The anterior cingulate cortex is a brain region critical for integrating cognitive and emotional functions in support of adaptive, goal-directed behaviour, Reference Devinsky, Morrell and Vogt1,Reference Bush, Luu and Posner2 and abnormalities in this region play a significant role in the pathophysiology of bipolar disorder. Reference Harrison3 Early attention focused on a subcallosal region of the anterior cingulate cortex ventral to the genu (also termed subgenual prefrontal cortex), following initial magnetic resonance imaging (MRI) evidence of a left-lateralised volumetric reduction in this region in patients with chronic unipolar depression or bipolar disorder. Reference Drevets, Price, Simpson, Todd, Reich and Vannier4 However, other reports of right-sided subcallosal reductions Reference Sharma, Menon, Carr, Densmore, Mazmanian and Williamson5 or no group differences, Reference Brambilla, Nicoletti, Harenski, Sassi, Mallinger and Frank6,Reference Zimmerman, DelBello, Getz, Shear and Strakowski7 coupled with findings of volumetric reductions in other subregions Reference Lyoo, Kim, Stoll, Demopulos, Parow and Dager8,Reference Fornito, Malhi, Lagopoulos, Ivanovski, Wood and Saling9 and even grey matter increases, Reference Bearden, Thompson, Dalwani, Hayashi, Lee and Nicoletti10,Reference Adler, DelBello, Jarvis, Levine, Adams and Strakowski11 have led to confusion regarding the precise nature of abnormalities observed in people with bipolar disorder.
One possible reason for such inconsistencies is that much of the work to date has focused on patients with chronic illness, making it difficult to determine whether the observed changes are a result of prolonged illness duration or are apparent from earlier illness stages. Studying patient samples with first-episode psychoses can help mitigate these effects, although the findings of the only four such studies that have been published have been variable: Hirayasu et al Reference Hirayasu, Shenton, Salisbury, Kwon, Wible and Fischer12 replicated earlier findings Reference Drevets, Price, Simpson, Todd, Reich and Vannier4 by identifying a volumetric reduction in the left subcallosal anterior cingulate cortex of patients with a positive family history; Kubicki et al Reference Kubicki, Shenton, Salisbury, Hirayasu, Kasai and Kikinis13 and Farrow et al Reference Farrow, Whitford, Williams, Gomes and Harris14 found no differences in grey matter using voxel-based techniques; and Adler et al Reference Adler, DelBello, Jarvis, Levine, Adams and Strakowski11 found evidence of increased grey matter in caudal anterior cingulate cortex regions.
A second reason for inconsistencies in the literature is that no study to date has adequately accounted for the substantial interindividual variability in sulcal and gyral anatomy of the anterior cingulate cortex, which differs between patients with bipolar disorder and healthy populations, Reference Fornito, Malhi, Lagopoulos, Ivanovski, Wood and Velakoulis15 and has been shown to affect volumetric and other morphometric measures in the region. Reference Paus, Otkay, Caramanos, MacDonald, Zijdenbos and D'Avirro16–Reference Fornito, Yucel, Wood, Proffitt, McGorry and Velakoulis18 Such findings indicate that spurious differences in group comparisons might emerge if patients and controls are not well matched for sulcal anatomy. To address these limitations, we applied a novel cortical surface-based protocol for parcellating the anterior cingulate cortex into functionally relevant subdivisions while accounting for the region's anatomical variability Reference Fornito, Wood, Whittle, Fuller, Adamson and Saling17,Reference Fornito, Whittle, Wood, Velakoulis, Pantelis and Yucel19 to MRI data obtained from a sample of patients with bipolar disorder experiencing their first psychotic episode. Importantly, we individually matched patients and controls for age, gender and sulcal variability to ensure that any identified changes could not be attributed to group differences in cortical folding patterns. Our surface-based approach enabled calculation of regional grey matter volume, surface area and cortical thickness with sub-millimeter resolution, allowing us to detect relatively subtle changes that may be occurring in earlier stages of the disorder. Following current theories emphasising a primary role for subcallosal abnormalities in the pathophysiology of bipolar disorder, Reference Drevets, Ongur and Price20 we expected that patients would show particularly pronounced grey matter reductions in this region.
Method
Participants
The patient sample comprised 26 patients with bipolar I disorder experiencing a first psychotic episode who were recruited from the Early Psychosis Prevention and Intervention Centre, Melbourne, Australia. All patients were in a manic state at presentation. DSM–IV 21 diagnoses were confirmed over a minimum 6-month follow-up period and assigned based on medical record review and the Structured Clinical Interview for DSM–IV (SCID). Reference First, Spitzer, Gibbon and Williams22 None of the patients had a history of childhood-onset bipolar disorder. Further details regarding recruitment procedures are published elsewhere. Reference Velakoulis, Wood, Wong, McGorry, Yung and Phillips23 Twelve (eight male) patients were taking atypical antipsychotics at the time of scanning and nine (five male) were taking typical agents (mean chlorpromazine equivalents at time of scanning: 188.04 mg/day, s.d. = 119.16). Seven patients (four male) were taking lithium: two adjunctively with typical antipsychotics, four with atypicals and one as monotherapy. Two patients were medication-free. Treatment data were not available for two patients. All patients were experiencing psychotic symptoms at the time of scanning.
Prior psychiatric treatment in this sample was as follows: 16 none, 3 depression (all were on antidepressants at MRI; maximum treatment duration was 2 months), 1 post-traumatic stress disorder (not treated at study intake). One patient reported experiencing two manic episodes 5 years prior to the study but did not seek treatment. Family history of psychiatric illness was as follows: 11 none, 1 bipolar, 1 bipolar and schizophrenia, 1 schizophrenia, 4 depression, 2 depression plus anxiety. Clinical histories were unavailable for five patients (excluding these participants did not change the findings, so their data were retained for the final analysis).
Healthy controls with no personal history of mental or neurological illness and no family history of psychosis were selected from our larger database in order to individually match them to each patient for age, gender and anterior cingulate cortex sulcal morphology (see below). Exclusion criteria for all participants included a history of steroid misuse, substantial head injury and impaired thyroid function. Participants were also excluded if they had ever met diagnostic criteria for a substance use/dependence disorder, given evidence that such disorders can affect grey matter. Reference Szeszko, Robinson, Sevy, Kumra, Rupp and Betensky24 Patients with comorbid psychiatric, neurological or significant medical conditions were also excluded from the study (see Velakoulis et al Reference Velakoulis, Wood, Wong, McGorry, Yung and Phillips23 for more details). All participants gave written, informed consent in accordance with local ethics committee guidelines. Demographic details are presented in Table 1.
Table 1 Sample characteristics
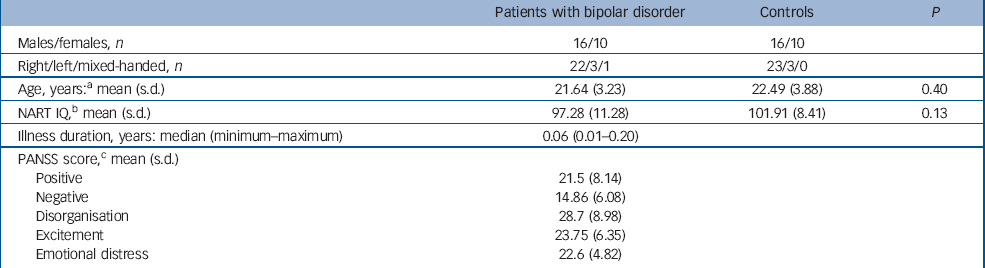
| Patients with bipolar disorder | Controls | P | |
|---|---|---|---|
| Males/females, n | 16/10 | 16/10 | |
| Right/left/mixed-handed, n | 22/3/1 | 23/3/0 | |
| Age, years:a mean (s.d.) | 21.64 (3.23) | 22.49 (3.88) | 0.40 |
| NART IQ,b mean (s.d.) | 97.28 (11.28) | 101.91 (8.41) | 0.13 |
| Illness duration, years: median (minimum—maximum) | 0.06 (0.01-0.20) | ||
| PANSS score,c mean (s.d.) | |||
| Positive | 21.5 (8.14) | ||
| Negative | 14.86 (6.08) | ||
| Disorganisation | 28.7 (8.98) | ||
| Excitement | 23.75 (6.35) | ||
| Emotional distress | 22.6 (4.82) |
Magnetic resonance imaging
Image acquisition
Scans were acquired using a GE Signa 1.5 T scanner at the Royal Melbourne Hospital, Victoria, Australia. A three-dimensional volumetric SPGR sequence generated 124 contiguous coronal slices. Imaging parameters were: time to echo (TE), 3.3 ms; time to repetition (TR), 14.3 ms; flip angle, 30°; matrix size, 256×256; field of view, 24×24 cm; voxel dimensions, 0.938×0.938×1.5 mm. Data were transferred from DAT tape to a Linux Debian 3.1 workstation and coded to ensure participant confidentiality and masked rating.
Image pre-processing
Prior to classifying sulcal morphology, each participants' image was stripped of extracerebral tissue and aligned to the N27 template via a 6° rigid-body transformation using tools contained in the FSL software package (www.fmrib.ox.ac.uk/fsl). No rescaling or warping was performed, but the images were resampled to 1 mm Reference Harrison3 voxels in the process.
Classification of sulcal variability
There are two major variations in sulcal and gyral anatomy that can affect region-of-interest boundaries and alter regional morphometry: the incidence and extent of the paracingulate sulcus, and the confluence of the cingulate sulcus with the superior rostral sulcus. Reference Fornito, Whittle, Wood, Velakoulis, Pantelis and Yucel19 The paracingulate sulcus runs dorsal and parallel to the cingulate sulcus in 30–60% of cases. Reference Yücel, Stuart, Maruff, Velakoulis, Crowe and Savage25 We classified the paracingulate sulcus as ‘present’ if there was a clearly identifiable sulcus running dorsal and parallel to the cingulate sulcus that was at least 20 mm in length, or ‘absent’ if no such sulcus was apparent, according to reliable and established criteria (see Fornito et al Reference Fornito, Whittle, Wood, Velakoulis, Pantelis and Yucel19 and Yücel et al Reference Yücel, Stuart, Maruff, Velakoulis, Crowe and Savage25 ). The superior rostral sulcus was classified as ‘continuous’ with the cingulate sulcus if the two were connected rostral to the genu of the corpus callosum; all other cases were classified as ‘separate’ (see Fornito et al Reference Fornito, Whittle, Wood, Velakoulis, Pantelis and Yucel19 and online Fig. DS1). All sulcal classifications were performed on the N27-aligned images using Analyze 6.0 for Linux (Mayo Software, Englewood, Colorado, USA).
Prior work has shown that the appearance of a paracingulate sulcus affects the size and location of anterior cingulate cortex subregions. When a paracingulate sulcus is present, the paralimbic anterior cingulate cortex (ACCP; primarily Brodmann area 32) extends from the depths of the cingulate sulcus across the crown of the paracingulate gyrus to the paracingulate sulcus; when the paracingulate sulcus is absent, the ACCP is buried in the depths of the cingulate sulcus Reference Fornito, Whittle, Wood, Velakoulis, Pantelis and Yucel19,Reference Vogt, Nimchinsky, Vogt and Hof26 (online Fig. DS1). Limbic ACC (ACCL; Brodmann area 24) is generally located between the callosal and cingulate sulci, although its size is also affected by paracingulate sulcus variability. Reference Paus, Otkay, Caramanos, MacDonald, Zijdenbos and D'Avirro16,Reference Fornito, Wood, Whittle, Fuller, Adamson and Saling17,Reference Fornito, Whittle, Wood, Velakoulis, Pantelis and Yucel19 This effect can be quite large, with one study suggesting that the appearance of a paracingulate sulcus leads to 39% decrease in ACCL volume and 88% increase in ACCP volume in some cases. Reference Fornito, Whittle, Wood, Velakoulis, Pantelis and Yucel19 Similar findings have been observed for regional surface area, although the relationship with cortical thickness is more complicated. Reference Fornito, Wood, Whittle, Fuller, Adamson and Saling17 Such findings, coupled with evidence that the paracingulate sulcus is less frequent in patients with bipolar disorder, Reference Fornito, Malhi, Lagopoulos, Ivanovski, Wood and Velakoulis15 indicate that spurious group differences may emerge if the comparison groups are not well matched for this morphological variation. To this end, we devised a four-category classification system that described the incidence of the paracingulate sulcus in both hemispheres for each individual. The four categories were: present in the left and absent in the right hemisphere (n = 9); present in both hemispheres (n = 6); absent in both hemispheres (n = 6); or absent in the left and present in the right hemisphere (n = 5). Each patient was classified according to this system and controls were then randomly selected from a larger database and individually matched to each patient on the basis of this classification, gender and age. Since paracingulate sulcus and superior rostral sulcus classifications tend to be related, this procedure also resulted in good matching for superior rostral sulcus morphology. In the right hemisphere, 8 patients and 8 controls were classified as having a continuous superior rostral sulcus, and the remaining 18 patients and 18 controls were classified as having a separate superior rostral sulcus. In the left hemisphere, 7 patients and 12 controls had a continuous classification, whereas 19 patients and 14 controls were classified as showing a separate pattern. Although there can still be considerable variation in the rostro-caudal extent of the paracingulate sulcus within participants classified as ‘present’, we have previously shown that such variations do not have a major influence on the measures examined in this study. Reference Fornito, Wood, Whittle, Fuller, Adamson and Saling17
Cortical surface reconstruction
The white (i.e. grey/white matter boundary) and pial (grey/cerebrospinal fluid boundary) surfaces of the cortical ribbon were tessellated with a triangular mesh comprising ∼150 000 vertices per hemisphere using methods described in detail elsewhere Reference Dale, Fischl and Sereno27–Reference Han, Jovicich, Salat, van der Kouwe, Quinn and Czanner29 and as implemented in the Freesurfer software package (http://surfer.nmr.mgh.harvard.edu). The surfaces were reconstructed using the raw, unaligned images in native space to avoid unnecessary interpolation and resampling, and were visually inspected and corrected for accuracy as per guidelines on the Freesurfer website. Reconstructing these surfaces enabled calculation of surface area, grey matter volume and mean cortical thickness for each of six anterior cingulate cortex regions per hemisphere. Distinguishing between these three measures is important given that volume, the most widely used metric in anatomical MRI studies in patients with bipolar disorder, is a relatively diffuse measure that reflects the product of a cortical region's surface area and thickness. Consequently, changes in one or the other parameter can be obscured unless both are assessed independently – an important consideration when attempting to characterise relatively subtle changes early in the illness course.
Each of the measures were calculated according to previously described methods. Reference Fischl and Dale28 Briefly, cortical thickness was calculated at each surface point by finding the vertex on the pial surface closest to a given point on the white surface (and vice versa) and averaging these two values. As the surfaces are generated with sub-voxel resolution, the resulting thickness measures are estimated with sub-millimeter precision and have been validated against post-mortem specimens. Reference Rosas, Liu, Hersch, Glessner, Ferrante and Salat30 The thickness values for each surface point in the regions of interest were averaged to obtain the mean thickness for that region. Regional surface area was calculated by summing the area of the triangle faces included in each region of interest for the white and pial surfaces separately, and then taking the average of the two. Grey matter volumes were calculated as the product of surface area and thickness at each surface point, averaged across all pial and white matter vertices in each region of interest.
Region-of-interest delineation
The parcellation protocol divides the ACCL and ACCP into dorsal, rostral and subcallosal regions, yielding six regions of interest per hemisphere. Boundaries separating the dorsal, rostral and subcallosal regions were designed to approximate previously identified functional subdivisions within the area. Reference Devinsky, Morrell and Vogt1,Reference Bush, Luu and Posner2 Boundaries distinguishing between the ACCL and ACCP varied in accordance with paracingulate sulcus and superior rostral sulcus variability and were based on post-mortem work documenting how cytoarchitectonic areas in the region vary in accordance with sulcal morphology Reference Vogt, Nimchinsky, Vogt and Hof26 (online Fig. DS1). A more detailed justification and description of these boundaries has been provided elsewhere. Reference Fornito, Whittle, Wood, Velakoulis, Pantelis and Yucel19 We have recently shown that reliabilities for this method are satisfactory (all >0.8, with most >0.9). Reference Fornito, Wood, Whittle, Fuller, Adamson and Saling17
Intracranial volume
Intracranial volume was calculated for each individual to control for any group differences in brain size using a previously described, reliable method. Reference Velakoulis, Wood, Wong, McGorry, Yung and Phillips23
Statistical analyses
All analyses were performed using SPSS 12.0 for Windows. Regional grey matter volumes, cortical thickness and surface area were analysed with mixed within-participant and between-participants ANOVA, with hemisphere (left or right), region (dorsal, rostral and subcallosal) and cortex (ACCL or ACCP) as within-participant factors, and diagnosis and gender as between-participants factors. Gender was included as a factor in the analyses to determine whether diagnostic differences varied for males and females. There is growing evidence that gender interacts with diagnosis in affecting brain morphology, including the anterior cingulate cortex, in other psychotic disorders, Reference Narr, Thompson, Sharma, Moussai, Zoumalan and Rayman31,Reference Goldstein, Seidman, O'Brien, Horton, Kennedy and Makris32 but such effects have seldom been investigated in bipolar disorder. Volume, area and thickness were treated as independent measures analysed using separate models to simplify interpretation of the findings. This approach allows inferences regarding group differences within each measure, but does not allow any conclusions regarding relative differences across measures (see Limitations for further discussion). As sphericity assumptions were invariably violated, main effects and interactions were evaluated using Greenhouse–Geisser corrected degrees of freedom, with α=0.05. Post hoc contrasts were evaluated against a Bonferroni-adjusted α to correct for multiple comparisons. Effect sizes, expressed as Cohen's d, are also reported for these contrasts (negative values indicate a decrease in the patient group). Only effects involving diagnosis are reported, as these were the primary focus of the current study.
We corrected grey matter volume and surface area estimates for intracranial volume using equations described by Free et al. Reference Free, Bergin, Fish, Cook, Shorvon and Stevens33 For cortical thickness, we report results of analyses of uncorrected values since the anatomical significance of the relationship between thickness and intracranial volume is unclear. Reference Rakic34 (We note that separate analyses that did covary for intracranial volume yielded similar findings.)
Results
Anterior cingulate cortex morphometry
Group means for grey matter volume, surface area and cortical thickness are presented in Table 2. For grey matter volume, there was no main effect of diagnosis (F(1,48) = 0.887, P = 0.351) or diagnosis×gender interaction (F(1,48) = 0.164, P = 0.687), nor did diagnosis interact with any of the within-participant factors. Similarly, there was no significant main effect of diagnosis (F(1,48) = 1.248, P = 0.270), diagnosis×gender interaction (F(1,48) = 0.263, P = 0.610) or interaction between diagnosis and any within-participant factor for surface area.
Table 2 Mean (s.d.) volume, surface area and cortical thickness for each group in each region of interesta
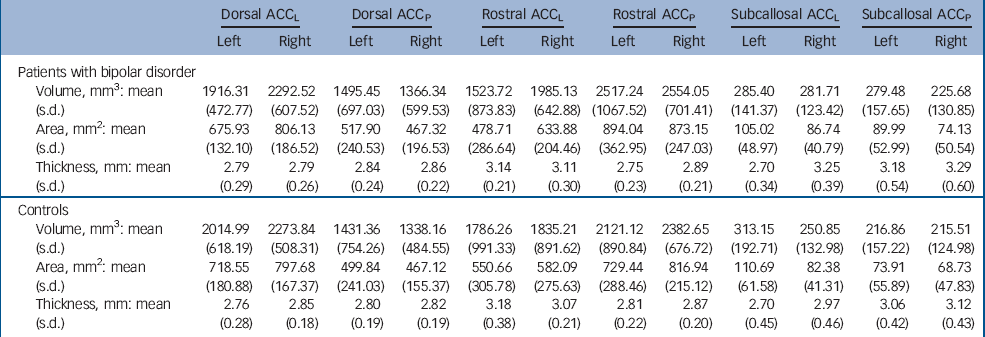
| Dorsal ACCL | Dorsal ACCP | Rostral ACCL | Rostral ACCP | Subcallosal ACCL | Subcallosal ACCP | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | |
| Patients with bipolar disorder | ||||||||||||
| Volume, mm3: mean (s.d.) | 1916.31 (472.77) | 2292.52 (607.52) | 1495.45 (697.03) | 1366.34 (599.53) | 1523.72 (873.83) | 1985.13 (642.88) | 2517.24 (1067.52) | 2554.05 (701.41) | 285.40 (141.37) | 281.71 (123.42) | 279.48 (157.65) | 225.68 (130.85) |
| Area, mm2: mean (s.d.) | 675.93 (132.10) | 806.13 (186.52) | 517.90 (240.53) | 467.32 (196.53) | 478.71 (286.64) | 633.88 (204.46) | 894.04 (362.95) | 873.15 (247.03) | 105.02 (48.97) | 86.74 (40.79) | 89.99 (52.99) | 74.13 (50.54) |
| Thickness, mm: mean (s.d.) | 2.79 (0.29) | 2.79 (0.26) | 2.84 (0.24) | 2.86 (0.22) | 3.14 (0.21) | 3.11 (0.30) | 2.75 (0.23) | 2.89 (0.21) | 2.70 (0.34) | 3.25 (0.39) | 3.18 (0.54) | 3.29 (0.60) |
| Controls | ||||||||||||
| Volume, mm3: mean (s.d.) | 2014.99 (618.19) | 2273.84 (508.31) | 1431.36 (754.26) | 1338.16 (484.55) | 1786.26 (991.33) | 1835.21 (891.62) | 2121.12 (890.84) | 2382.65 (676.72) | 313.15 (192.71) | 250.85 (132.98) | 216.86 (157.22) | 215.51 (124.98) |
| Area, mm2: mean (s.d.) | 718.55 (180.88) | 797.68 (167.37) | 499.84 (241.03) | 467.12 (155.37) | 550.66 (305.78) | 582.09 (275.63) | 729.44 (288.46) | 816.94 (215.12) | 110.69 (61.58) | 82.38 (41.31) | 73.91 (55.89) | 68.73 (47.83) |
| Thickness, mm: mean (s.d.) | 2.76 (0.28) | 2.85 (0.18) | 2.80 (0.19) | 2.82 (0.19) | 3.18 (0.38) | 3.07 (0.21) | 2.81 (0.22) | 2.87 (0.20) | 2.70 (0.45) | 2.97 (0.46) | 3.06 (0.42) | 3.12 (0.43) |
For cortical thickness, there was no main effect of diagnosis (F(1,48) = 0.705, P = 0.405) or diagnosis×gender interaction (F(1,48) = 1.001, P = 0.322), although there was a significant diagnosis×hemisphere×gender interaction (F(1,48) = 5.790, P = 0.020). As can be seen in Fig. 1, this effect was largely driven by male patients showing increased thickness in the right subcallosal areas. Post hoc contrasts revealed that male patients showed a significant thickness increase in the right subcallosal ACCL, with no other differences reaching statistical significance (Table 3).

Fig. 1 Mean thickness for male and female patients and controls in each limbic (ACCL) and paralimbic (ACCP) anterior cingulate cortex subregion. Error bars represent standard deviations. Left column presents data for left hemisphere regions; right column presents data for right hemisphere regions. *P<0.05, corrected.
Table 3 Results of post hoc contrasts comparing patients and controls in each anterior cingulate cortex subregion separately for males and females
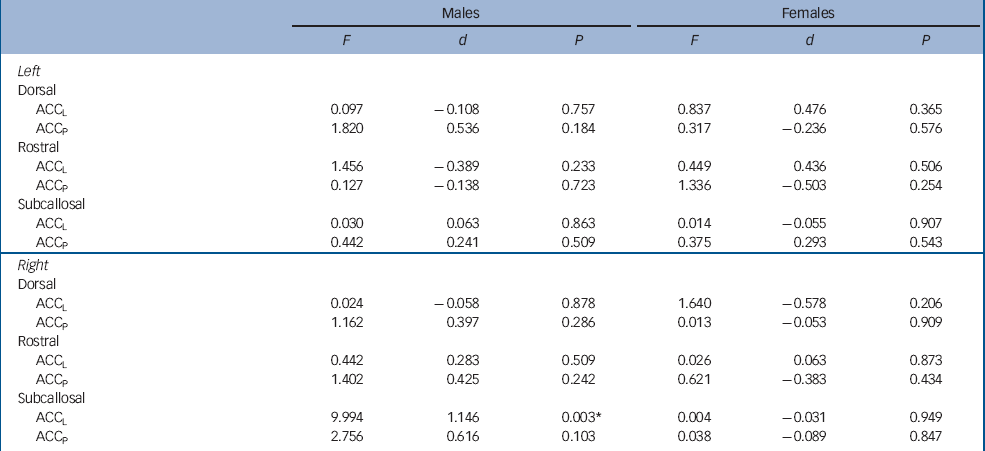
| Males | Females | |||||
|---|---|---|---|---|---|---|
| F | d | P | F | d | P | |
| Left | ||||||
| Dorsal | ||||||
| ACCL | 0.097 | -0.108 | 0.757 | 0.837 | 0.476 | 0.365 |
| ACCP | 1.820 | 0.536 | 0.184 | 0.317 | -0.236 | 0.576 |
| Rostral | ||||||
| ACCL | 1.456 | -0.389 | 0.233 | 0.449 | 0.436 | 0.506 |
| ACCP | 0.127 | -0.138 | 0.723 | 1.336 | -0.503 | 0.254 |
| Subcallosal | ||||||
| ACCL | 0.030 | 0.063 | 0.863 | 0.014 | -0.055 | 0.907 |
| ACCP | 0.442 | 0.241 | 0.509 | 0.375 | 0.293 | 0.543 |
| Right | ||||||
| Dorsal | ||||||
| ACCL | 0.024 | -0.058 | 0.878 | 1.640 | -0.578 | 0.206 |
| ACCP | 1.162 | 0.397 | 0.286 | 0.013 | -0.053 | 0.909 |
| Rostral | ||||||
| ACCL | 0.442 | 0.283 | 0.509 | 0.026 | 0.063 | 0.873 |
| ACCP | 1.402 | 0.425 | 0.242 | 0.621 | -0.383 | 0.434 |
| Subcallosal | ||||||
| ACCL | 9.994 | 1.146 | 0.003* | 0.004 | -0.031 | 0.949 |
| ACCP | 2.756 | 0.616 | 0.103 | 0.038 | -0.089 | 0.847 |
Medication effects
Excluding the seven patients taking lithium did not change the cortical thickness finding (hemisphere×gender×diagnosis interaction: F(1,34) = 8.796, P = 0.005). To investigate whether antipsychotic class had any effect, patients taking typicals were matched to those taking atypicals for paracingulate sulcus morphology using the procedures described above. Any patients who could not be matched were excluded from the analysis, resulting in eight patients taking typicals being compared with eight taking atypicals. This analysis revealed no significant main effect of antipsychotic class on cortical thickness (F(1,14) = 0.131, P = 0.723) and no interactions between medication class and any of the within-participant factors.
Discussion
This study implemented a novel surface-based approach to characterise anatomical abnormalities of the anterior cingulate cortex in patients with bipolar disorder experiencing their first episode of psychosis. Relative to controls matched for age, gender and paracingulate sulcus, male patients showed a significant thickness increase in the right subcallosal ACCL. No significant differences were identified for grey matter volume or surface area, or for any measure in the female group. The fact that only thickness, but not surface area or volume, was different highlights the importance of distinguishing between these measures when assessing anatomical changes in psychiatric disorders and suggests thickness may be a particularly sensitive metric for detecting such changes, especially in early illness stages. Importantly, this is the first study to match patients and controls for sulcal variability, indicating these differences are unlikely to be caused by group biases in cortical folding patterns.
Past findings
Differences in subcallosal areas were expected, although the direction of the difference (i.e. grey matter increase in patients) was not. Several authors have emphasised a primary role for subcallosal grey matter reductions, particularly in the left hemisphere, in the pathophysiology of bipolar disorder, Reference Harrison3,Reference Hirayasu, Shenton, Salisbury, Kwon, Wible and Fischer12,Reference Drevets, Ongur and Price20 but past MRI studies of the anterior cingulate cortex in bipolar disorder have reported variable findings with respect to the laterality, subregion and nature (grey matter increase or decrease) of the abnormality. Reference Drevets, Price, Simpson, Todd, Reich and Vannier4–Reference Lyoo, Kim, Stoll, Demopulos, Parow and Dager8,Reference Hirayasu, Shenton, Salisbury, Kwon, Wible and Fischer12 The majority of these studies have been conducted in patients with prolonged illness, although four other studies of patients with first-episode affective psychosis have also yielded contradictory findings. Reference Adler, DelBello, Jarvis, Levine, Adams and Strakowski11–Reference Kubicki, Shenton, Salisbury, Hirayasu, Kasai and Kikinis13 Direct comparison between these reports and our findings is complicated by differences in the methods used and the fact that we had insufficient power to appropriately examine the effects of family history. This is an important consideration, given that two independent studies have previously found left subcallosal reductions in familial patients. Reference Drevets, Price, Simpson, Todd, Reich and Vannier4,Reference Hirayasu, Shenton, Salisbury, Kwon, Wible and Fischer12 By way of speculation, the fact that the majority of our sample did not report a family history of affective illness suggests that left-sided abnormalities may be more prominent in familial cases, whereas right-sided changes may characterise sporadic patients.
Prior neglect of the potential effect of paracingulate sulcus variability on the findings is also likely to contribute to inconsistent findings in the literature. We have previously shown that paracingulate sulcus variability can have a large and complex effect on regional volume, surface area and thickness in the anterior cingulate cortex Reference Fornito, Wood, Whittle, Fuller, Adamson and Saling17,Reference Fornito, Whittle, Wood, Velakoulis, Pantelis and Yucel19 and that, as a group, patients with bipolar disorder are less likely to display this sulcus than healthy controls. Reference Fornito, Malhi, Lagopoulos, Ivanovski, Wood and Velakoulis15 This means that spurious group differences in estimates of regional grey matter such as volume and thickness can arise if patients and controls are not well matched for this anatomical variation. Consequently, inconsistent and contradictory findings of anterior cingulate cortex changes in patients with bipolar disorder may partly reflect differences in regional cortical folding patterns rather than true changes in the relative amount of grey matter in the region.
When such sulcal variability is accounted for, our findings suggest that first-episode psychosis in bipolar disorder is associated with a thickness increase in the right subcallosal ACCL of male patients. Relatively few studies of bipolar disorder have examined gender effects on anterior cingulate cortex morphometry in detail. Drevets et al Reference Drevets, Price, Simpson, Todd, Reich and Vannier4 found a left subcallosal anterior cingulate cortex reduction while statistically covarying for gender in patients with chronic bipolar disorder, Reference Drevets, Price, Simpson, Todd, Reich and Vannier4 whereas another study of a similar region in a predominantly female sample found no group differences, Reference Sassi, Brambilla, Hatch, Nicoletti, Mallinger and Frank35 suggesting the differences are more pronounced in males. Kaur et al Reference Kaur, Sassi, Axelson, Nicoletti, Brambilla and Monkul36 failed to identify a gender× diagnosis interaction in their study of the entire anterior cingulate gyrus, although they studied a paediatric sample.
To date, only three other studies have found grey matter increases in the anterior cingulate cortex of patients with bipolar disorder. Adler et al Reference Adler, Levine, DelBello and Strakowski37 reported an increase in a region corresponding to our rostral ACCL/ACCP in a sample of patients with long-term illness. More recently, Bearden et al Reference Bearden, Thompson, Dalwani, Hayashi, Lee and Nicoletti10 found evidence of increased grey matter in the anterior cingulate cortex of patients with established bipolar disorder, although they attributed their findings to the effects of lithium, since a subsample of patients not taking lithium at the time of scanning showed no evidence of such changes, and a significant, inverted U-shaped relationship was observed between anterior cingulate cortex grey matter and duration of exposure to lithium. However, another recent study of patients with bipolar disorder experiencing their first episode of mania Reference Adler, DelBello, Jarvis, Levine, Adams and Strakowski11 also reported increased anterior cingulate cortex grey matter in a sample where only 1 patient (out of 33) was being treated with lithium. When coupled with our finding that excluding patients taking lithium did not change the results, this suggests not all grey matter increases can be attributed to the effects of this drug. It should be noted, however, that the grey matter increase reported by Adler et al Reference Adler, DelBello, Jarvis, Levine, Adams and Strakowski11 was in the caudal, rather than subcallosal anterior cingulate cortex. Reasons for this discrepancy include that Adler et al did not consider the influence of paracingulate sulcus variability on their findings and that they did not examine gender×diagnosis interactions. Furthermore, our sample was composed entirely of patients with bipolar disorder experiencing psychotic symptoms, whereas Adler et al Reference Adler, DelBello, Jarvis, Levine, Adams and Strakowski11 did not report how many of their patients were experiencing such symptoms. This may be an important distinction, given emerging evidence for a distinct phenotype in this subgroup of patients. Reference Glahn, Bearden, Barguil, Barrett, Reichenberg and Bowden38 Further investigation of the neuroanatomical changes associated with psychotic and non-psychotic bipolar disorder will therefore be an important avenue of future research.
The gender-specific nature of our findings, coupled with the direction and laterality of the thickness difference (i.e. we found a right-sided increase, rather than decrease, in our patient group), is interesting in light of recent evidence regarding the role of the subcallosal anterior cingulate cortex in regulating the body's physiological stress response, particularly as mediated through the hypothalamic–pituitary–adrenal (HPA) axis. The subcallosal anterior cingulate cortex is heavily connected with the hypothalamus, amygdala and brainstem autonomic nuclei. Reference Sesack, Deutch, Roth and Bunney39 Rodent work has shown that lesions to the right, but not left, ventral medial prefrontal cortex (which includes the subcallosal anterior cingulate cortex) significantly decrease corticosterone levels in response to external stressors, Reference Sullivan and Gratton40 suggesting that the right subcallosal anterior cingulate cortex plays an important role in activating the HPA axis. It is, therefore, plausible that although lesions to this area reduce HPA activation, increased thickness may reflect a hyperfunctional abnormality that produces an excessive stress response. Heightened HPA activity has been demonstrated in patients with first-episode psychosis, Reference Ryan, Collins and Thakore41 and although the precise mechanism through which a thickness increase might lead to this excessive stress response remains unclear, a link between the two is suggested by evidence that other brain regions implicated in HPA axis activation, such as the amygdala and pituitary gland, are also enlarged in patients with first-episode affective psychosis. 21,Reference Pariante, Dazzan, Danese, Morgan, Brudaglio and Morgan42 In this context, two notable findings are that: (a) patients with bipolar disorder show increased anatomical connectivity between the subcallosal anterior cingulate cortex and amygdala; Reference Houenou, Wessa, Douaud, Leboyer, Chanraud and Perrin43 and (b) volume of the pituitary gland, which is correlated with peripheral measures of HPA activation, Reference Axelson, Doraiswamy, Boyko, Rodrigo Escalona, McDonald and Ritchie44 is increased in ultra-high-risk individuals who eventually develop an affective psychosis, with the enlargements being most pronounced in individuals scanned in closer temporal proximity to psychosis onset. Reference Garner, Pariante, Wood, Velakoulis, Phillips and Soulsby45 It is unclear whether an increase in anterior cingulate cortex thickness such as that observed in our data would also precede psychosis onset, although these findings do support the notion that size increases in brain regions implicated in HPA regulation are associated with an elevated stress response. Chronic hyperactivity of this circuit may account for why reports of volumetric reductions in the anterior cingulate cortex are more common in samples with chronic illness, Reference Drevets, Price, Simpson, Todd, Reich and Vannier4,Reference Sharma, Menon, Carr, Densmore, Mazmanian and Williamson5,Reference Sassi, Brambilla, Hatch, Nicoletti, Mallinger and Frank35 given that prolonged elevation of corticosterone levels has been associated with decreased volume in both rodents and humans. Reference MacLullich, Ferguson, Wardlaw, Starr, Deary and Seckl46,Reference Cerqueira, Catania, Sotiropoulos, Schubert, Kalisch and Almeida47
Taken together, these findings suggest that relative hypertrophy in brain regions critical for regulating HPA axis activation (i.e. the anterior cingulate cortex, amygdala and pituitary) are associated with an elevated stress response around the time of psychosis onset that ultimately causes volumetric contractions in later illness stages. In this context, the gender-specific nature of our findings may be related to gender differences in HPA reactivity, which is known to be elevated in males relative to females. Reference Kajantie and Phillips48 It should be noted, however, that left-sided reductions have been observed in patients with familial bipolar disorder, Reference Hirayasu, Shenton, Salisbury, Kwon, Wible and Fischer12 with Drevets et al Reference Drevets, Ongur and Price20 positing that such changes may disinhibit right-sided activation of physiological stress responses in familial patients. Thus, although the effects of paracingulate sulcus variability on these earlier findings are unknown and the precise effects of family history remain to be characterised, it is possible that lateralised pathology of the subcallosal anterior cingulate cortex differs in sporadic and familial cases. The net effect of both would be to increase HPA reactivity, albeit through distinct mechanisms. Future work examining the role of stress, gender and family illness history in influencing the longitudinal course of neuroanatomical abnormalities in bipolar disorder will be critical for characterising these relationships further.
Limitations
In this study, we defined ‘first-episode’ as first contact with psychiatric services for a psychotic episode and we did not control for the effects of prodromal symptoms, duration of untreated psychosis or pre-psychotic affective episodes. The effects of such characteristics can only be unambiguously determined through prospective work, although one recent voxel-based study found no effect of duration of untreated psychosis (retrospectively assessed) on anterior cingulate cortex grey matter, Reference Lappin, Morgan, Morgan, Hutchison, Chitnis and Suckling49 suggesting that any such influences on our region-of-interest measures may be minimal. We also note that there was no significant correlation between thickness in this region and duration of psychosis in our patient sample (Spearman's rho = −0.121, P = 0.574).
Excluding patients taking lithium did not significantly alter our findings, suggesting they do not reflect grey matter increases known to be related to mood stabiliser treatment. Reference Bearden, Thompson, Dalwani, Hayashi, Lee and Nicoletti10 Similarly, we found no evidence for an effect of antipsychotic class, although our power for detecting such an effect was limited. Past studies of such effects have yielded inconsistent findings, with one voxel-based investigation reporting that, relative to drug-free patients with psychosis (comprising both schizophrenia and affective psychosis), those taking typical antipsychotics had less anterior cingulate cortex grey matter, whereas those taking atypicals showed no significant changes in this region. Reference Dazzan, Morgan, Orr, Hutchinson, Chitnis and Suckling50 In contrast, recent work using region-of-interest techniques has suggested that anterior cingulate cortex volume is positively correlated with exposure to typical antipsychotics and negatively correlated with exposure to atypicals. Reference McCormick, Decker, Nopoulos, Ho and Andreasen51 However, this study also failed to find a difference in the rate of volumetric change between patients with schizophrenia taking typicals or atypicals in the first 2–3 years following psychosis onset, suggesting that the effects of differential antipsychotic exposure on anterior cingulate cortex morphometry are minimised in the first few years of illness. Although we cannot determine whether similar changes to those reported in this paper would be observed in a medication-naïve sample, the fact that the median illness duration in our sample was ∼3 weeks (the longest was 10 weeks) suggests that any effects of antipsychotic medication were minimal.
We deemed our retrospective paracingulate sulcus matching procedure to be an efficient means for matching patients and controls on this important parameter since prospective matching would require very large samples (i.e. its morphology is only ascertainable after scanning). It should be noted that controls, but not patients, were selected to meet the matching criteria, meaning that no biases were introduced into the patient sample, as this group represented all patients for whom data were available. Although the matching procedure may have introduced some sampling bias in the control group, this bias would have minimised any differences between patients and controls, since the controls were being chosen explicitly because their brain had a similar morphology to that of the patient group. (Preliminary analyses indicated no significant differences between controls selected and not selected with respect to National Adult Reading Test Reference Nelson and Wilson52 IQ.) Matching patients and controls for both paracingulate sulcus and superior rostral sulcus morphology would require very large samples and was not possible in the current study. However, since these two morphological variations tend to be related, our paracingulate sulcus matching also resulted in good matching for superior rostral sulcus morphology. Importantly, superior rostral sulcus morphology was perfectly matched in the right hemisphere, where the primary thickness differences were, suggesting superior rostral sulcus differences cannot account for our findings.
Our attempt to account for the functional and anatomical diversity of the anterior cingulate cortex resulted in a highly multivariate data-set with many comparisons being made. Our analyses therefore attempted to strike an appropriate balance between control of type I and type II errors, while minimising complexity. To simplify analysis and interpretation of the findings, we treated grey matter volume, area and thickness as independent measures that were analysed using separate models. As we treated these measures independently, we evaluated main effects and interactions in these models using uncorrected significance thresholds, although the post hoc contrasts used to characterise the main finding of this work were tested using very stringent criteria (i.e. Bonferroni correction for 12 comparisons). This, coupled with the large effect size associated with the subcallosal difference (Cohen's d >1), indicates the effect is robust and unlikely to be a false positive. However, our failure to find differences in other regions may have reflected a lack of power. Table 3 indicates there were several regions in which moderate effect sizes were obtained (including in female patients), suggesting they warrant further investigation in larger samples. Such work should also investigate the relationship and interactions between changes in volume, area and cortical thickness, rather than treating them independently.
Future study
Our novel surface-based approach enabled us to derive a detailed characterisation of anterior cingulate cortex abnormalities in the early stages of bipolar psychosis. By explicitly accounting for individual differences in sulcal morphology, we ensured that our results are not an artifact of group differences in local cortical folding patterns. Our findings provide an impetus for future research examining the longitudinal course of neuroanatomical abnormalities in bipolar disorder, while also considering the influences of family illness history and gender differences in stress responses.
Acknowledgement
The authors thank Dr Sue M. Cotton for helpful comments regarding this manuscript.







eLetters
No eLetters have been published for this article.