Iodine is an essential trace element needed for the synthesis of the thyroid hormones, thyroxine (T4) and triiodothyronine (T3), with iodine comprising 65 and 59 % of their weights, respectively. Thyroid hormones regulate the metabolic processes in most cells and play a determining role in the process of early growth and development of most organs as well, especially that of the brain(1).
Despite a worldwide successful implementation of iodine fortification and supplementation programmes over the last four decades, iodine deficiency remains a public health problem in Europe. In 2004, it was estimated that of the 2 billion people around the world at risk of iodine deficiency, 20 % live in Europe(Reference De Benoist, McLean and Andersson2). On the basis of the national medians of urinary iodine concentrations (UIC) for forty countries, it has been estimated in 2003 that the populations of nineteen countries had adequate iodine nutrition, twelve had mild iodine deficiency (MID), one had moderate iodine deficiency and eight countries had insufficient data(1). Over the last two decades, extraordinary progress has been achieved in decreasing the prevalence of iodine deficiency disorders by increasing the number of people with access to iodised salt(Reference Zimmermann, Jooste and Pandav3). In Europe, however, compared to other regions in the world, still only nine out of forty countries had household coverage of iodised salt of at least 90 % in 2003(1). Moreover, legislation on iodised salt varies from country to country, which creates difficulties for the food trade and results in an increased number of non-iodised foods on the market(Reference Zimmermann and Andersson4). Recently in 2010, however, it has been reported that the number of children with low iodine intakes in Europe has decreased by approximately 30 % since 2003 and that the number of European countries in which iodine deficiency remains a public health problem decreased from twenty-three to fourteen(Reference Zimmermann and Andersson4).
Several surveys in the last 50 years repeatedly indicated that Belgium has been affected by MID(Reference Delange, Van Onderbergen and Shabana5–Reference Bourdoux7). In 1998, a representative survey in Belgian school children showed a median UIC of 80 μg/l(Reference Delange, Van Onderbergen and Shabana5), lower than the optimal urinary median values of 100–199 μg/l in the population(8). A survey among 401 healthy subjects aged between 40 and 60 years, of Belgian, Moroccan, Turkish and Congolese descent residing in Brussels, revealed a median UIC of 68 μg/l with a frequency of 73 % below 100 μg/l(Reference Moreno-Reyes, Carpentier and Macours9).
The main consequences of MID in the adult population are a higher prevalence of multinodular goitre and thyroid nodules, which may be responsible for hyperthyroidism(1, Reference Laurberg, Nohr and Pedersen10–Reference Baltisberger, Minder and Burgi14). MID may prevent children from reaching their full intellectual potential(Reference Zimmermann15). Correction of mild-to-moderate iodine deficiency in primary school children was shown to improve cognitive and motor function(Reference Gordon, Rose and Skeaff16, Reference Zimmermann, Connolly and Bozo17). In addition, MID represents an economic burden to the Belgian healthcare system, as previously reported(Reference Vandevijvere, Annemans and Van Oyen18).
Optimising iodine intake was chosen among several other nutritional issues as a priority by the Ministry of Health in its ‘National Nutrition and Health Plan for Belgium’ (NNHP-B) for the period 2005–10(Reference Moreno-Reyes, Van and Vandevijvere19). The first intervention was started in 2009: from April onwards, the bakery sector agreed with the Federal Public Service on Health, Food Chain Safety and Environment to use only iodised salt (10–15 parts per million (ppm)) in the production of bread. Presently, this has not yet been regulated by law, but there has been a lot of publicity among bakers to encourage them to use iodised salt instead of regular salt in the production of bread. The approach is selective: the use of iodised salt is only encouraged in the production of bread. Considering the estimated daily intake of 80 μg of iodine in Belgium(Reference Delange, Van Onderbergen and Shabana5), the overall strategy of the NNHP-B aims at an initial increase of daily iodine intake of 30 μg. In a next phase, taking into account the results of the monitoring programme, a second increase of 40 μg of daily iodine intake is aimed at in order to attain 150 μg of daily iodine intake over a period of 10 years. The usual daily bread consumption of the Belgian adult population is 120 g/d(Reference De Vriese, Huybrechts and Moreau20). Taking into account a salt content of 12·5 g/kg of bread, more or less 1·5 g salt/d (about 2·0 g salt/d) is consumed via consumption of bread. If salt iodisation in bread is performed with 10–15 mg iodine/kg salt (10–15 μg iodine/g salt), as is recommended by the Belgian Superior Health Council, then this will lead to a daily iodine intake of more or less 30 μg iodine/d, via consumption of bread. Further, in general, people are encouraged to buy and use iodised household salt instead of non-iodised salt; and pregnant women are advised to use food supplements.
The objective of the present study was to calculate the distribution of iodine intake among Flemish preschool children and to identify the major dietary sources contributing to the iodine intake. Further, the impact of a more generalised utilisation of iodised salt in bread on iodine intake of preschool children was assessed.
Methods
Survey population
The present study used data from the Flanders Preschool Dietary Survey (data collected from October 2002 until February 2003) in which usual dietary intake was calculated from 3-d estimated dietary records and a forty-seven-item FFQ, completed by a proxy (parent/caregiver). The target population comprised preschool children aged 2·5–6·5 years. The sampling design, methods, response rate (50 %) and representativity of the study sample have been described previously(Reference Huybrechts, Matthys and Pynaert21). In brief, schools were used as primary sampling units and classes as secondary sampling units. A total of 2095 children from forty-three schools were invited to participate. The percentage of under-reporters was low ( < 2 % of the children when using the individual Goldberg cut-offs adopted for children) and has been described in depth elsewhere(Reference Huybrechts and De Henauw22). They have not been excluded from the analyses in this paper.
The Ethical Committee of the Ghent University Hospital (Ghent, Belgium), De Pintelaan 185, 9000 Ghent granted ethical approval for the study. Informed written consent was obtained from all parents.
Assessment of dietary iodine intake
For the purposes of the present analyses, only food diaries with three completed record days were included, resulting in a final sample of 696 (66 % of the 1052 collected) diaries.
Using a systematic procedure, the following food composition tables (listed in order of priority) were used to perform nutrient calculations: the Belgian food composition table Nutriënten België (NUBEL)(23), the food composition table of the Belgian Institute Paul Lambin (Brussels, Belgium)(24), Dutch food composition database Nederlands Vodingsstoffenbestand (NEVO)(25), German food composition database (Bundes Lebensmittel Schlüssel (BLS); version II.3.1, 2005)(Reference Dehne, Klemm and Henseler26) and the McCance and Widdowson's UK food composition tables(27). Within the context of this study, the systematic procedure refers to the use of the Belgian food composition tables as the basic tables and thereafter when insufficient information was available in these Belgian databases, the other databases were used. The Belgian food composition databases (NUBEL and Institute Paul Lambin) contained only little information with regard to iodine concentrations in foods. For milk, there was a considerable difference in iodine concentrations between the German BLS food composition database ( ± 10 μg/100 ml) and the McCance and Widdowson's food composition database ( ± 30 μg/100 ml). Therefore, for dairy products, both the German BLS food composition database and the McCance and Widdowson's UK food composition database were used in two different scenarios.
In total, 936 foods and composite dishes were reported in the food diaries of the 696 children which were included in the study sample. All recipes disaggregated into ingredients in the diaries were encoded as ingredients in the database. In order to enable classification of foods into food groups of the Flemish Food-Based Dietary Guidelines(28), eight extra composite dishes had to be disaggregated into ingredients using standard recipes (nasi goreng, nasi goreng with egg, spaghetti Bolognese, chicken ragout, turkey ragout, lasagne, macaroni ham/cheese sauce, and stew). The ingredients could then be classified in the existing food groups.
Hypothetical iodine intakes via dietary supplements were calculated using data from the general questionnaire, in which the frequency of consumption of dietary supplements (every day, 5–6 d/week, 2-4 d/week, 1 d/week and 1-3 d/month) was asked. No iodine supplements were reported among the dietary supplements reported, but multi-minerals, multi-vitamins and multi-mineral–vitamin supplements were included. Because no quantity was asked with regard to dietary supplement intakes, standard units were used (e.g. one tablet or one capsule) to quantify the daily intakes. Since no detailed information was collected about brand and composition of the supplements, the iodine composition of the different brands of a supplement were aggregated to calculate the mean composition of the different dietary supplements captured in the questionnaire. The supplement composition data used for this aggregation procedure were based on the dietary supplement information, collected within the Belgian food consumption survey, while excluding dietary supplements for special use (e.g. pregnancy). Thereafter, frequency of dietary supplement consumption was multiplied with the aggregated supplement composition of each particular dietary supplement. An in-depth analysis reporting the frequency of consumption of dietary supplements among Flemish preschoolers is published elsewhere(Reference Huybrechts, Maes and Vereecken29).
Four scenarios were used to calculate habitual iodine intake in Flemish preschool children. In scenario 1, the percentage of bakers using iodised salt in bread in 2002 (the year of the preschool children survey) and iodine concentration values for dairy products from the German BLS composition table were used, while in scenario 2, the percentage of bakers using iodised salt in bread in 2011 (after the intervention) and iodine concentration values for dairy products from the German BLS composition table were used. In scenario 3, the percentage of bakers using iodised salt in bread in 2002 (the year of the preschool children survey) and iodine concentration values for dairy products from the McCance and Widdowson's composition table were used, while in scenario 4, the percentage of bakers using iodised salt in bread in 2011 (after the intervention) and iodine concentration values for dairy products from the McCance and Widdowson's composition table were used. As the Belgian nutrition policy encourages only the use of industrially iodised salt (10–15 ppm) in the production of bread, for all other foods only natural iodine was taken into account. The use of industrially added iodised salt in other foods is assumed to be negligible as iodised salt is a bit more expensive and its use in other food products is not encouraged by the government in Belgium. For bread, a concentration value of 12·5 ppm was used in the calculations. The percentage of bakers using iodised household salt in 2002 (before the intervention) was 12 % and in 2011 (after the intervention) 44 %. These data were obtained from ESCOSALT (http://www.esco-salt.com/en/index.html), one of the main suppliers of iodised salt to the bakers in Belgium.
All scenarios took into account the use of iodised household salt, using a concentration value of 17·5 ppm in the calculations, as iodised salt with 15–20 ppm of iodine is most frequently found on the Belgian market. The percentage of children for whom parents use salt in the preparation of recipes and/or add salt afterwards to the meals of the children, and the percentage of parents stating that they are using iodised salt in the household, were obtained from a recently completed survey among school-aged children (6–12 years old). From these data, it was derived that the percentage of children using iodised household salt was 26 % (S Vandevijvere, unpublished results).
Statistical analyses
The Statistical Package SAS 9.2 (SAS Institute Inc., Cary, NC, USA) was used to perform statistical analyses. A simulation model, developed by Verkaik-Kloosterman et al. (Reference Verkaik-Kloosterman, van't Veer and Ocké30) combining deterministic and probabilistic approaches was used to estimate habitual iodine intake in Flemish preschoolers, according to the four scenarios. The details of this method can be found elsewhere(Reference Verkaik-Kloosterman, van't Veer and Ocké30). Briefly, iodine intake from four different potential dietary sources was estimated separately: (1) iodine found naturally in foods, (2) iodine added to bread via iodised salt, (3) iodine from discretionarily added iodised household salt and (4) iodine via iodine-containing dietary supplements. Iodine intake via natural sources was calculated in a deterministic way by multiplying the consumed amount of a food by a point estimate of the natural iodine level in that food. Iodine intake via iodised salt used in the production of bread was calculated using a probabilistic approach.
A random sample of 12 % (scenarios 1 and 3) and 44 % (scenarios 2 and 4) of preschool children was drawn among the consumers of bread. The selected children were assumed to only consume bread made with iodised salt during the three consecutive days of registration. The sampling was repeated for 100 iterations, to take into account this uncertainty. For each iteration, the daily intake of iodine from industrially added iodised salt to bread was calculated per subject by multiplying the consumed amount of bread in g with 25 μg iodine/100 g of bread. A similar probabilistic approach was used to estimate daily iodine intake from discretionarily used iodised household salt. A random sample of 26 % of all preschool children was drawn independently for 100 iterations. These children were assumed to use iodised household salt on all observed days. Foods consumed raw and foods already containing industrially added iodised salt were excluded. It was assumed that users of iodised household salt add it to all of the following food groups in the following amounts (amounts derived from Verkaik-Kloosterman et al. (Reference Verkaik-Kloosterman, van't Veer and Ocké30)): 0·4 g/100 g to potatoes, rice, cereals and pasta, 0·6 g/100 g to vegetables, legumes and prepared meals, 0·8 g/100 g to sauces and 1·8 g/100 g to meat, meat replacers, fish and eggs. A difference with the method of Verkaik-Kloosterman et al. (Reference Verkaik-Kloosterman, van't Veer and Ocké30) was that in this study, iodine intake via food supplements was not included in the model but presented separately, as the data were derived from an FFQ and thus already presented usual intake.
Population habitual iodine intake distribution was estimated for each of the 100 iterations separately and the distributions were compared to the estimated average requirement (EAR) of 65 μg/d(31), the RDA of 90 μg/d(31, 32) and to the tolerable upper intake level (UL) of 300 μg/d(32) to estimate the proportion of the population at risk of inadequate or potentially excessive iodine intake. The distributions were calculated using statistical modelling (Iowa State University method, developed at Iowa State University (Ames, IA, USA)) in order to correct for day-to-day variability in the 3 d estimated dietary records(Reference Guenther, Kott and Carriquiry33, Reference Nusser, Carriquiry and Dodd34). The program used to calculate usual intakes was the Software for Intake Distribution Estimation (C-side; Iowa State University)(35).
Mean usual iodine intakes were compared among boys and girls, supplement users and non-supplement users and both age groups according to the two-group Student's t test.
The population proportion formula was used to determine the percentage contribution of each food group to the intake of iodine(Reference Krebs-Smith, Kott and Guenther36), which includes summing the amount of the component provided by the food for all individuals divided by the total intake of that component from all foods for the entire study population(Reference Krebs-Smith, Kott and Guenther36–Reference Royo-Bordonada, Gorgojo and de Oya38).
Results
Characteristics of the study population
The total study sample of 696 children included 49 % boys and 46 % girls; 28 % of the children were between 2·5 and 3 years old and 67 % were between 4 and 6·5 years old (Table 1). For thirty-four children, information about sex and age was missing. The proportion of children studied in each province (30 % in Antwerp, 24 % in East-Flanders, 22 % in West-Flanders, 15 % in Flemish Brabant and 9 % in Limburg), compared well with the proportions derived from the target population, namely preschool children in Flanders (28, 23, 18, 18 and 13 %, respectively).
Table 1 Characteristics of the study population participating in the Flemish preschool dietary survey 2002
(Number of children and percentages, n 696)
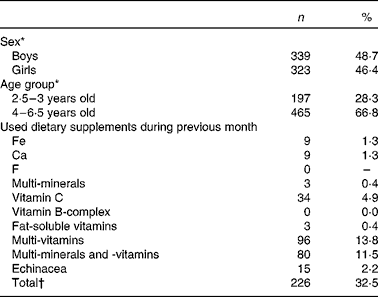
* There were thirty-four missing values for sex and thirty-four missing values for age group.
† The total number of children using dietary supplements is not equal to the sum of the children using the different types of dietary supplements as some of the children were using more than one type of dietary supplement.
Dietary iodine intake
In scenario 1, with only 12 % of the bakers using iodised salt (before the intervention) in bread and with iodine concentrations in dairy products from the German BLS food composition table, the mean dietary iodine intake was 103·9 (sd 0·7) μg/d, while in scenario 2, with 44 % of the bakers using iodised salt in bread, the iodine intake increased to 109·0 (sd 0·8) μg/d (Table 2). In scenario 3, with only 12 % of the bakers using iodised salt (before the intervention) in bread and with iodine concentrations in dairy products from the McCance and Widdowson's food composition table, the mean dietary iodine intake was 159·0 (sd 0·7) μg/d, while in scenario 4, with 44 % of the bakers using iodised salt in bread, the iodine intake increased to 164·1 (sd 0·7) μg/d (Table 3). In general, dietary iodine intake (excluding intake via food supplements) was higher among boys than girls (P < 0·001), higher among the youngest age group compared to the older age group (P < 0·001) and higher among non-supplement users compared to supplement users (P < 0·001). Additional usual iodine intake via food supplements (all days, total population) was on average 4·2 (sd 9·9) μg/d and 37·0 μg/d at the 95th percentile. For iodine-containing supplement users only, the iodine intake via food supplements was on average 16·9 μg/d (Tables 2 and 3).
Table 2 Distribution of usual iodine intake via food supplements (μg/d) and usual dietary iodine intake (including iodine intake via iodised household salt) (μg/d) with 12 % (scenario 1, 2002) and 44 % (scenario 2, 2011) of the bakers using iodised salt in the production of bread, using the iodine concentration values for dairy products from the Bundes Lebensmittel Schlüssel food composition table (Flanders preschool dietary survey 2002)
(Mean values, standard deviations, 5th percentile, 50th percentile and 95th percentile of the 100 iterations in each simulation for scenarios 1 and 2, n 696)

* Number of preschool children with three valid completed dietary record days.
† Iodine-containing supplements.
Table 3 Distribution of usual iodine intake via food supplements (μg/d) and usual dietary iodine intake (including iodine intake via iodised household salt) (μg/d) with 12 % (scenario 3, 2002) and 44 % (scenario 4, 2011) of the bakers using iodised salt in the production of bread, using the iodine concentration values for dairy products from the McCance and Widdowson's food composition table (Flanders preschool dietary survey 2002)
(Mean values, standard deviations, 5th percentile, 50th percentile and 95th percentile of the 100 iterations in each simulation for scenarios 3 and 4, n 696)

* Number of preschool children with three valid completed dietary record days.
† Iodine-containing supplements.
In scenario 1, on average 12·4 (sd 9·0) % of the children had an iodine intake below the EAR of 65 μg/d and on average 0·28 (sd 0·05) % of the children had an iodine intake above the UL of 300 μg/d. For scenarios 2, 3 and 4, these percentages were on average 9·1 (sd 8·8) and 0·33 (sd 0·07), 5·0 (sd 3·8) and 3·9 (sd 0·2), and 3·8 (sd 3·6) and 4·2 (sd 0·2), respectively (Fig. 1). The percentage of preschool children below the RDA of 90 μg/d was 41·5 (sd 1·0) % in scenario 1, 35·0 (sd 1·1) % in scenario 2, 14·1 (sd 0·4) % in scenario 3 and 11·6 (sd 0·6) % in scenario 4 (Fig. 1).

Fig. 1 Distribution of habitual dietary iodine intake (μg/d) (including iodine intake via iodised household salt, excluding iodine intake via food supplements) according to the four scenarios (scenario 1, ![]() ; scenario 2,
; scenario 2, ![]() ; scenario 3,
; scenario 3, ![]() ; scenario 4,
; scenario 4, ![]() ), compared with the estimated average requirement (
), compared with the estimated average requirement (![]() ) of 65 μg/d, the RDA (
) of 65 μg/d, the RDA (![]() ) of 90 μg/d and with the tolerable upper intake level (
) of 90 μg/d and with the tolerable upper intake level (![]() ) of 300 μg/d (Flanders preschool dietary survey 2002, n 696). Scenario 1: 12 % of bakers using iodised salt (2002) in the production of bread and iodine concentration values for dairy products from the Bundes Lebensmittel Schlüssel (BLS) German food composition table. Scenario 2: 44 % of bakers using iodised salt (2011) in the production of bread and iodine concentration values for dairy products from the BLS German food composition table. Scenario 3: 12 % of bakers using iodised salt (2002) in the production of bread and iodine concentration values for dairy products from the McCance and Widdowson's food composition table. Scenario 4: 44 % of bakers using iodised salt (2011) in the production of bread and iodine concentration values for dairy products from the McCance and Widdowson's food composition table. Values are means of 100 iterations per simulation. (A colour version of this figure can be found online at journals.cambridge.org/bjn).
) of 300 μg/d (Flanders preschool dietary survey 2002, n 696). Scenario 1: 12 % of bakers using iodised salt (2002) in the production of bread and iodine concentration values for dairy products from the Bundes Lebensmittel Schlüssel (BLS) German food composition table. Scenario 2: 44 % of bakers using iodised salt (2011) in the production of bread and iodine concentration values for dairy products from the BLS German food composition table. Scenario 3: 12 % of bakers using iodised salt (2002) in the production of bread and iodine concentration values for dairy products from the McCance and Widdowson's food composition table. Scenario 4: 44 % of bakers using iodised salt (2011) in the production of bread and iodine concentration values for dairy products from the McCance and Widdowson's food composition table. Values are means of 100 iterations per simulation. (A colour version of this figure can be found online at journals.cambridge.org/bjn).
On average, 11·6 (sd 8·6) % of the preschool children not using iodine-containing supplements (n 523) had a total iodine intake below the EAR, and on average, 0·30 (sd 0·09) % of them had a total iodine intake exceeding the UL in scenario 1. For scenarios 2, 3 and 4, these percentages were 8·46 (sd 8·32) and 0·34 (sd 0·10), 4·57 (sd 3·68) and 4·59 (sd 0·36), and 3·49 (sd 3·50) and 4·93 (sd 0·34), on average, respectively (Fig. 2). For iodine-containing supplement users (n 173), both the percentage of preschool children with an iodine intake below the EAR and above the UL were lower than in the case of non-supplement users, without taking into account iodine intake via supplements.

Fig. 2 Percentage of (a) non-iodine-containing supplement users and (b) iodine-containing supplement users with iodine intake (derived from food and iodised salt intake alone) below the estimated average requirement (EAR) of 65 μg/d and above the upper tolerable intake level (UL) of 300 μg/d for the four different scenarios. Scenario 1: 12 % of bakers using iodised salt (2002) in the production of bread and iodine concentration values for dairy products from the Bundes Lebensmittel Schlüssel (BLS) German food composition table. Scenario 2: 44 % of bakers using iodised salt (2011) in the production of bread and iodine concentration values for dairy products from the BLS German food composition table. Scenario 3: 12 % of bakers using iodised salt (2002) in the production of bread and iodine concentration values for dairy products from the McCance and Widdowson's food composition table. Scenario 4: 44 % of bakers using iodised salt (2011) in the production of bread and iodine concentration values for dairy products from the McCance and Widdowson's food composition table. Percentages presented as P50 of 100 iterations, error bar presents P5–P95 range of 100 iterations (Flanders preschool dietary survey 2002, n 696). ![]() , Percentage with intake < EAR;
, Percentage with intake < EAR; ![]() , percentage with intake >UL. (A colour version of this figure can be found online at journals.cambridge.org/bjn).
, percentage with intake >UL. (A colour version of this figure can be found online at journals.cambridge.org/bjn).
Mean iodine intake (excluding food supplements) at the first percentile for supplement users was 44·9 μg/d, while at percentile 2·5 it was 50·27 μg/d. Taking into account average iodine intake via food supplements, the percentage of children with an intake below the EAR in scenario 1 decreased to below 2·5 %, while still none of the children exceeded the UL (Fig. 2).
Dietary sources of iodine intake
Table 4 lists the food and beverage categories and their relative contributions to daily iodine intake in preschool children for scenario 1 (before the intervention, 2002) and scenario 2 (after the intervention, 2011). In scenario 1, sugared dairy products, milk, bread and fish (22·5, 13·7, 7·4 and 3·9 %, on average, respectively) were the main contributors to iodine intake of preschool children. In scenario 2, sugared dairy products, milk, bread and fish (21·4, 13·1, 8·4 and 7·0 %, on average, respectively) were the main contributors. In scenario 1, iodised household salt contributed on average 9·1 % to total dietary iodine intake, while in scenario 2 this percentage was, on average, 8·7 % (excluding food supplements; Table 4).
Table 4 Percentage contribution of dietary sources to total dietary iodine intake (including iodised household salt, excluding food supplements) for scenarios 1* and 2† (Flanders preschool dietary survey 2002)
(Mean values, standard deviations, 5th percentile, 50th percentile and 95th percentile of 100 iterations, n 696)

* 12 % of bakers using iodised salt (2002) in the production of bread and iodine concentration values for dairy products from the Bundes Lebensmittel Schlüssel (BLS) German food composition table.
† 44 % of bakers using iodised salt (2011) in the production of bread and iodine concentration values for dairy products from the BLS German food composition table.
‡ Intervention of a more generalised use of iodised salt in the production of bread by the bakers.
Discussion
It was found that at present, with 44 % of the bakers using iodised salt in bread (10–15 ppm), dependent on iodine concentrations in milk, between 4 and 9 % of the preschool children would have an iodine intake lower than the EAR and between 0·3 and 5 % of the children would have an iodine intake higher than the UL. Average estimated dietary iodine intake in preschool children in all scenarios was higher than the median urinary concentration (80 μg/l), as found by Delange et al. (Reference Delange, Van Onderbergen and Shabana5) in the nationwide study from 1998. The latter study was performed among older children (6–12 years). In the 1998 study, it was found that median UIC was almost uniformly lower in girls than in boys for all ages combined (76 μg/l in girls and 85 μg/l in boys). Also, in the present study, boys were found to have a higher iodine intake than girls.
As preschool children drink a lot of milk, it is not surprising that milk and dairy products contributed the most to total dietary iodine intake. Concentrations of iodine in milk in food composition databases vary from 8 up to 30 μg/100 ml; therefore, usual dietary iodine intake was calculated according to two scenarios in this study, as it is difficult to know the real iodine concentrations in milk consumed in Belgium. The differences in iodine intake were considerable between the use of the BLS German database and the McCance and Widdowson's food composition database: iodine intake increased by more or less 35 % on average when the McCance and Widdowson's table was used (P < 0·001). Iodine concentrations in milk from healthy dairy cattle were measured in Belgium by Guyot et al. (Reference Guyot, Saegerman and Lebreton39) and amounted, on average, to 145 μg/l. This was based only on twelve samples, but shows that the real concentrations lie somewhere between 8 and 30 μg/100 ml, such as that used in our study. It also suggests scenarios 1 and 2, using the German composition table, are probably more representative for the current situation in Belgium than scenarios 3 and 4.
After a more generalised utilisation of iodised salt in bread, the iodine intake increased by 3·1 % using the McCance and Widdowson's table and 4·7 % using the BLS German database, when the percentage of bakers using iodised salt in bread increased from 12 to 44 % (P < 0·001 in both cases). Bread becomes an important source of iodine intake in scenario 2 where 44 % of the bakers already use iodised salt. According to scenario 2, the iodine intake via the consumption of bread was about 9 μg/d; this is still far below the average iodine intake via bread of 30 μg/d as aimed by the NNHP-B.
Although the situation is getting better compared to previous studies(Reference Delange, Van Onderbergen and Shabana5, Reference Moreno-Reyes, Carpentier and Macours9), the percentage of bakers using iodised household salt should continue to increase in future and the use of iodised instead of non-iodised household salt should be further encouraged. Moreover, in view of the necessary salt reduction among the population, because salt intakes are too high(Reference Vandevijvere and Van Oyen40, Reference Vandevijvere, De Keyzer and Chapelle41), iodine intake could decrease again and the iodine policy might have to be adapted.
Assessment of iodine status in the population is usually performed via monitoring of UIC, but iodine intake is an important determinant of thyroid disorders in populations(Reference Laurberg, Cerqueira and Ovesen11). Even minor differences in iodine intake between populations could be associated with differences in the occurrence of thyroid disorders(Reference Vandevijvere, Dramaix and Moreno-Reyes42). Not many European countries report dietary iodine intakes in the literature. A survey on iodine intake in Dutch school children found a mean optimal daily iodine intake of 171 μg in boys and 143 μg in girls, which is in good agreement with the measured median urinary concentration of 167 μg/l in boys and 145 μg/l in girls. Bread consumption was the main contributor to iodine intake in the Netherlands(Reference Wiersinga, Podoba and Srbecky43). In a scenario with 12, 25 and 50 % of salt reduction in industrially processed foods, the iodine intake remained adequate for a large part of the Dutch population(Reference Verkaik-Kloosterman, van 't Veer and Ocké44). In the Netherlands, the use of iodised salt is allowed to more food groups in higher amounts than in Belgium. Furthermore, the use of iodised household salt is higher in the Netherlands than in Belgium.
In Iceland, optimal iodine intakes were found(Reference Gunnarsdottir, Gunnarsdottir and Steingrimsdottir45) and dairy products (43 %) and fish (24 %) contributed most to total iodine intake in adolescents. However, recently fish and milk consumption considerably decreased in Iceland, especially among young people. Moreover, iodised salt is not commonly used, and so future monitoring will be necessary(Reference Gunnarsdottir, Gunnarsdottir and Steingrimsdottir45, Reference Gunnarsdottir, Gustavsdottir and Thorsdottir46).
In Slovenia, the iodine intake of a national representative sample of 2581 adolescents, aged 14–17 years, was determined using the FFQ. The iodine intake was 189·7 (sd 2·6) μg/d. Table salt was by far the biggest dietary source of iodine and Na for both sexes(Reference Stimec, Kobe and Smole47).
In Portugal, the dietary intake of 7–9-year-old children was measured using a 24 h dietary recall. The proportion of children with an intake below the estimated average intake/adequate intake was very low (=10 %) for iodine(Reference Valente, Padez and Mourao48).
Although, willingness to participate leads to some selection bias, these data represent a more general population of preschool children in Flanders, compared to other food consumption surveys mostly restricted to local areas. Nonetheless, as shown previously(Reference Huybrechts, Matthys and Pynaert21), the study sample was subject to some selection bias, with lower socio-economic classes being slightly underrepresented. Furthermore, only 66 % of the diaries could be utilised in the analysis, which could affect representativity of the study population.
As with other dietary assessment methodologies, diet records are prone to a degree of misreporting, which possibly had an influence on the classification of compliance and non-compliance with the recommendations. However, the percentage of under-reporters in the final sample for analysis was less than 2 %. In addition, a 3 d diet record does not necessarily reflect an individual's usual intake. For this reason, statistical modelling (Iowa State University method) that accounts for within-individual variability(35) was used in order to calculate the usual iodine intake.
This study used a new, recently developed(Reference Verkaik-Kloosterman, van't Veer and Ocké30) simulation model to estimate habitual dietary iodine intake in Belgium. The novelty of this model is that it makes use of a combination of deterministic and probabilistic techniques to take into account observed individual dietary patterns and several uncertainties in the data. A particular problem with estimating iodine intake is the lack of detailed data about the discretionary use of iodised kitchen salt and iodisation of industrially processed foods. In this study, probabilistic methods were used to take into account part of these shortcomings. A total of 100 iterations were found to be sufficient by Verkaik-Kloosterman et al. (Reference Verkaik-Kloosterman, van't Veer and Ocké30), as in their study, duplicate simulation, differing only in which participants are drawn in the samples of iodised salt users or consumers of foods containing industrially added iodised salt, lead to comparable results.
Unfortunately, it was impossible to correct for seasonal variations, because the fieldwork was conducted only during autumn and wintertime. No data were found about the potential seasonal influences on nutrient intake in this population group in Belgium. However, from the results of the National Food Consumption Survey (2004), it could be concluded that seasonal variations have a small effect on nutrient and food intakes(Reference De Vriese, Huybrechts and Moreau49), probably due to the widespread availability of most foods round the year. Moreover, it has been shown in literature that concentrations of iodine in milk may be lower during winter than in summer(Reference Soriguer, Gutierrez-Repiso and Gonzalez-Romero50). However, in this study, iodine concentrations from food composition databases were used over all seasons. In addition, it should be noted that food composition data used for calculating nutrient intake might also introduce some bias in dietary surveys reporting nutrient intake. Therefore, the authors would like to emphasise the growing requirement for good-quality food composition data. In this study, the authors have used two different scenarios for the iodine content in milk products, using two different food composition tables, although the scenarios using the German food composition table with lower iodine concentrations in milk are likely to be more representative for the situation in Belgium. In this study, point estimates of salt iodine concentrations were used rather than distributions of possible concentrations because data about the distribution of salt iodine concentrations are currently lacking.
Conclusion
Dietary iodine intakes could still be improved in Flemish preschool children, especially compared to most other West-European industrialised countries. Milk and dairy products contributed most to total iodine intake. The use of adequately iodised household salt and the more generalised use of iodised salt by bakers should be further encouraged. Further, the situation should be monitored using nationwide surveys on iodine concentrations in urine samples among children.
Acknowledgements
The authors thank all the parents and teachers who participated in this project and generously volunteered their time and knowledge. The Flanders Preschool Dietary Survey was funded by the Belgian Nutrition Information Center. The contributions of the authors were as follows: S. V. performed and interpreted the statistical analyses and drafted the manuscript. All other authors helped in the evaluation of the results and in the writing of the manuscript. Moreover, I. H. was responsible for the study protocol and the fieldwork. All authors read and approved the manuscript. The authors declare no conflicts of interest.








