-Badri (1999, personal communication) found no difference in exposure to definite complications in those with early-onset (8/16; 50%) or late-onset (5/14; 36%) disorder. Pooled data failed to find differences in age at first presentation to psychiatric services (Reference Browne, Byrne and MorrisBrowne et al, 2000; CCPS, updated) or age at first psychiatric admission in individuals with bipolar disorder who were or were not exposed to obstetric complications (Reference Stober, Kocher and FranzekStober et al, 1997; CCPS, updated). Dalen (Reference Dalen1965), Browne et al (Reference Browne, Byrne and Morris2000) and El-Badri (1999, personal communication) noted that patients with family history of bipolar disorder were equally likely to have been exposed to obstetric complications as those without such a family history (exposure rate 18% v. 14.5%).
The relationship between exposure to obstetric complications and the course of bipolar disorder is unclear. One study (Reference Vocisano, Klein and KeefeVocisano et al, 1996) reported a non-significant increase in exposure to complications among individuals with functionally deteriorated bipolar disorder (n=6; 40%) compared with individuals with non-functionally deteriorated disorder (n=2; 18%). (Functionally deteriorated disorder was defined as disorder in which patients were continually hospitalised or were out-patients dependent on others for necessities such as food or clothing, had no useful work or employment, and did not have full symptom remission over the previous 5 years.) No directly comparable data were available, but the CPPS (updated) study noted that individuals with bipolar disorder and a history of exposure to obstetric complications had significantly fewer psychiatric admissions (n=9; mean 2.3, s.d.=1.3) than those without exposure (n=40; mean 4.5, s.d.=3.1; 95% CI for difference in means -4.3 to -0.07). However, lengths of admission and out-patient attendances were not recorded, so it was not possible to apply the classification of Vocisano et al (Reference Vocisano, Klein and Keefe1996).
Incidence studies
Brown et al (Reference Brown, Van Os and Driessens2000) reported the incidence of bipolar disorder among the offspring of mothers who were or were not exposed to famine during the Dutch ‘hunger winter’ of 1944-5. Relative to unexposed individuals (those born in the same region before the German invasion), the risk of developing an ICD-9 affective disorder requiring hospitalisation was significantly increased for people whose mothers were exposed during the second trimester (RR=1.5, 95% CI 1.2-1.9) and third trimester (RR=1.4, 95% CI 1.2-1.8) of pregnancy. Separate evaluations of the RR for unipolar disorders (including ICD-9 manic-depressive psychosis, depressed type) and bipolar disorders revealed similar trends in both disorders, but the risks were statistically significant for unipolar disorders but not for bipolar disorder (second trimester RR for bipolar disorder=1.4, 95% CI 0.9-2.1, P=0.08; third trimester RR=1.3, 95% CI 0.9-1.9, P=0.1).
Machon et al (Reference Machon, Mednick and Huttunen1997) found a statistically significant increase in the risk of developing an affective disorder in the offspring of women who were pregnant during the 1957 Greater Helsinki influenza epidemic compared with the offspring of unexposed mothers (10/56 v. 36/442; RR=2.19, 95% CI 1.15-4.17; P=0.033). The association was strongest for exposure during the second trimester of pregnancy. The risk (RR=2.89, 95% CI 1.03-8.09) was statistically significant for the development of an ICD-8 unipolar disorder requiring hospitalisation (exposed 7% v. unexposed 0.5%; Fisher's exact test, P=0.002), with a similar but non-significant increase in the risk of bipolar disorder (exposed 5% v. unexposed 1.6%; RR=1.21, 95% CI 0.27-8.49). In a smaller-scale study, Cannon et al (Reference Cannon, Cotter and Coffey1996) noted a comparable statistically significant increase in the risk of unipolar disorder in those exposed to the epidemic in utero (RR=1.59, 95% CI 1.15-2.19), but a non-significant increase in the risk of bipolar disorder (RR=1.1.5, 95% CI 0.4-2.95).
In a prospective study of over 1000 individuals from a 1959-66 birth cohort, Zornberg et al (Reference Zornberg, Buka and Tsuang2000) found a non-significant increase in the risk (RR=2.0, 95% CI 0.4-8.6) of developing bipolar disorder in individuals who had experienced hypoxic ischaemia-related foetal or neonatal complications (4/174) compared with those who had not experienced such complications (6/519).
DISCUSSION
This systematic review did not find any robust evidence that overall exposure to obstetric complications is associated with the subsequent development of bipolar disorder. However, unlike the extensive research on obstetric complications in schizophrenia, relatively few studies have been undertaken that are large-scale, methodologically sound and report on well-defined complications in precisely ascertained populations of patients. Although the findings of the pooled analyses must be interpreted cautiously, we noted that meta-analyses based only on studies of the highest quality (e.g. employing prospective ascertainment of obstetric complications using a robust assessment tool and careful descriptions of family history of mood disorders and of bipolar disorder caseness) still did not yield statistically significant results. Therefore, we will briefly review the findings but then highlight important limitations of the currently available literature and the implications for future research.
The findings
The meta-analyses undertaken found that individuals who develop bipolar disorder are no more likely to have been exposed to any one of a range of non-specific obstetric complications than either healthy controls or individuals who develop unipolar disorder. Furthermore, those who develop bipolar disorder have significantly less exposure to obstetric complications than those who develop schizophrenia. Examination of the limited information on individual complications demonstrated some interesting trends, but only parity - specifically being born to a mother with a history of three or more previous pregnancies - increased the risk of subsequent development of bipolar disorder compared with healthy controls. The studies do not differentiate between live and still births, but other research suggests the latter may be associated with affective disorders (e.g. Reference Done, Johnstone and FirthDone et al, 1991). Although exposure to famine or infection in utero, particularly during the second trimester, increased the risk of developing an adult mood disorder relative to unexposed individuals, the RR was significant for unipolar disorders, not bipolar disorder. No association was found between exposure to obstetric complications and age at onset or indicators of course and prognosis of bipolar disorder.
Limitations
The principal limitation of the available research concerns small sample sizes (median n=44) and a lack of statistical power with consequent risk of type II error. There are also a number of problems with the design of many of the studies. The basic principle of a case-control study is that the cases have the disorder of interest and that the controls do not. It is also important to know whether cases have a familial or genetic liability to the disorder in question. Three studies failed to rule out a diagnosis of bipolar disorder in the control group (Reference Stober, Kocher and FranzekStober et al, 1997; Reference Brown, Van Os and DriessensBrown et al, 2000; CCPS, updated) and few studies reported on whether cases or controls were screened for any family history of bipolar or other affective disorders, particularly in the mother, nor whether the mothers had any prenatal mood disorder, which might be associated with problems such as preterm delivery or low birth weight (Reference O'Keane and ScottO'Keane & Scott, 2005). Four studies failed to control for factors such as socio-economic status and prenatal care (Reference Verdoux and BourgeoisVerdoux & Bourgeois, 1993a ; El-Badri, 1999, personal communication; CCPS, updated; Gunduz et al, updated) and few explored other demographic, illness history or lifestyle factors (e.g. maternal cigarette and alcohol consumption) that may increase the risk of obstetric complications or moderate the estimated effect size for different complications. The absence of such details represents a significant problem when using meta-analysis because the OR cannot be adjusted adequately for confounders. However, when studies that failed to provide these data were excluded, the meta-analyses still failed to produce additional significant results. Eagles et al (Reference Eagles, Gibson and Bremner1990) previously detailed other methodological issues, such as failure to control for intra-uterine environment, that can be surmounted by comparing cases and controls that are biologically related. It is noteworthy that the only research group that recruited unaffected siblings as a comparison group (Kinney et al, Reference Kinney, Yurgelun-Todd and Levy1993, Reference Kinney, Yurgelun-Todd and Maureio1998) reported highly significant ORs and that these findings contrasted markedly with the other studies of bipolar disorder cases and healthy controls.
There are particular issues that need to be considered when attempting to distinguish whether exposure to obstetric complications or to an individual complication are differentially associated with the development of bipolar or unipolar disorders. First, a significant proportion of cases of recurrent unipolar disorder may experience a manic or hypomanic episode and be reclassified as bipolar disorder cases at a later date. Thus studies that reported significant RR for affective disorders and for unipolar disorders but not for bipolar disorder (e.g. Reference Machon, Mednick and HuttunenMachon et al, 1997; Reference Brown, Van Os and DriessensBrown et al, 2000; Wals et al, updated) might have underestimate the magnitude of risk for bipolar disorder because many individuals had not been through the peak period of risk of its development. Second, the diagnostic criteria for affective disorders have changed over time. For example, in earlier revisions of the International Classification of Diseases (ICD-8 and ICD-9), the diagnosis of ‘manic-depressive psychosis’ included cases of severe unipolar disorder as well as bipolar disorder. Also, mood disorders with vegetative symptoms could be classified as ‘affective psychosis’ even in the absence of typical psychotic symptoms. These anomalies in diagnostic criteria may contribute to the wide variations in the magnitude of any association between exposure to obstetric complications and unipolar disorders, bipolar disorder or broadly defined affective disorders.
As highlighted in many earlier publications concerning obstetric complications in schizophrenia, information gathered retrospectively through interviews with the mothers of those with the disorder may be subject to biased recall. Furthermore, there are significant problems relating to the quality and accuracy of scales specifically designed to record obstetric complications (Reference McNeil, Cantor-Graae and SjostromMcNeil et al, 1994). Exposure to any obstetric complication in the studies reviewed varied from 12% to 95% in bipolar disorder cases; 4% to 60% in healthy controls; 10% to 28% in unipolar disorder cases; and 15% to 48% in schizophrenia cases. The high rate of complications in healthy controls (median 28%) and the considerable variability in rates of exposure to complications within diagnostic groups (an eight-fold difference across the 11 bipolar disorder samples) clearly calls into question the reliability and validity of the scales and the phenomena being measured. This finding reflects problems previously encountered in schizophrenia research, but as suggested by Cannon et al (Reference Cannon, Jones and Murray2002b ), the concept of ‘obstetric complications’ will be rendered meaningless unless there is agreement on more concise definitions of the nature, timing, duration or intensity of the exposures to be scrutinised.
The longitudinal studies we reviewed displayed fewer methodological flaws than the case-control studies. However, the potential weakness of this approach is the assumption that all the participants with bipolar disorder were exposed in foetal life to the complication or event being investigated. Although this - the so-called ‘ecological fallacy’ - is not relevant to most of the complications we studied, it is to variables such as exposure to influenza or the Dutch ‘hunger winter’.
Implications for future research
This review suggests that increased methodological rigour in future studies will need to be accompanied by detailed proposals regarding the individual complication that might potentially have a causal role in affective disorders and a priori hypotheses outlining the mechanisms by which pregnancy, labour or perinatal complications affect individual risk of developing bipolar disorder (Reference O'Keane and ScottO'Keane & Scott, 2005). The traditional view of obstetric complications as a broad array of discrete, non-specific and largely unrelated events that may lead to structural brain abnormalities does not match the empirical evidence from contemporary studies (Reference Cannon, Jones and MurrayCannon et al, 2002b ; Reference Mortensen, Pedersen and MelbeyeMortensen et al, 2003). Maternal psychiatric disorders, genetic factors and perinatal events may all affect the likelihood of experiencing an obstetric complication, as well as affecting the timing of its occurrence. Furthermore, early abnormalities in foetal development may be associated with the occurrence of complications at a later stage in pregnancy that initially appear unrelated. This is illustrated by the meta-analysis by Geddes et al (Reference Geddes, Verdoux and Takei1999), which suggests that the complications consistently associated with the development of schizophrenia, although apparently unrelated, may share a common pathophysiology, namely foetal hypoxia. In mood disorders, we would propose a greater focus on research on in utero exposures to adversity that have functional consequences for foetal brain development rather than on perinatal complications that are a direct source of cerebral insult. For example, longitudinal studies demonstrate that impaired foetal growth and low birth weight are associated with increased risk of mood disorder (Reference Brown, Van Os and DriessensBrown et al, 2000; Thompson et al, 2000; Reference Wals, Reichart and ManonWals et al, 2003). It has been suggested that this relationship is mediated by hormonal reprogramming in which plasma levels of hormones or the set points of the foetal hypothalamic-pituitary-adrenal axis are altered permanently, with diminished ability to inhibit stress-induced glucocorticoid secretion (Reference BarkerBarker, 1997). Furthermore, prenatal mood disorder in the mother may be implicated in impaired foetal growth and low birth weight. The exploration of maternal and foetal stressors during critical periods of development will be more complex than a simple count of the number of exposures to obstetric complications, but may lead to the identification of causal factors and the greater understanding of non-genetic risk factors for bipolar disorders.
Acknowledgements
The authors thank Dr Gunduz, Dr El-Badri and Dr Wals for allowing access to previously collected data, for providing additional unpublished data or allowing us to review pre-publication manuscripts.







eLetters
No eLetters have been published for this article.