Epidemiological studies link high polyphenol intake with reduced risk of oxidative stress-related diseases like diabetes, hypertension and CVD(Reference Buijsse, Feskens and Kok1–Reference Pereira, Parker and Folsom3). In particular, consumption of cocoa and dark chocolate (DC) has been shown to improve endothelium function, insulin sensitivity, blood pressure (BP) in healthy individuals, hypertensives with or without glucose intolerance(Reference Grassi, Lippi and Necozione4–Reference Grassi, Desideri and Necozione6) and obese subjects(Reference Davison, Coates and Buckley7). Cocoa and DC are rich sources of polyphenols providing on average more polyphenols per serving than red wine, green tea or black tea(Reference Lee, Kim and Lee8). These polyphenols confer potent antioxidant properties to cocoa and DC(Reference Lee, Kim and Lee8, Reference Richelle, Tavazzi and Offord9) in addition to the ability to regulate NO(Reference Grassi, Lippi and Necozione4–Reference Davison, Coates and Buckley7).
Obesity is known to be associated with insulin resistance and elevated BP(Reference Olson, Schmitz and Leon10). One of the underlying factors linked to these cardiovascular risk factors is abnormal cortisol metabolism(Reference Vicennati and Pasquali11, Reference Duclos, Pereira and Barat12). Cortisol is a counterregulatory hormone that is essential in the long-term maintenance of blood glucose(Reference Newton13) and which could also unfavourably influence BP and lipid profile(Reference Duclos, Pereira and Barat12–Reference Kidambi, Kitchen and Grim15). When present in excess, cortisol induces overproduction of reactive oxygen species(Reference Bjelaković, Beninati and Pavlović16, Reference Iuchi, Akaike and Mitsui17) leading to reduced endothelial NO synthase expression(Reference Liu, Mladinov and Pietrusz18). In obesity, particularly abdominal obesity, postprandial hypercortisolism and enhanced peripheral metabolism of cortisol, characterised by increased urinary cortisone-to-cortisol ratio, are observed which are linked to insulin resistance and increased fasting insulin(Reference Vicennati and Pasquali11). Increased expression of subcutaneous adipose tissue 11β-hydroxysteroid dehydrogenase type 1 has also been reported, which is known to impair glucose-stimulated insulin secretion(Reference Alberti, Girola and Gilardini19). Since improved NO bioavailability is the main mechanism by which DC polyphenols reduce endothelium dysfunction, insulin resistance and hypertension(Reference Grassi, Lippi and Necozione4–Reference Davison, Coates and Buckley7), this preliminary study aimed to assess and compare the effect of DC containing two different doses of polyphenols on fasting capillary whole blood glucose levels, total cholesterol, BP, urinary free cortisol and cortisone excretion in healthy overweight and obese subjects. The other objective was to observe whether improvements in fasting blood glucose, total cholesterol and BP could be correlated with changes in urinary free cortisol or cortisone excretion. A secondary objective was to monitor Mg intake and excretion since DC is known to contain large quantities of Mg, which, in turn, could influence BP, insulin action and the metabolic syndrome(Reference Meisel20–Reference Song, Choi and Oh22).
Methods
Study design
The study used a randomised, cross-over design where each subject acted as their own control. Following a 1-week run-in phase, eligible subjects were randomly assigned to one of the two polyphenol doses: 500 mg polyphenols DC or 1000 mg polyphenols DC. Participants followed each intervention for 2 weeks, after which they were crossed-over to the next intervention separated by a 1-week washout period (Fig. 1). The study included healthy non-smoker volunteers, aged 19–50 years with BMI ≥ 25 kg/m2(23), no history of diabetes, hypertension or CVD. People taking dietary supplements, BP or cholesterol-lowering drugs, or those with soya and nut allergies were excluded. Smokers were excluded to minimise confounding factors since nicotine consumption is known to enhance hypothalamic–pituitary–adrenal axis activity, hence resulting in elevated cortisol levels(Reference Lovallo24, Reference Rohleder and Kirschbaum25). Participants gave written consent, completed a lifestyle questionnaire before being screened for fasting blood glucose, total cholesterol, BP and BMI to determine their eligibility. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by Queen Margaret University Ethics Committee.

Fig. 1 Diagram showing random allocation of subjects into the different dietary interventions. BP, blood pressure; DC, dark chocolate.
Diet
Table 1 provides a summary of the nutrient composition of the two Acticoa DC used in the present study, which were kindly supplied by Barry Callebaut (Lebbeke, Belgium). The 500 mg dose was previously shown to reduce fasting glucose (FG) levels and BP by Grassi et al. (Reference Grassi, Lippi and Necozione4, Reference Grassi, Necozione and Lippi5) and Taubert et al. (Reference Taubert, Berkels and Roesen26). However, due to the great variation in epicatechin and catechin levels between the chocolate used in the present study and the one used by Grassi et al. (Reference Grassi, Lippi and Necozione4, Reference Grassi, Necozione and Lippi5), a higher DC dose of 1000 mg was also chosen. This 1000 mg dose was selected to provide similar quantities of polyphenol to what is consumed by the Kuna population of Panama, who are known to consume large quantities of cocoa and to have low incidence of hypertension(Reference Bayard, Chamorro and Motta27). This dose will also provide about 43·2 % of the epicatechin and catechin dose used by Grassi et al. (Reference Grassi, Lippi and Necozione4, Reference Grassi, Necozione and Lippi5). Subjects were instructed to distribute DC doses throughout the day to achieve a high steady-state concentration. They were also instructed to maintain their usual diet throughout the study but to refrain from polyphenol-rich foods and beverages that supply ≥ 15 mg/kg epicatechin and ≥ 4 mg/l epicatechin(Reference Olthof, Hollman and Katan28–Reference Olthof, Hollman and Buijsman30). Subjects completed a 3-d (two weekdays and one weekend) diet and physical activity(Reference Bouchard, Tremblay and Leblanc31) diary during the run-in phase and at the end of each dietary intervention. The Photographic Atlas of Food Portion Sizes was used to assist subjects in describing their portion sizes(Reference Nelson, Atkinson and Meyer32). Diet diaries were validated by interviewing the subjects using a validated questionnaire(Reference Lindroos, Lissner and Sjostrom33). The diet diaries were analysed and energy, fat, protein, carbohydrate and magnesium intake were estimated using Windiet software (Windiet Research, Univation Ltd, Robert Gordon University, Aberdeen, UK). Compliance with the study's protocol was assessed by direct interviewing, returning of empty chocolate foils and assessment of diet diaries.
Table 1 Nutritional composition of 20 g of 500 and 1000 mg polyphenol dark chocolate (DC)

Measurements
To measure fasting blood glucose and total cholesterol, 12-h fasting capillary whole blood samples were obtained and analysed using a calibrated Accutrend GC system (Roche diagnostics, Mannheim, Germany). Participants were instructed to consume the last DC dose 12 h before analyses(Reference Taubert, Roesen and Lehmann34), avoid heavy physical activity and alcohol intake 24 h before testing and consume the same diet the day before each test(Reference Olthof, Hollman and Buijsman30). Waist circumference, hip circumference and BMI were measured. Data on waist and hip circumference were used to calculate waist-to-hip ratio, where waist-to-hip ratio >1·0 in men and >0·85 in women indicate abdominal obesity(Reference Farin, Abassi and Reaven35). Both waist circumference and BMI serve as good indicators of the degree of insulin resistance in overweight and obese individuals(Reference Farin, Abassi and Reaven35) while waist-to-hip ratio serves as a predictor of hypertension(Reference Fuchs, Gus and Moreira36) and hypothalamic-pituitary–adrenal axis hyperactivity, characterised by high baseline plasma cortisol and low 24-h urinary cortisol excretion in obese women(Reference Vicennati and Pasquali11). An automated A&D Medical UA-767 BP monitor (A&D Medical, San Jose, CA, USA) was used to measure BP according to Grassi et al. (Reference Grassi, Necozione and Lippi5). This monitor was previously validated and was shown to achieve grade A for both systolic and diastolic BP according to the British Hypertension Society standard(Reference Verdecchia, Angeli and Poeta37).
Urine samples were obtained for estimating 24-h urinary Mg excretion. Urinary Na and K excretion were also monitored since they serve as direct measures of Na and K intake, which could act as confounding factors in relation to BP. The 24-h urine collections were validated by measuring creatinine excretion(Reference Rios, Gonthier and Remesy38, Reference Roura, Andres-Lacueva and Estruch39). Analyses of urine Na, K and Mg concentrations were conducted using an automated platform (Olympus, Essex, UK) at the Clinical Biochemistry Laboratory, Royal Infirmary of Edinburgh, Scotland, UK. Urinary cortisol and cortisone levels were analysed in duplicates using ELISA according to the method described by Al-Dujaili & Bryant(Reference Al-Dujaili and Bryant40) and Al-Dujaili(Reference Al-Dujaili41). The data were then used to calculate urinary cortisol-to-cortisone ratio. This ratio serves as a measure of renal 11β-hydroxysteroid dehydrogenase type 2 activity(Reference Palermo, Shackleton and Mantero42). Monitoring the activity of this enzyme helps detect changes in peripheral metabolism of cortisol(Reference Palermo, Shackleton and Mantero42). All tests were carried out at baseline, before and after each intervention.
Statistical methods
All data are expressed as means and standard deviations. Mixed between–within subjects ANOVA or split-plot ANOVA was performed for multiple comparison, where time (baseline, week 1, week 2) was the within-group variable and intervention group (500 mg, 1000 mg DC) was the between-group variable and the continuous variable were FG, SBP, DBP BMI, weight, waist circumference, hip circumference and waist-to-hip circumference. A P-value ≤ 0·05 was considered statistically significant. Within each intervention group (500, 1000 mg DC), changes in fasting blood glucose levels, SBP and DBP were analysed using repeated measures ANOVA with Bonferroni post hoc tests. A separate split-plot ANOVA was also performed to detect any carry-over effects between the two interventions and to ensure changes in FG, SBP and DBP following each treatment were not affected by the sequence of DC administration (1000 mg followed by 500 mg v. 500 mg followed by 1000 mg). Two-tailed paired sample t tests were used to assess changes between baseline and post-intervention total cholesterol, urinary free cortisol or cortisone, urinary cortisol-to-cortisone ratio and mineral excretion. Similarly, differences in response to both DC doses among the various ethnic groups were assessed using one-way between-groups ANOVA with FG, SBP and DBP as the dependent variables and ethnicity as the factor. An independent sample t test was also used to compare the response to DC polyphenols between the abdominally obese individual and the peripherally obese individuals. The relationship between fasting blood glucose levels, total cholesterol, SBP, DBP, BMI, weight, waist circumference, hip circumference, waist-to-hip circumference, urinary Mg, Na and K levels, 24-h urinary free cortisol, cortisone and cortisol-to-cortisone ratio were assessed using Pearson product-moment correlation coefficient, r. The coefficient of determination was estimated by obtaining r 2. All statistical analyses were performed using SPSS for Windows, version 16.0.0 (SPSS Inc., Chicago, IL, USA). The sample size was calculated using G-power software version 3.0.8 (Heinrich Heine University, Dusseldorf, Germany) to detect 0·3 mmol/l reduction in FG with baseline sd = 0·5 mmol/l and post-DC sd = 0·04 mmol/l, which is similar to the reduction reported by Grassi et al. (Reference Grassi, Desideri and Necozione6).
Results
The study included fourteen healthy volunteers (eight males (five Caucasians, two Asians, one African) and six females (five Caucasians and one Hispanic)), 21–50 years old, mean age 26·4 (sd 11·5) years) with a BMI of 27·7 (sd 2·5) kg/m2. Of these participants, thirteen were peripherally obese and one was abdominally obese (African).
Mixed between–within subjects ANOVA revealed a significant reduction in fasting capillary blood glucose concentrations (P = 0·002), SBP (P < 0·0001) and DBP (P < 0·0001) following DC consumption. These effects were independent of the sequence of DC administration and no significant interaction between time, intervention group and sequence of DC administration was observed (FG F(2,11) = 1·057, P = 0·380; SBP F(2,11) = 0·431, P = 0·660; DBP F(2,11) = 0·653, P = 0·539; Figs. 2 and 3). No significant differences between the effect of 1000 and 500 mg polyphenols DC on fasting capillary blood glucose (P>0·05) and BP (P>0·05) were observed indicating that both doses have a similar efficacy.
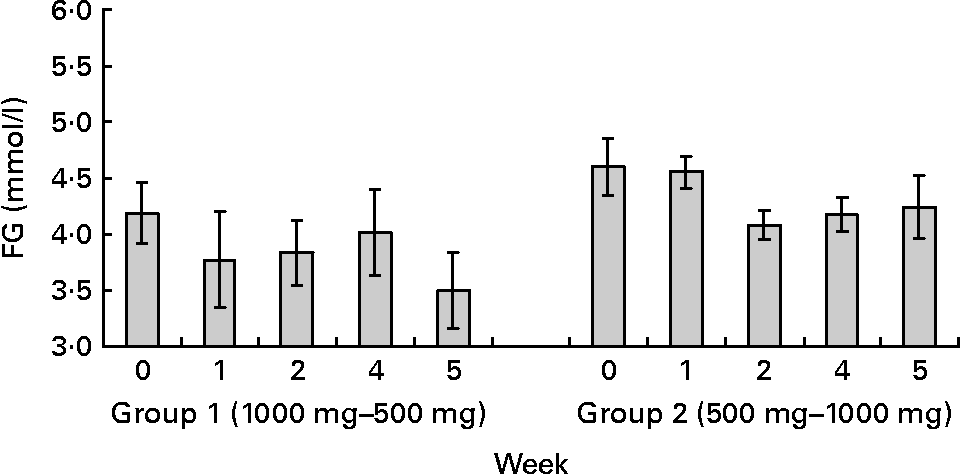
Fig. 2 Capillary fasting glucose (FG) levels at baseline (week 0), and at the end of 1and 2 weeks of each of the polyphenols doses. Group 1 received 1000 mg polyphenols dark chocolate (weeks 1–2) followed by 500 mg polyphenols dark chocolate (weeks 4–5). Group 2 received 500 mg polyphenols dark chocolate (weeks 1–2) followed by 1000 mg polyphenols dark chocolate (weeks 4–5). Changes in FG were independent of the sequences of chocolate administration (P>0·05). Values are means with their standard errors represented by vertical bars.

Fig. 3 Systolic blood pressure (SBP) (a) and diastolic blood pressure (DBP) (b) at baseline (week 0), and at the end of 1 and 2 weeks of each of the polyphenols doses. Group 1 received 1000 mg polyphenols dark chocolate (weeks 1–2) followed by 500 mg polyphenols dark chocolate (weeks 4–5). Group 2 received 500 mg polyphenols dark chocolate (weeks 1–2) followed by 1000 mg polyphenols dark chocolate (weeks 4–5). Changes in SBP and DBP were independent of the sequences of chocolate administration (P>0·05). Values are means with their standard errors represented by vertical bars.
To explore the results further, a one-way repeated measures ANOVA was conducted to compare fasting blood glucose levels, SBP and DBP at baseline, week 1 and week 2 for each of the two dietary interventions. A significant effect of DC on fasting blood glucose levels (F(2,12) = 4·305, P = 0·039), SBP (F(2,12) = 12·330, P = 0·001) and DBP (F(2,12) = 13·937, P = 0·001) was observed after consumption of 1000 mg DC. Post hoc comparisons using Bonferroni test indicated that mean fasting blood glucose levels and SBP at week 2 were significantly decreased after chocolate ingestion (FG 3·97 (sd 0·54) v. baseline 4·42 (sd 0·70) mmol/l; SBP 112·12 (sd 9·68) v. baseline 119·38 (sd 10·51) mmHg). Mean DBP levels were significantly lower at week 1 (74·45 (sd 7·17) mmHg) and week 2 (74·57 (sd 7·39) mmHg) compared to baseline (78·62 (sd 7·74) mmHg).
A significant effect of 500 mg DC on FG levels (F(2,12) = 5·026, P = 0·026), SBP (F(2,12) = 11·971, P = 0·001) and DBP (F(2,12) = 7·709, P = 0·007) was also observed. Post hoc comparisons indicated that the mean FG levels at week 2 were significantly different from baseline (3·92 (sd 0·86) v. 4·42 (sd 0·30) mmol/l). Mean SBP was also reduced at the end of week 1 (114·24 (sd 9·53) mmHg) and week 2 (112·40 (sd 9·51) mmHg) as compared to baseline (119·38 (sd 10·51) mmHg). Similar findings were observed with DBP (Week 1 = 74·62 (sd 4·27) and Week 2 = 73·00 (sd 5·06) v. baseline 78·62 (sd 7·74) mmHg).
Total cholesterol did not change significantly after 1000 mg (P = 0·191) or 500 mg polyphenols DC (P = 0·246). There was a trend towards a reduction in 24-h urinary free cortisone levels in both the 1000 and 500 mg DC groups, although this reduction did not reach statistical significance even after adjustment for weight. No changes in anthropometrical data (Table 2), 24-h urinary free cortisol, cortisol-to-cortisone ratio, 24-h urinary Mg, Na, K were observed (Table 3). Likewise, one-way ANOVA revealed no significant effect of ethnicity on changes in FG, SBP, DBP in both DC groups, with the exception of one female subject (Hispanic), who experienced a greater reduction in SBP following both polyphenol doses as compared to Caucasians (1000 mg, P = 0·009; 500 mg, P < 0·0001), Asians (1000 mg, P = 0·014; 500 mg P = 0·003) and African (1000 mg, P = 0·0001; 500 mg, P < 0·009). The subject with abdominal obesity showed an increase in urinary free cortisol following 1000 mg polyphenols (+52·44 nmol/d) compared to peripherally obese individuals, who demonstrated a reduction ( − 11·05 nmol/d; P = 0·037). This subject also had a greater reduction in DBP following 500 mg DC compared to other individuals ( − 15·7 mmHg v. − 3·10 mmHg; P = 0·017).
Table 2 Effect of either 500 or 1000 mg polyphenol dark chocolate (DC) on anthropometrical measurements
(Mean values and standard deviations)

Table 3 Results for 24-h urine collections
(Mean values and standard deviations)

DC, dark chocolate.
Pearson's product-moment correlations revealed a significant correlation between changes in 24-h urinary free cortisol, cortisone and changes in 24-h Na excretion (Table 4). There were no significant correlations between age and changes in FG, SBP, DBP following both DC doses (P>0·05). Addition of DC to the diet did not affect Mg intake or excretion significantly. Moreover, no significant correlations were found between changes in Mg intake or excretion and the reductions in fasting blood glucose and BP seen following DC consumption. Energy expenditure, energy, macronutrient and mineral intake did not change significantly through the study period (Fig. 4).
Table 4 Pearson product-moment correlations between changes in urinary glucocorticoid levels and changes in selected parameters


Fig. 4 Energy (I), macronutrient (II) and mineral intake (III) at baseline and at the end of each intervention: (a) 20 g dark chocolate with 1000 mg polyphenols and (b) 20 g dark chocolate with 500 mg polyphenols. Values are means with their standard errors represented by vertical bars. (![]() ), Energy expenditure; (
), Energy expenditure; (![]() ) energy intake; (
) energy intake; (![]() ) fat intake; (
) fat intake; (![]() ) protein intake; (
) protein intake; (![]() ) carbohydrate intake; (
) carbohydrate intake; (![]() ) Mg intake; (
) Mg intake; (![]() ) Na intake; (
) Na intake; (![]() ) K intake.
) K intake.
Discussion
The present study demonstrates that polyphenol-rich DC reduces fasting blood glucose levels and BP in overweight and obese individuals. These findings are consistent with previous observations that polyphenol-rich DC intake improved insulin resistance, insulin sensitivity, FG levels and BP in healthy individuals(Reference Grassi, Lippi and Necozione4), hypertensives(Reference Grassi, Necozione and Lippi5), glucose-intolerant hypertensives(Reference Grassi, Desideri and Necozione6) and obese subjects(Reference Davison, Coates and Buckley7). The results are also in agreement with studies on diabetic obese mice, where reductions in blood glucose and fructosamine levels were reported following consumption of cacao liquor procyanidins(Reference Tomaru, Takano and Osakabe43).
Enhanced vascular function is thought to be the main mechanism by which DC polyphenols improve glucose and BP homeostasis(Reference Grassi, Lippi and Necozione4–Reference Davison, Coates and Buckley7, 23, Reference Taubert, Roesen and Lehmann34, Reference Karim, McCormick and Kappagoda44–Reference Faridi, Nijke and Dutta47), although other mechanisms like decreased and delayed carbohydrate digestion and absorption might also be involved(Reference Quesada, Bartolomé and Nieto48, Reference McDougall, Shpiro and Dobson49). The present study investigated whether polyphenol-rich DC could alter cortisol metabolism and whether improvements in glucose and BP seen in obese individuals following DC consumption are linked to improved cortisol metabolism. The hypothesis was based on that cortisol plays an important role in glucose and BP homeostasis, probably through a mechanism involving increased reactive oxygen species production and decreased NO bioavailability, and that in obesity several alteration in cortisol metabolism are observed, which are, in turn, linked to increased insulin resistance and hypertension. The study demonstrates that both 500 and 1000 mg polyphenol DC decrease 24-h urinary free cortisol and cortisone levels. However, these reductions were not significant and are not associated with reductions in fasting blood glucose or BP. Such findings differ from previous findings, wherein polyphenols increased(Reference Song, Lorenzo and Reidenberg50–Reference Sardi, Geda and Nerici53) or decreased(Reference Arion, Canfield and Ramos54, Reference Hemmerle, Burger and Below55) cortisol levels(Reference Lamuela-Raventós and Andrés-Lacueva56). The lack of significance could be related to a number of factors. For instance, the sample size might have not been sufficiently large to detect a significant change. In this case, using several parameters of cortisol metabolism including its measurement in urine, saliva and blood might have helped detect any such effect. Additionally, the study population consisted mainly of subjects with peripheral obesity rather than those with abdominal obesity, who exhibit more prominent abnormalities in cortisol metabolism as indicated by the association between high waist circumference or waist-to-hip ratio and high urinary cortisol or cortisone-to-cortisol ratio(Reference Vicennati and Pasquali11, Reference Fraser, Ingram and Anderson57). In addition, differences in Na intake were not controlled for and could have acted as confounding factors(Reference Chamarthi, Kolatkar and Hunt58). This could be observed in the association between changes in urinary free cortisol or cortisone and changes in Na excretion, and the association between changes in Na intake and changes in cortisol-to-cortisone ratio. Dietary factors have been reported to influence cortisol metabolism. High-fat low-carbohydrate diets stimulate cortisol regeneration by 11β-hydroxysteroid dehydrogenase type 1, while reducing cortisol inactivation in liver(Reference Stomson, Johnstone and Homer59). Na loading, on the other hand, decreases plasma cortisol levels by enhancing cortisol elimination(Reference Litchfield, Hunt and Jeunemaitre60) possibly via a mechanism involving increased hepatic blood flow(Reference Kerstens, Kleij and Bonnstra61). The latter may explain the association between increased urinary free cortisol excretion and urinary Na levels. However, subjects did not report significant changes in Na intake during the study, which overall suggest that DC polyphenols influence glucose and BP homeostasis mainly via the NO pathway.
The present study demonstrates that DC with 500 mg polyphenols is as effective in reducing fasting blood glucose levels in overweight and obese individuals as 1000 mg polyphenol DC with a similar macronutrient composition. Furthermore, the results indicate that DC polyphenols reduce blood glucose levels after 2 weeks of commencing a polyphenol-rich DC diet. These findings are important since in relation to glucose metabolism, inconsistencies still exist regarding the treatment duration and dose required to achieve a glucose-lowering effect. For example in their pilot study, Stote et al. (Reference Stote, Clevidence and Baer62) failed to show any significant improvement in glucose levels, insulin resistance and insulin sensitivity following 5 d of twice daily consumption of procyanidin-rich cocoa beverage containing 22–900 mg procyanidins by insulin-resistant men and women. Similarly, Taubert et al. (Reference Taubert, Roesen and Lehmann34) failed to demonstrate any improvement in glucose or insulin levels following 18 weeks of daily ingestion of 6·3 g DC containing 30 mg polyphenols. Conversely, Davison et al. (Reference Davison, Coates and Buckley7) showed reduced insulin resistance following consumption of a cocoa beverage containing 902 mg flavanols twice daily for 12 weeks in overweight and obese subjects. Together, these findings suggest that a longer duration and a higher dose of polyphenols could be required to achieve a significant reduction in glucose levels. The present study reinforces this hypothesis while demonstrating that increasing polyphenol dose does not necessarily results in further reductions in glucose and BP levels since a saturation effect may occur with increasing DC polyphenol content(Reference Grahame-Smith and Aronson63). It also highlights the need to identify the minimum polyphenol dose at which maximal health benefits could be achieved, since a reduction in the polyphenol content of chocolate implies reduced bitterness(Reference Luna, Crouzillat and Cirou64, Reference Counet, Ouwerx and Rosoux65), which could render the chocolate more palatable and acceptable to the general population. In relation to BP, 20 g DC with 500 mg polyphenols reduced SBP and DBP to a similar extent as 20 g DC with 1000 mg polyphenol. Moreover, the reduction in BP observed following the 20 g DC (500 mg polyphenols) was comparable to the reduction reported in a previous study on normotensive subjects (7 and 3 mmHg reduction in SBP and DBP, respectively)(Reference Grassi, Lippi and Necozione4). This might suggest that reducing the portion of DC while maintaining a similar total phenol content results in equivalent reductions in BP. This could provide several advantages since reducing the portion of DC would permit delivery of high quantity of polyphenols in a less energy-dense form, which is essential if DC is to be included as part of a healthy balanced diet.
In contrast to Fraga et al. (Reference Fraga, Actis-Goretta and Ottaviani66) and Grassi et al. (Reference Grassi, Necozione and Lippi5), we did not observe any significant changes in total cholesterol. Such results are to be expected since our subjects had normal baseline total blood cholesterol levels as compared to Grassi et al. (Reference Grassi, Necozione and Lippi5) (baseline total cholesterol = 5·4 (sd 0·6) mmol/l). Moreover, Grassi et al. (Reference Grassi, Necozione and Lippi5) suggested that both the catechin and the fat component of DC account for its beneficial effect on total cholesterol. Similar assumptions were made in relation to stearic acid in DC(Reference Ding, Hutfless and Ding67–Reference McKeown, Jacque and Zhang69). Since in the present study a lower DC portion was used, the lack of significant change in total blood cholesterol could be related to the lower levels of linoleic, oleic and stearic acids present in this DC. There was also a lack of correlation between the reported energy intake and physical activity, which is similar to the findings of Davison et al. (Reference Davison, Coates and Buckley7), who argued that obese individuals might underreport energy intake and overreport physical activity.
In conclusion, the present study confirms previous reports of improved FG levels and BP following DC consumption. It also demonstrates that these effects do not appear to be mediated through changes in cortisol metabolism. Further studies are needed to identify the optimal dose of polyphenols required to improve glucose metabolism and to examine additional parameters that could be influenced by polyphenols.
Acknowledgements
We would like to thank all the volunteers who participated in the present study. We would also like to thank Barry Callebaut, Belgium, for their continuous support and for providing us with Acticoa dark chocolate. S. A. contributed to study design, data acquisition, data analysis and manuscript preparation. C. H. assisted with urinary electrolyte analysis and E. A., S. A. assisted with glucocorticoid analyses. Both E. A., S. A. and L. F. supervised the study, reviewed and edited the manuscript.
None of the authors had any personal or financial conflict of interest.










